Abstract
Silk-elastin-like protein polymers (SELPs), consisting of the repeating units of silk and elastin blocks, combine a set of outstanding physical and biological properties of silk and elastin. Due to the unique properties, SELPs have been widely fabricated into various materials for the applications in drug delivery and tissue engineering. However, little is known about the fundamental self-assembly characteristics of these remarkable polymers. Here we propose a two-step self-assembly process of SELPs in aqueous solution for the first time and report the importance of the ratio of silk to elastin blocks in a SELP’s repeating unit on the assembly of the SELP. Through precise tuning of the ratio of silk to elastin, various structures including nanoparticles, hydrogels and nanofibers could be generated either reversibly or irreversibly. This assembly process might provide opportunities to generate innovative smart materials for biosensors, tissue engineering and drug delivery. Furthermore, the newly developed SELPs in this study may be potentially useful as biomaterials for controlled drug delivery and biomedical engineering.
Keywords: SELPs, self-assembly, biosynthesis, nanostructures, protein polymer
INTRODUCTION
Nature has evolved a variety of complex yet elegant and highly functional materials that form fascinating structures across a multitude of length scales.1,2 Genetically engineered protein-based biopolymers that mimic the remarkable designs in nature have attracted much attention. For example, bioengineered spider silk protein was produced and spun into a fiber displaying mechanical properties comparable to those of native silk.3 Similarly, elastin-like proteins were assembled into organized fibrous structures with properties similar to those of native elastin.4,5 Recently, a class of silk-elastin-like protein polymers (SELPs), consisting of repeating units of silk and elastin, have been synthesized6,7 and fabricated into various structures, such as microdiameters fibers, nanofibrous scaffolds, displaying unique mechanical properties that combine high tensile strength of silk and resilence of elastin.8,9 The potential applications of these polymers in drug delivery and tissue engineering are under investigation.10–14 However, despite the growth in applications-related studies with SELPs, little is known about the fundamental self-assembly characteristics of these remarkable polymers.15,16
Silk blocks or domains (GAGAGS) mimicking the natural silkworm heavy chain tend to assemble into insoluble tightly packed secondary structures, β-sheets,17,18 whereas elastin blocks (GXGVP) are highly hydrated by water below the transition temperature. Due to the fact that the silk and elastin blocks exhibit large differences in solubility,19–21 we speculated that SELPs may result in phase separation, where assembly into micellar-like particles occurs with a core of less soluble silk blocks and a hydrated corona composed of more soluble elastin blocks. Moreover, we speculated that the ratio of silk to elastin blocks in a SELP’s repeating unit might play an important role in the assembly of the SELP.
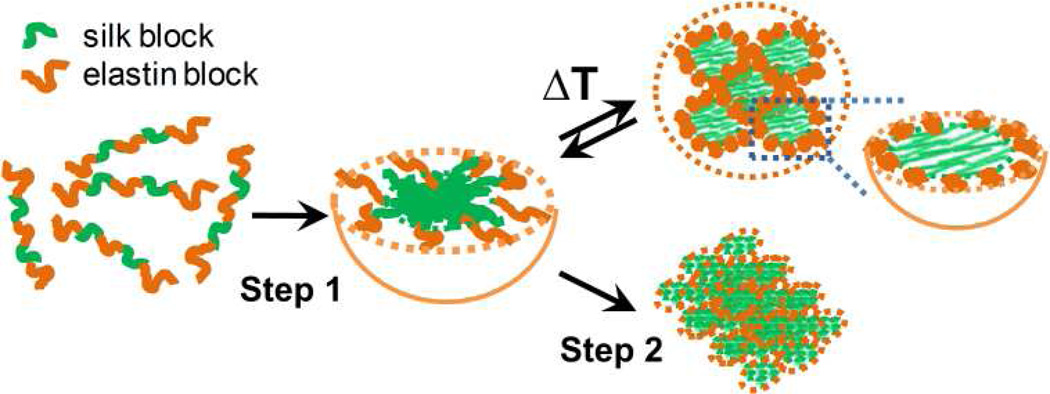
Elastin blocks undergo a reversible phase transition at a transition temperature (Tt), which is affected by the chemical identify of the second amino acid (X) in the pentapeptide repeat. They are soluble in aqueous solutions at a temperature below the Tt and undergo an entropically driven, temperature induced contraction and self assembly into aggregates at a temperature above the Tt.19–21 Therefore, we hypothesized that SELPs would, with an increase in temperature, self-assemble into higher-order nanostructures driven by a combination of hydrophobic interactions19–21 and hydrogen bonding,22 and that the ratio of silk to elastin blocks might tune this self-assembly process reversibly or irreversibly (Scheme 1).
Scheme 1.
Proposed two-step self-assembly of silk-elastin-like protein polymers. Step 1: spontaneous formation of micellar-like particles; step 2: thermal responsive self-assembly of the particles into reversible coacervates or irreversible gel states.
EXPERIMENTAL SECTION
MATERIALS
Synthetic oligonucleotides and genes were obtained from Invitrogen (Carlsbad, CA) and GenScript (Piscataway, NJ), respectively. E. coli competent cells of DH5α and BL21Star (DE3) were purchased from Invitrogen. Restriction endonucleases and T4 DNA ligase were obtained from New England Biolabs (Beverly, MA). The Plasmid Miniprep Kit and QIAquick Gel Extraction kits and nickel-chelated Sepharose resin were purchased from Qiagen (Valencia, CA). Plasmid pET-19b and the QuikChange site directed mutagenesis kit were obtained from Novagen, Inc. (Madison, WI) and Stratagene (La Jolla, CA), respectively. General reagents for protein expression and purification were obtained from Sigma (St. Louis, MO) and Fisher Scientific (Fairlawn, NJ).
METHODS
Synthesis of Polymers
Construction of Expression Vector
All molecular biology procedures were conducted according to standard protocols. The tailor-made expression vector, pET-19b3, was constructed for the expression of silk-elastin-like protein polymers under the T7 promoter. First, plasmid pET-19b was subjected to site-directed mutagenesis to eliminate three BanII sites on the plasmid. The first round of mutagenesis was performed to eliminate the BanII site in the intragenetic region of the lacI gene with mutagenesis primers FlacIm (5’-CGCGCTGTTAGCGGGACCATTAAGTTCTGTC-3’) and RlacIm (5’-GACAGAACTTAATGGTCCCGCTAACAGCGCG-3’), which results in a synonymous mutation in the lacI gene. The remaining BanII sites were removed in the second round of mutagenesis using primers FBan2 (5’-GGGGAAGATCGGGCTAGCCACTTCAGGCTCATGAGCGCTT-3’) and RBan2 (5’-AAGCGCTCATGAGCCTGAAGTGGCTAGCCCGATCTTCCCC-3’). The resulting plasmid, pET-19b2 was further modified to insert a DNA linker in the multiple cloning sites. The DNA linker was designed to contain terminal NdeI and BamHI restriction sites that permit insertion of the oligonucleotide linker into pET-19b2. The linker DNA sequence was also designed to contain an internal BanII restriction site, which permits insertion of the DNA sequence of a SELP protein. The oligonucleotides F19b (5’-TATGGGTGCTGGTGCGGGCTCTTAAG-3’) and R19b (5’-GATCCTTAAGAGCCCGCACCAGCACCCA-3’) were annealed and ligated with NdeI-BamHI digested plasmid pET-19b2. The resulting plasmid containing the correct linker DNA sequence was designated as pET-19b3 (Supporting Information Figure S1).
Construction of Expression Plasmids
DNA sequences were designed to encode each of the three silk-elastin-like sequences: SE8Y[(GAGAGS)(GVGVP)4(GYGVP)(GVGVP)3], S2E8Y[(GAGAGS)2(GVGVP)4(GYGVP)(GVGVP)3] and S4E8Y[(GAGAGS)4(GVGVP)4(GYGVP)(GVGVP)3]. The monomer DNA sequences were purchased as synthetic genes that were cloned into EcoRV site of the vector pUC57 from GenScript. The BanII restriction sites were designed to flank each of the monomer DNA sequences. The monomer DNA sequences were liberated by digesting the pUC57 derivatives with BanII, isolated by preparative gel electrophoresis, and purified using the QIAquick Gel Extraction kit. The purified monomer DNA was then self-ligated with T4 DNA ligase for 8 h at 16°C to yield DNA multimers. Next, the BanII and alkaline phosphatase-treated pET-19b3 plasmid was added to the reaction mixture and incubated for additional 16 h. The ligation mixture was then used to transform chemically competent cells of E. coli DH5α. The resulting transformants contained recombinant plasmids that carried repetitive genes of varying lengths. These expression plasmids were identified by restriction digest analysis with the enzymes NcoI and BamHI and confirmed by dideoxy sequencing with both forward and reverse primers based on the T7 promoter and terminator sequences (Tufts Core Facility). The pET-19b3-derived expression plasmid also encodes an N-terminal hexahistidine tag that permits protein purification via metal chelating affinity chromatography.
Protein Expression and Purification
The plasmids pSE8Y, pS2E8Y and pS4E8Y were used to transform chemically competent cells of E. coli strain BL21Star (DE3). The recombinant strains were grown at 37°C in a 4-L flask containing 1 L of Luria-Bertani medium in a shaking incubator at 250 rpm. Cells were induced with 1 mM isopropyl-β-d-thiogalactopyranoside when the optical density at 600 nm reached approximately 0.7. At 6 h after induction, cells were harvested by centrifugation at 9,000 g for 20 min at 4°C. The supernatant was decanted, and the cell pellets were resuspended in denaturing lysis buffer (100 mM NaH2PO4, 10 mM Tris•HCl, 8 M urea, pH 8.0) overnight. The resuspension was centrifuged at 9,000 g for 30 min and 15°C. The resulting supernatant was loaded onto a nickel chelating resin column that had been equilibrated with the denaturing lysis buffer. The column was washed and eluted with buffers (100 mM NaH2PO4, 10 mM Tris•HCl, 8 M urea) at pH 6.3 and 4.5, respectively. The purified proteins were dialyzed (MWCO 3.5 kDa) against deionized water for 5 days and concentrated using an Amicon® Ultra-15 centrifugal filter unit with Ultracel-30 membrane (Millipore, Billerica, MA). Protein concentrations were measured using a Pierce BCA Protein Assay kit (Product # 23225; Thermo Scientific, Rockford, IL). The purity of the proteins was monitored via SDS-PAGE. The molecular weights of the purified proteins were confirmed via matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Tufts Core Facility).
Structure Characterization of Polymers
UV-Visible Spectrophotometry
The inverse temperature phase transition of the protein polymers was characterized by monitoring the absorbance of an aqueous protein solution of 0.5 mg/mL at 300 nm as a function of temperature on an Aviv 14DS UV-Vis spectrophotometer equipped with a Peltier temperature controller (Aviv Biomedical, Lakewood, NJ).6,7 Reversibility of the transition was examined by fist heating from 20°C to 100°C at a rate of 3°C/min, and then cooling to 20°C at the same rate. Absorbance readings were taken after equilibrating a polymer solution at the desired temperature for 30 s. The inverse transition temperature (Tt) was defined as the temperature corresponding to 50% of the maximum value.
Differential Scanning Calorimetry (DSC)
DSC experiments were performed on a differential scanning calorimeter (Nano DSC II Model 6100; Calorimetry Sciences Corporation, Lindon, UT). Sample solutions (1 mg/mL) were heated from 10°C to 100°C at a rate of 1 °C/min and then cooled to 10°C at the same rate.
Circular Dichroism (CD)
CD spectra were recorded on an Aviv model 62DS spectrophotometer equipped with a Peltier temperature controller (Aviv Biomedical). Cuvettes with 1-mm path length were used. Protein solutions (0.1 mg/mL in distilled water) were equilibrated for 30 min at 4°C before measurement. Temperature-dependent CD scans were performed from 4 to 95°C with 10 min equilibration at each temperature. The reversibility of the CD spectra was measured by scanning over a decreasing temperature range with the same equilibration period. Spectra were obtained from 260 to 190 nm at a resolution of 0.5 nm and at a scanning speed of 50 nm/min. The CD spectra represented the average of three measurements and were smoothed using SigmaPlot data smoothing program. CD data are reported as mean residue ellipticity ([θ], deg cm2 dmol−1).
Dynamic Light Scattering (DLS)
DLS was carried out on a DynaPro Titan instrument (Wyatt Technology, Santa Barbara, CA) equipped with a temperature controller. All samples were filtered through a 0.2 µm Whatman Anotop filter before measurement. The protein solutions (0.2 mg/mL) were introduced into quartz cuvettes and the samples stabilized at the desired temperature for 10 minutes prior to measurement. To obtain the hydrodynamic radii, the intensity autocorrelation functions were analyzed using the Dynamics software (Wyatt Technology, Santa Barbara, CA).
Atomic Force Microscopy (AFM)
AFM was performed in tapping mode using a Dimension 3100 Scanning Probe Microscope with Nanoscope III and IV controllers (Digital Instruments, Santa Barbara, CA) and equipped with rotated tapping-mode etched silicon probes (RTESP; Nanodevices, Santa Barbara, CA). The commercial silicon tip probe had a spring constant of 1–5 N m−1, a resonance frequency of 60–100 kHz. Both topography and phase signal images were recorded with 512 × 512 data points. All samples (0.2 mg/mL) were casted on silicon surfaces at room temperature unless noted otherwise and allowed to dry for two days. Particle size measurements were obtained from several micrographs acquired in the AFM.
Scanning Electron Microscope (SEM)
All samples (0.02 mg/mL) were casted on silicon surfaces and allowed to dry for two days at room temperature. Next, the specimens were sputter-coated with gold by double side of carbon for enhancing surface conductivity, and then examined using a Zeiss Supra 55 VP SEM (Oberkochen, Germany). Particle size measurements were obtained from several micrographs acquired in the SEM.
RESULTS AND DISCUSSION
Biosynthesis of SELPs with Different Ratios of Silk to Elastin
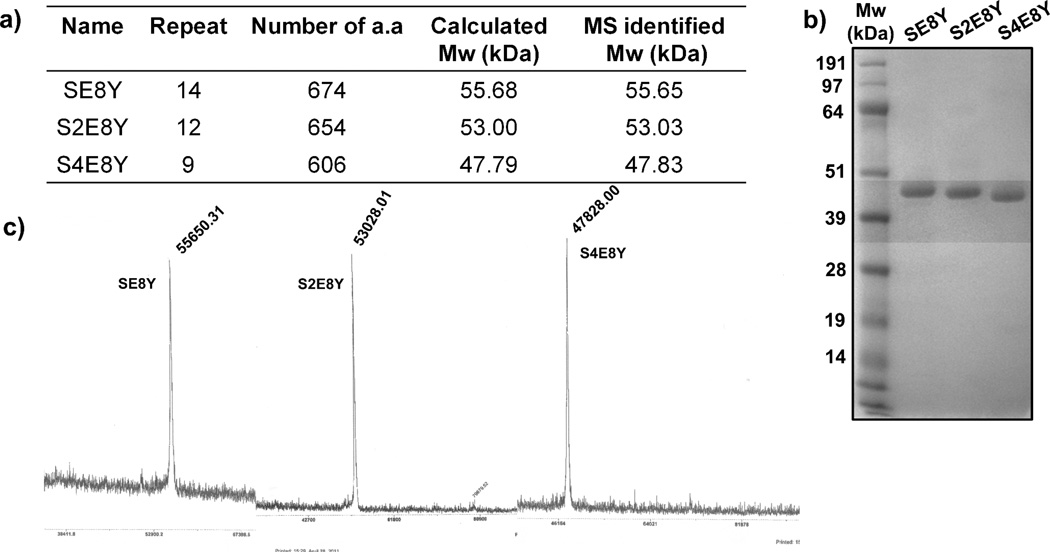
To test our hypothesis, three SELPs were genetically engineered with varying ratios of silk to elastin blocks in a monomer repeat. The three monomer genes were chemically synthesized and multimerization of each monomer gene was achieved through the “concatemerization” strategy employing the restriction enzyme site BanII. Each of the multimer genes was then cloned into a tailor-made plasmid, which was derived from a commercial plasmid by removing the endogenous BanII sites and adding a linker sequence for convenient cloning (Figure S1). This seamless cloning strategy avoided the introduction of extra amino acid residues at the junctions between monomers. Expression plasmids were generated that encoded the three SELPs (SE8Y, S2E8Y, S4E8Y) with silk to elastin ratios at 1:8, 1:4, and 1:2, and molecular weights of 55.7 kDa, 53.0 kDa and 47.8 kDa, respectively (Figure 1a and Figure 2a). Tyrosine (Y) was employed in the second amino acid position of the fifth elastin block in each monomer. This hydrophobic amino acid permitted elastin blocks to display conformational transitions in the physiological temperature range based on previous studies.23,24 The three SELPs were recombinantly expressed in the bacterial host E. coli BL21 Star(DE3) and purified using immobilized-metal-affinity chromatography. The purified yields of the SE8Y, S2E8Y and S4E8Y are 50 mg/L, 80 mg/L and 85 mg/L, respectively. All the purified protein polymers were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and further confirmed by mass spectrometry (Figure 1b and c).
Figure 1.
Characterization of purified silk-elastin-like protein polymers (SELPs). (a) The repeats of the monomer genes and molecular weight (Mw) along with amino acid numbers of the encoded SELPs. (b) Coomassie-stained 4–12% SDS-PAGE gel analysis of purified SELPs. (c) MALDI-TOF spectrum of SELPs.
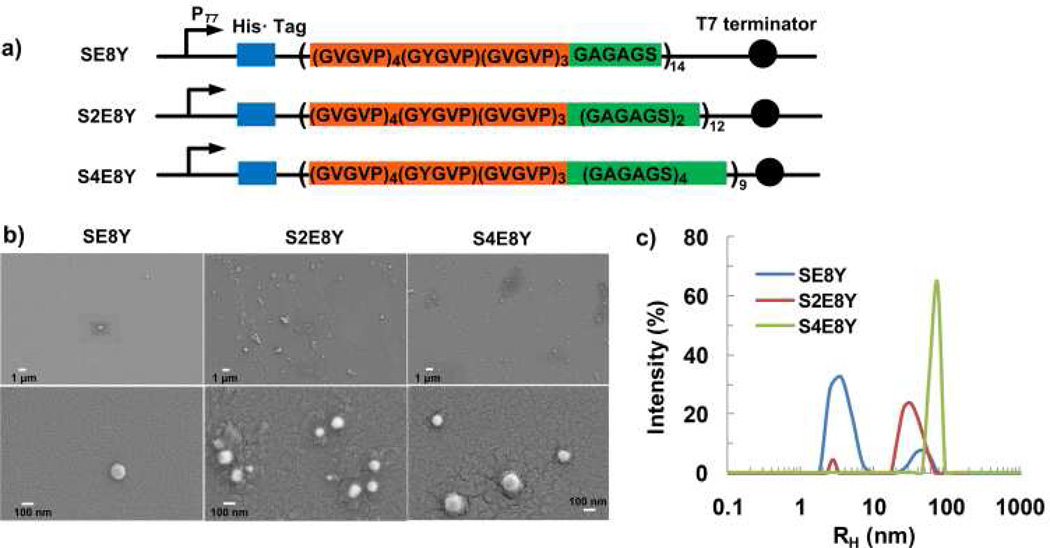
Figure 2.
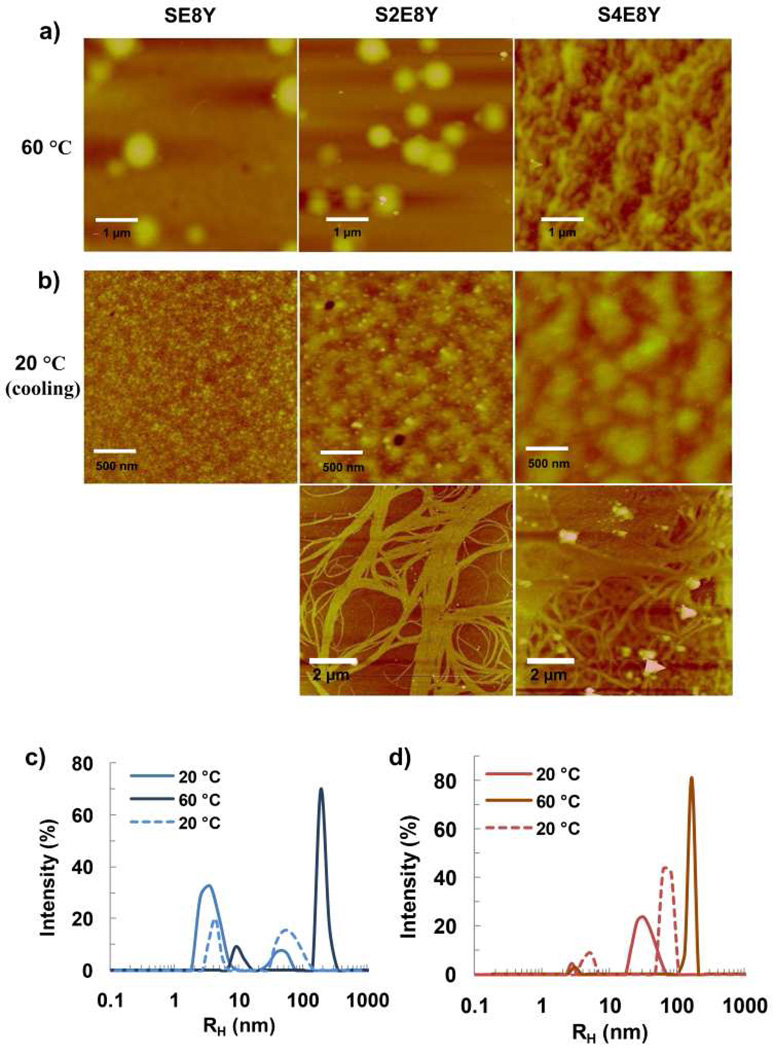
a) Constructs of silk-elastin-like proteins that contain varying ratios of the silk to elastin blocks in each monomer repeat. b) Representative SEM images of the spherical micellar-like particles derived from self-assembly of SE8Y, S2E8Y, and S4E8Y in aqueous solutions. c) DLS size distribution profiles for the SE8Y, S2E8Y, and S4E8Y solutions at 20°C.
Spontaneous Self-assembly of SELPs into Micellar-like Particles
To examine the solution behaviour of the three SELPs, we performed scanning electron microscopy (SEM) of specimens deposited from dilute aqueous solution onto silicon wafer at 20°C. Results of SEM indicated the formation of globules suggestive of micellar-like particles with average radius of 60 ± 15 nm, 55 ± 10 nm and 70 ± 12, respectively. The SEM analysis also revealed that the assembly capability of SELPs depended on the silk to elastin ratio (Fig. 2b). Similar structures have also been observed in native silk solutions collected from silk glands of the silkworm and with regenerated silk solution, also arising due to the block copolymer nature of these proteins.25 We further determined the size distribution of the particles by dynamic light scattering (DLS). Notably, scattered light at a hydrodynamic radius (Rh) of around 4.5 nm is suggestive of the presence of free chains in solution.24, 26 SE8Y showed a strong intensity of scattered light at Rh of 4.5 nm and a weak intensity of scattered light at Rh of 40 ± 13 nm (Figure 2c), indicating the formation of only small amounts of particles. An increase in the silk to elastin ratio from 1:8 to 1:4 decreased drastically the intensity of scattered light at 4.5 nm, and increased intensity of scattered light at 38 ± 14 nm, which suggested that many free chains assembled into particles in S2E8Y. When the silk to elastin ratio was further increased to 1:2, there was only one sharp peak at an Rh of 68 ± 12 nm, suggestive of the formation of uniform particles in S4E8Y. Notably, the mean particle sizes from SEM were slightly larger than that obtained from DLS, which is probably attributed to the small number (tens to hundreds) of particles we counted from the SEM images compared with millions from DLS. Another reason could be that during sample preparation for SEM imaging, the morphology, shape, and consequently the sizes of particles can be altered, such as due to dehydration. Collectively, the results suggested that the silk to elastin ratio played an important role in self-assembly and particle size of SELPs. These results are consistent with the model (Scheme 1), where the silk blocks self-assemble to form the core decorated with the elastin blocks. When the length of the silk blocks is reduced, e.g. in the case of SE8Y, the association between the silk blocks decreases, which leads to the formation of less micellar-like particles observed from the SEM and DLS. Notably, elastin-like proteins are not able to form micellar-like structures themselves below their transition temperatures.24, 28
Thermal Responsive Properties of SELPs
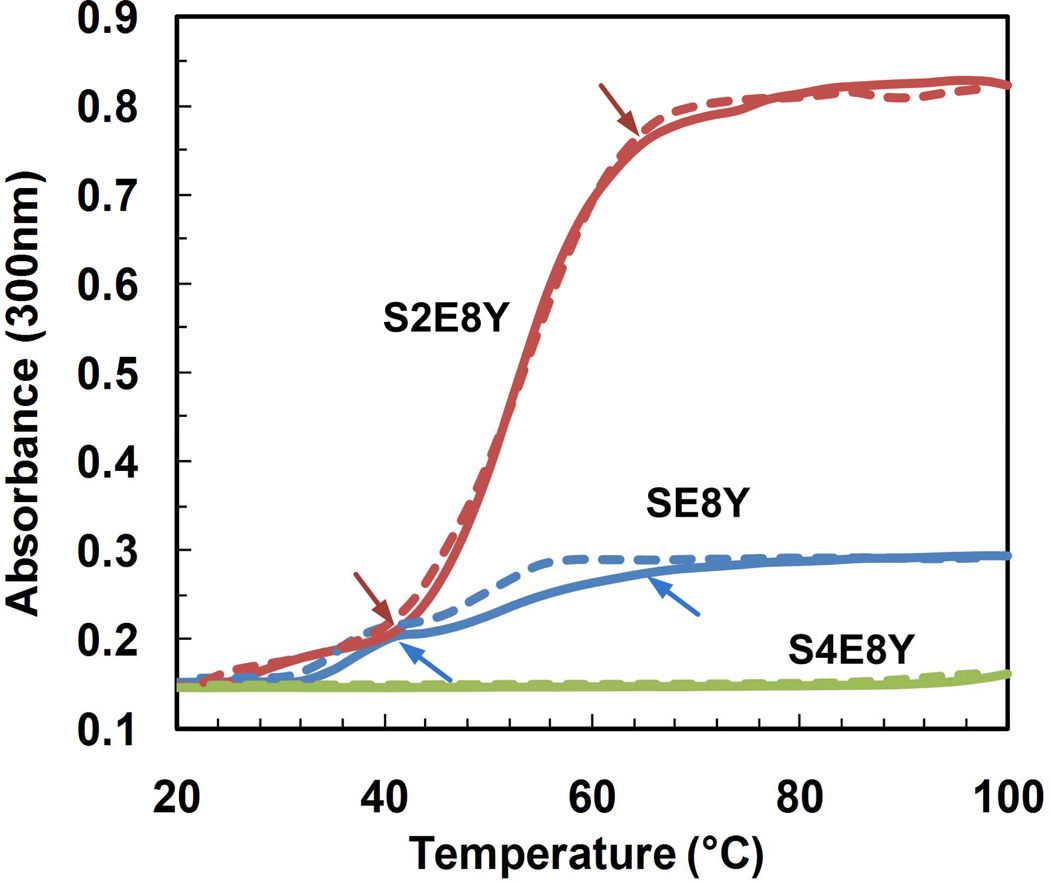
It was previously reported that the thermal transition properties of elastin-like peptides might change greatly upon fusion to other proteins.27 To test whether the SELPs still had these properties, we monitored changes in the optical density (OD) of protein solutions at 300 nm upon heating from 20°C to 100°C. 28 Interestingly, SE8Y and S2E8Y exhibited a two-step thermal transition and this transition was fully reversible (Figure 3). The first transition occurred below 40°C and the second transition below 60°C. We believe that the first increase in absorbance was caused by the self-assembly of the free chains of SELPs into small micellar-like particles. As indicated in the earlier DLS studies, free chains of SE8Y and S2E8Y polymers still existed in the solution at 20°C. Upon heating above 40°C, particle-particle interactions induced the formation of large coacervates, as indicated by the high OD values. This two-step thermal transition was also observed in an amphiphilic AB diblock of elastin polypeptides when an appropriate ratio of hydrophilic to hydrophobic block was obtained.28 Notably, the OD change for S2E8Y was significantly higher than that for SE8Y despite of the same protein concentrations used. This is mainly attributed to more particles formed in the former case since the particle sizes in both cases did not show significant difference from the AFM and DLS analyses (Fig. 6). In contrast, S4E8Y did not show any obvious transitions in the temperature range examined. A likely explanation was that a high ratio of silk to elastin blocks masked the role of elastin blocks in the polymer.
Figure 3.
Turbidity profiles of the silk-elastin-like protein solutions at 0.5 mg/mL as a function of temperature. Turbidity profiles were obtained by monitoring optical density at 300 nm as the aqueous solutions were heated (solid lines) and cooled (dash lines) at a rate of 3°C/min. Arrows indicate the locations of the transition.
Figure 6.
a) Representative AFM images of the nanostructures derived from self-assembly of SE8Y, S2E8Y and S4E8Y in aqueous solutions upon heating to 60°C and b) cooling back to 20°C. DLS size distribution profiles for c) SE8Y and d) S2E8Y particles formed upon heating from 20°C to 60°C and cooling back (dash lines).
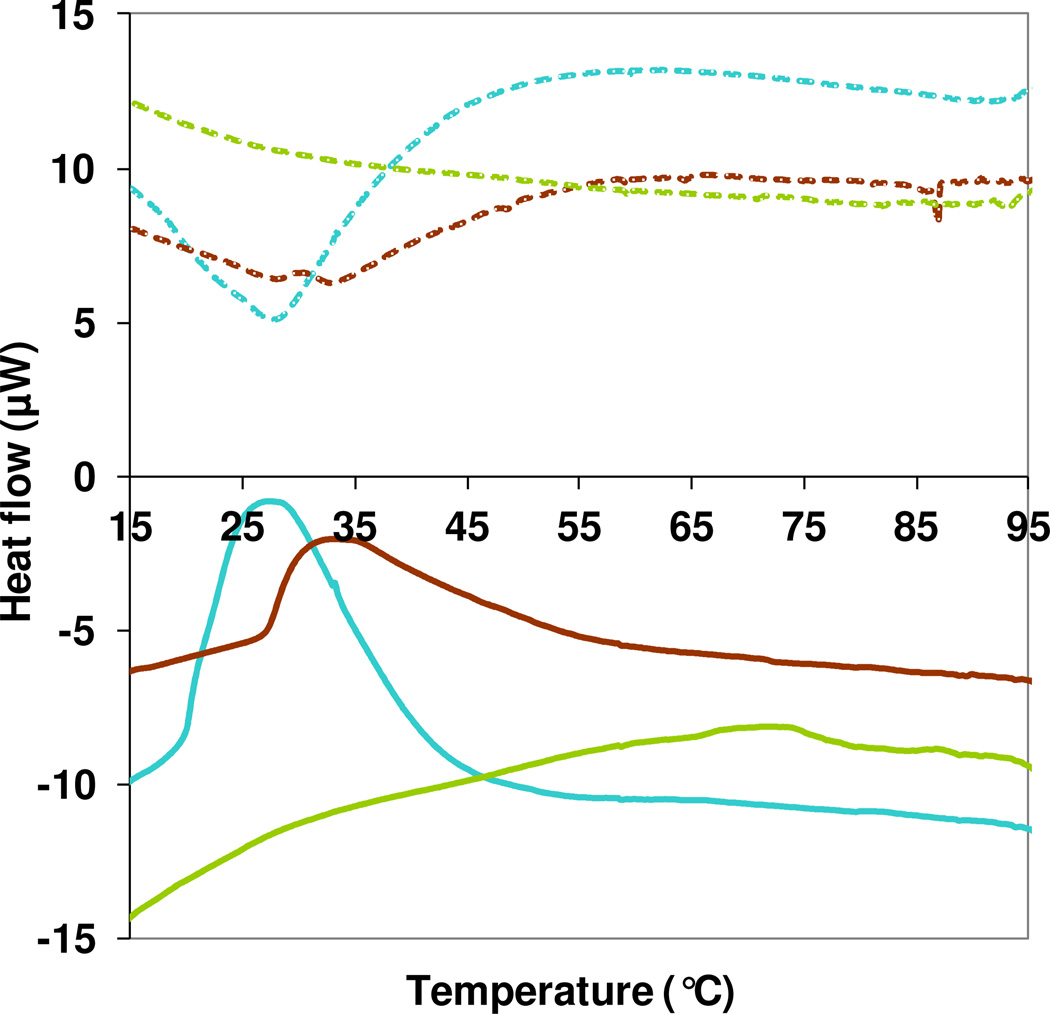
Differential scanning calorimetry (DSC) was performed to further confirm the thermal transition properties of SELPs.29 Interestingly, we observed only one positive peak at approximately 27°C and 33°C for SE8Y and S2E8Y, respectively (Figure 4). These two temperatures corresponded to the first transition temperatures of the two polymers, as measured by UV spectrometry. This indicated that the first transition for SE8Y and S2E8Y was exothermic, which is probably a crystallization process of silk blocks with enthalpically driven beta sheet formation. However, the second transition observed from UV spectrometry study was not detected on the DSC curves, which might be attributed to the net result of a complex process reflecting different thermal contributions from the silk and elastin blocks. A similar phenomenon was observed in a previous study for a silk elastin-like protein polymer.30 On the other hand, no transition was observed for S4E8Y, which is consistent with the UV spectrometry study. The absence of the exothermic peak for S4E8Y probably indicated that the beta sheet formation had occurred at some point prior to the analysis, which is supported by the DLS data in Figure 2. Moreover, DSC also confirmed the reversibility of the transitions, as seen from UV spectrometry studies (Figure 4).
Figure 4.
Differential scanning calorimetry (DSC) of protein polymers SE8Y (blue line), S2E8Y (dark red line) and S4E8Y (green line), showing heat flow as a function of temperature. The concentrations of all samples were 1 mg/mL with a heating (solid lines) and cooling (dash lines) rate of 1°C/min.
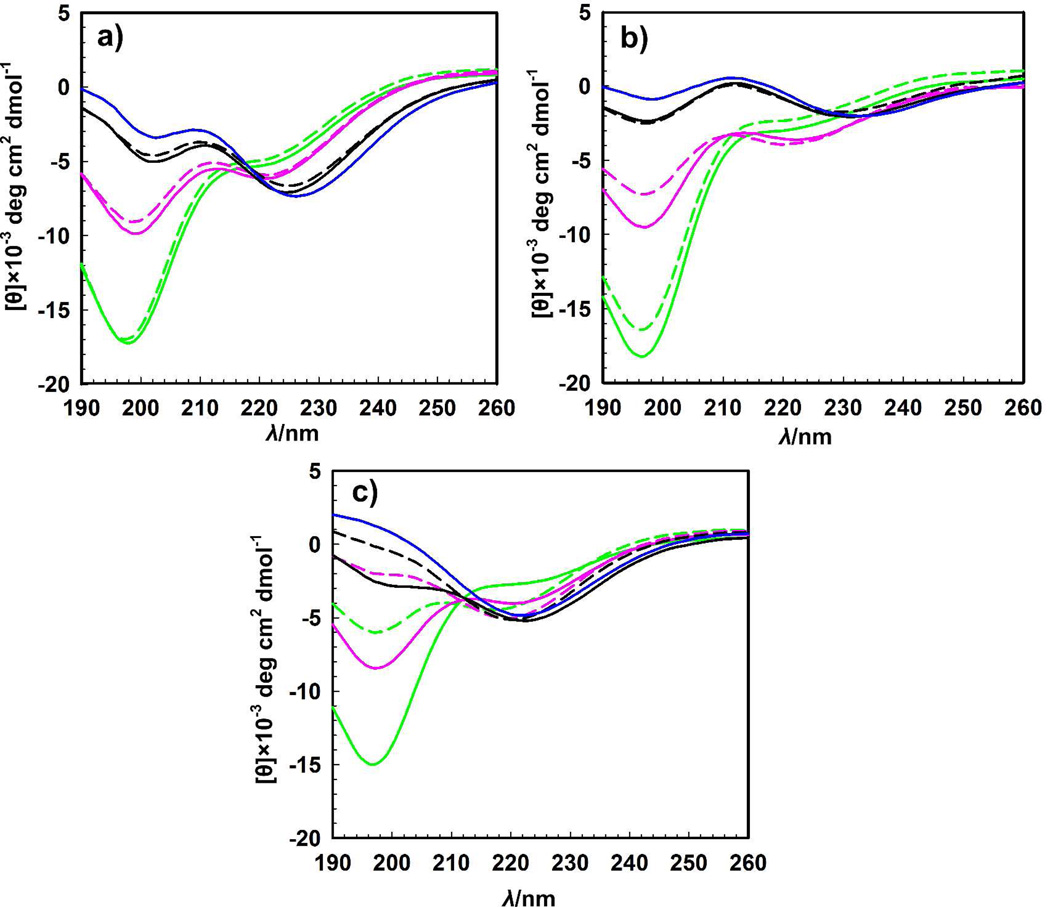
To further study the conformational changes of the SELPs upon heating, secondary structure was assessed by circular dichroism (CD) spectroscopy.31 CD spectra of SE8Y and S2E8Y showed that at higher temperatures, the negative signal at 197 nm weakened, whereas the magnitude of the signal at 210 nm increased (Figure 5), suggestive of an induction of a type II β-turn conformation.24 These transitions are similar to those previously observed for elastin-like polymers,19,20 which indicates that the elastin blocks might dominate the temperature-dependent conformational transitions in the present work, as anticipated. In contrast, CD spectra of S4E8Y at 95°C revealed a positive ellipticity at 190 nm and a negative ellipticity at 220 nm, indicating the induction of a β-sheet conformation in the polymer, a feature usually observed for silk block polymers. Nonetheless, these results suggested that all three polymers became more structured as the temperature increased. On examination of the reversibility of the conformational changes, we also profiled CD spectra of the polymers upon cooling. It was found that a higher silk to elastin ratio led to lower reversibility, which may be due to irreversible physical cross-linking of the silk blocks. These results demonstrated that the thermal transitions of SELPs could be reversible or irreversible by adjusting the ratio of silk to elastin blocks.
Figure 5.
Temperature-dependent CD spectra of a) SE8Y, b) S2E8Y and c) S4E8Y. Scans were performed at increasing (solid lines) and then decreasing temperatures (dash lines) with 10 min equilibration at each temperature: 4°C (green line), 37°C (pink line), 60°C (black line), and 95°C (blue line).
Thermal Responsive Self-assembly of SELPs into Diverse Nanostructures
We performed atomic force microscopy (AFM) to study the morphological changes of the SELPs upon heating to 60°C, the temperature at which both SE8Y and S2E8Y had undergone the two-step thermal transitions (Figure 6a). This analysis revealed the formation of spherical particles with average Rh of 241 ± 13 and 212 ± 16 nm for SE8Y and S2E8Y respectively. It appeared that these particles were formed from the coalescence of the smaller micellar-like particles that had self-assembled at lower temperature, which can be inferred from the presence of smaller particles in the AFM images. For S4E8Y, gel states were observed instead of obvious particles, which might be due to the cross-linking of the silk blocks upon heating.32 When the aqueous solutions of the three polymers were cooled back to 20°C, SE8Y displayed uniform small micellar-like particles, S2E8Y existed as worm-like nanostructures composed of small spherical particles, whereas S4E8Y appeared as large, polydisperse aggregates (Figure 6b). In addition, aligned nanofibers were also observed for S2E8Y and S4E8Y, which might be due to cross-linking between the silk blocks (Figure 6b).
In the case of SE8Y, the size of the particles increased to a Rh of 220 ± 37 nm upon heating and reversed to the original size upon cooling (Figure 6c). For S2E8Y, the size of particles increased to 174 ± 28 nm upon heating and decreased upon cooling to 72 ± 13 nm, a size significantly larger than the original (Figure 6d). However, the turbidity measurement did not detect these changes, which revealed the limitation to use turbidity measurement for studying reversibility of phase transitions.
CONCLUSION
In summary, we have for the first time demonstrated that recombinant SELPs undergo a two-step self-assembly process in aqueous solutions. The first step is spontaneous formation of micellar-like particles with the silk blocks as the core structure, a process which is driven by hydrogen bonding between the silk blocks13 and controlled by adjusting the ratio of silk to elastin blocks. The formation of this micellar-like structure is supported by the hierarchical self-assembling model for silk.25 Here the silk proteins in aqueous solutions form micellar-like particles of 100–200 nm in size with hydrophobic domains (mainly GAGAGS) as a core and hydrophilic domains as a corona. This micellar-like structure has also been observed in the processing of SELPs into films and fibers.33,34 The second assembly step is driven by the hydrophobic interaction between elastin blocks above a specific transition temperature, which leads to the ordered association of SELPs molecules. Through precise tuning of the ratio of silk to elastin blocks, various nanostructures including nanoparticles, hydrogels or nanofibers were generated either reversibly or irreversibly. This assembly process provides opportunities to generate innovative smart materials for various applications. Furthermore, the newly developed SELPs may be potentially useful for controlled drug delivery and biomedical engineering.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the NIH P41 Tissue Engineering Resource Center (P41 EB002520) and the Air Force Office of Scientific Research. We thank Prof. Lori Setton for critical inputs to the paper, and Eileen Hwang and Sreevidhya Tarakkad-Krishnaji for help with DSC and SEM.
Footnotes
Supporting Information Available: A scheme for the construction of expression plasmids for recombinant silk elastin-like protein polymers. (Figure S1). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Omenetto FG, Kaplan DL. Science. 2010;329:528–531. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heim M, Keerl D, Scheibel T. Angew Chem. Int. Ed. Engl. 2009;48:3584–3596. doi: 10.1002/anie.200803341. [DOI] [PubMed] [Google Scholar]
- 3.Xia XX, Qian ZG, Ki CS, Park YH, Kaplan DL, Lee SY. Proc. Natl. Acad. Sci. U S A. 2010;107:14059–14063. doi: 10.1073/pnas.1003366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keeley FW, Bellingham CM, Woodhouse KA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:185–189. doi: 10.1098/rstb.2001.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel D, Menon R, Taite LJ. Biomacromolecules. 2011;12:432–440. doi: 10.1021/bm101214f. [DOI] [PubMed] [Google Scholar]
- 6.Gustafson JA, Ghandehari H. Adv. Drug Deliv. Rev. 2010;62:1509–1523. doi: 10.1016/j.addr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Nagarsekar A, Crissman J, Crissman M, Ferrari F, Cappello J, Ghandehari H. Biomacromolecules. 2003;4:602–607. doi: 10.1021/bm0201082. [DOI] [PubMed] [Google Scholar]
- 8.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 9.Urry DW, Urry KD, Szaflarski W, Nowicki M. Adv. Drug Deliv. Rev. 2010;62:1404–1455. doi: 10.1016/j.addr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Haider M, Cappello J, Ghandehari H, Leong KW. Pharm. Res. 2008;25:692–699. doi: 10.1007/s11095-007-9282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haider M, Leung V, Ferrari F, Crissman J, Powell J, Cappello J, Ghandehari H. Mol. Pharm. 2005;2:139–150. doi: 10.1021/mp049906s. [DOI] [PubMed] [Google Scholar]
- 12.Anumolu R, Gustafson JA, Magda JJ, Cappello J, Ghandehari H, Pease LF. ACS Nano. 2011;5:5374–5382. doi: 10.1021/nn103585f. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Qiu W, Huang Y, Teng W, Cohn CM, Cappello J, Wu X. Biomacromolecules. 2010;11:3219–3227. doi: 10.1021/bm100469w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nera Y, Stuart JA, Whited G, Sotzing GA. Polymer. 2009;50:5828–5836. [Google Scholar]
- 15.Chang J, Peng XF, Hijji K, Cappello J, Ghandehari H, Solares SD, Seog J. J. Am. Chem. Soc. 2011;133:1745–1747. doi: 10.1021/ja110191f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang W, Kim BH, Dandu R, Cappello J, Ghandehari H, Seog J. Langmuir. 2009;25:12682–12686. doi: 10.1021/la9015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canetti M, Seves A, Secundo F, Vecchio G. Biopolymers. 1989;28:1613–1624. doi: 10.1002/bip.360280910. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Lu Q, Sun L, Cebe P, Wang X, Zhang X, Kaplan DL. Biomacromolecules. 2010;11:3178–3188. doi: 10.1021/bm1010504. [DOI] [PubMed] [Google Scholar]
- 19.Urry DW, Hugel T, Seitz M, Gaub HE, Sheiba L, Dea J, Xu J, Parker T. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:169–184. doi: 10.1098/rstb.2001.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright ER, Conticello VP. Adv. Drug Deliv. Rev. 2002;54:1057–1073. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Alonso DO, Daggett V. J. Mol. Biol. 2001;305:581–592. doi: 10.1006/jmbi.2000.4306. [DOI] [PubMed] [Google Scholar]
- 22.Drummy LF, Phillips DM, Stone MO, Farmer BL, Naik RR. Biomacromolecules. 2005;6:3328–3233. doi: 10.1021/bm0503524. [DOI] [PubMed] [Google Scholar]
- 23.Luan CH, Parker TM, Gowda DC, Urry DW. Biopolymers. 1992;32:1251–1261. doi: 10.1002/bip.360320914. [DOI] [PubMed] [Google Scholar]
- 24.Kim W, Thévenot J, Ibarboure E, Lecommandoux S, Chaikof EL. Angew Chem. Int. Ed. Engl. 2010;49:4257–4260. doi: 10.1002/anie.201001356. [DOI] [PubMed] [Google Scholar]
- 25.Jin HJ, Kaplan DL. Nature. 2003;424:1057–1061. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- 26.Fujita Y, Mie M, Kobatake E. Biomaterials. 2009;30:3450–3457. doi: 10.1016/j.biomaterials.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Trabbic-Carlson K, Meyer DE, Liu L, Piervincenzi R, Nath N, LaBean T, Chilkoti A. Protein Eng. Des. Sel. 2004;17:57–66. doi: 10.1093/protein/gzh006. [DOI] [PubMed] [Google Scholar]
- 28.Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoti A. J. Am. Chem. Soc. 2008;130:687–694. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlos Rodriguez-Cabello J, Reguera J, Girotti A, Alonso M, Testera AM. Prog. Polym. Sci. 2005;30:1119–1145. [Google Scholar]
- 30.Cappello J, Crissman JW, Crissman M, Ferrari FA, Textor G, Wallis O, Whitledge JR, Zhou X, Burman D, Aukerman L, Stedronsky ER. J Control. Release. 1998;53:105–117. doi: 10.1016/s0168-3659(97)00243-5. [DOI] [PubMed] [Google Scholar]
- 31.Nuhn H, Klok HA. Biomacromolecules. 2008;9:2755–2763. doi: 10.1021/bm800784y. [DOI] [PubMed] [Google Scholar]
- 32.Hu X, Wang X, Rnjak J, Weiss AS, Kaplan DL. Biomaterials. 2010;31:8121–8131. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng W, Huang Y, Cappello J, Wu X. J Phys Chem B. 2011;115:1608–1615. doi: 10.1021/jp109764f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng W, Cappello J, Wu X. Biomacromolecules. 2009;10:3028–3036. doi: 10.1021/bm900651g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.