Abstract
This report describes a nickel-catalyzed allylic substitution process of simple alkenes whereby an important structural motif, a 1,4-diene, was prepared. A key for this success is the use of an appropriate Ni-phosphine complex and a stoichiometric amount of silyl triflate. Reactions of 1-alkyl-substituted alkenes consistently provided 1,1-disubstituted alkenes with high selectivity. Insight into the reaction mechanism as well as miscellaneous application of the developed catalytic process is also documented.
Keywords: Allylic substitution; 1,4-Diene; Homogeneous catalysis; Alkenes; Nickel
Introduction
Simple terminal alkenes are produced in metric megaton amounts each year, and they are inexpensive feedstock chemicals which serve as starting materials for the preparation of many classes of organic compounds.[1] Therefore, catalytic processes that convert simple terminal alkenes into more valuable compounds with concomitant C–C bond formation are highly desirable, such as polymerization of alkenes[2] and hydroformylation.[3] In these transformations, the alkene double bonds in the starting materials are converted into C–C single bonds. In contrast, olefin cross metathesis,[4] the Heck reaction[5] and the carbonyl-ene reaction[6] are widely used catalytic reactions of terminal alkenes leading to compounds bearing C=C double bonds. The Heck reaction and carbonyl-ene reaction, however, commonly employ conjugated terminal alkenes and electron-rich alkenes, respectively, especially in intermolecular variants; simple alkenes, such as alpha-olefins, are rarely used in these reactions.
In a pioneering paper in 2004, Ogoshi and coworkers reported intramolecular, Ni-mediated cyclization of alkenals in the presence of organophosphine and trimethylsilyl triflates (Me3SiOTf),[7a] and shortly thereafter we reported Ni-catalyzed intermolecular three-component couplings of alkenes, aldehydes, and silyl triflates.[8a] Over the past several years, both of our groups have actively investigated other related transformations.[7-9] In our ongoing efforts in this area, we have developed highly regioselective nickel-catalyzed three-component couplings of alkenes, aldehydes, and silyl triflates, enabling the access to both homoallylic alcohols[8b] and allylic alcohols[8c] by judicious choice of reaction conditions (equation 1). Nickel-catalyzed intermolecular coupling reactions of simple terminal alkenes with α,β-unsaturated carbonyl compounds has also been reported (equation 2).[8d] In these reactions, simple terminal alkenes serve as carbon nucleophiles under mild reaction conditions. The resulting functionalized alkenes can be employed as versatile building blocks for subsequent manipulations.
 |
(1) |
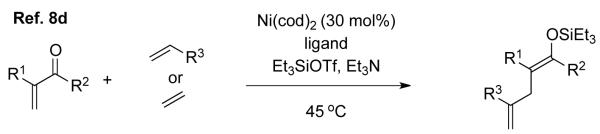 |
(2) |
The transition metal-catalyzed allylic substitution reactions (ASRs) provide a highly valuable tool in organic synthesis.[10] This methodology accommodates a wide range of carbon nucleophiles, such as activated methylene compounds,[11] enolates,[12] enamines,[13] Grignard reagents,[14] organozinc reagents[15] and alkenyl[16] or aryl[17] boron reagents. ASR of terminal alkenes, in principle, would enable the construction of 1,4-dienes (“skipped” dienes), a key structural motif prevalent in natural products.[18] Intermolecular allylic substitution reaction with non-metalated terminal alkenes, were reported by Tsukada et al.,[19] where only conjugated alkenes, styrene derivatives and butylacrylate, were employed (equations 3 and 4). Catalytic intramolecular ASRs of simple terminal alkenes were studied extensively by Oppolzer et al. with various transition metal catalysts (equation 5).[20] However, catalytic intermolecular ASR of simple terminal alkenes had not been reported before we started investigation.[21]
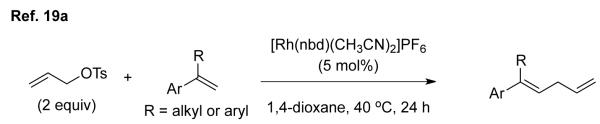 |
(3) |
 |
(4) |
 |
(5) |
In 2010, we described the first catalytic intermolecular ASR of simple alkenes.[22] In this process, several allylic alcohol derivatives were shown to react with terminal alkenes, including ethylene and propylene, in the presence of a catalytic amount of a nickel complex and a stoichiometric amount of an activator, triethylsilyl triflate, to give a variety of 1,4-dienes in high yield (equation 6). These reactions proceeded to completion at room temperature in less than 18 hours. An attractive feature of this process is the high branched-to-linear ratios in favor of 1,1-disubstituted alkenes when 1-alkyl-substituted alkenes are used. In this article, we describe the details of the developed Ni-catalyzed allylic substitution reactions including experiments that provide insight into the mechanism of this transformation, as well as a variety of its applications.
 |
(6) |
Results and Discussion
1. Nickel-Catalyzed Allylic Substitution of Ethylene
We initiated our research utilizing ethylene and a nickel catalyst, and found that the use of a stoichiometric amount of Lewis acid, Et3SiOTf, is indispensable for the ASR of ethylene to proceed. As is often the case with transition metal-catalyzed processes, the choice of phosphine ligand was found to have a profound impact. The effect of the phosphine ligand in the Ni-catalyzed ASR (10 mol%) of ethylene (1 atm) by cinnamyl methyl carbonate (1a) is summarized in Table 1, in which the results are arranged according to cone angle of the ligand.[23]
Table 1.
Ligand Screening for Ni-Catalyzed Allylic Substitution of Ethylene.

| Ligand | Cone angles[a] | νCO[b] | Conv. (%)[c] | Yield of 2a[d] | Yield of 3a[d] |
|---|---|---|---|---|---|
| PMe3 | 118 | 2064.1 | 42 | 3 | 4 |
| PMe2Ph | 122 | 2065.3 | 37 | 5 | trace |
| P(OEt)Ph2 | 133 | 2071.6 | 93 | 30 | 2 |
| PPh3 | 145 | 2068.9 | 25 | 4 | trace |
| P(p-anisyl)3 | 145 | 2066.1 | 71 | 48 | 1 |
| PCyPh2 | 153 | (2064.8)[f] | 100 | 89 | trace |
| PCy2Ph | 162 | (2060.6)[f] | 47 | 26 | trace |
| PCy3 | 170 | 2056.4 | 43 | 13 | trace |
| P(o-tolyl)3 | 194 | 2066.6 | 38 | 11 | trace |
| P(o-anisyl)3 | (>194)[e] | 2058.3 | 100 | 90 | 6 |
The stretching frequencies (νCO) of the terminal CO of Ni(CO)3L in CH2Cl2. Ref. 23.
Determined by GC.
Yields were determined by 1H NMR analysis using 1,4-dioxane as an internal standard.
Estimated values.
Calculated values using Tolman’s equation. Ref. 23. Abbreviation: Cy = cyclohexyl; tolyl = MeC6H4; anisyl = MeOC6H4.
Whereas a significant gap between conversion and yield of 2a was observed in most cases, probably due to decomposition of 1a in the presence of Et3SiOTf, high yields were obtained when PCyPh2 and P(o-anisyl)3 (anisyl = MeOC6H4) were used. In all cases, a small amount of conjugated diene 3a was observed as an inseparable side product.[24] At this stage in our investigations, there was no clear correlation between steric or electronic factors of the phosphine ligand and product yield.
With the optimal reaction conditions in hand, we then turned our attention to the scope of the leaving group of the allylic alcohol derivatives (Table 2). In contrast to typical Pd-catalyzed ASRs using allylic carbonates, allylic esters and allylic chlorides as coupling partners for an alkene, ethylene undergoes substitution with a wide range of allylating reagents, including electrophiles bearing classically poor leaving groups such as alkyl ethers,[25] trimethylsilyl ethers, and even allylic alcohols[26] (entries 1-4). Et3SiOTf is proposed to activate such allylic alcohol derivatives bearing classically poor leaving groups toward oxidative addition (vide infra). Cinnamyl derivatives bearing OAc and Cl as a leaving group also performed well in this transformation (entries 5, 6). It is worthy of note that in any studied case no reaction occurred in the absence of silyl triflate.
Table 2.
Scope of Leaving Group and Decrease of Catalyst Loading in Ni-Catalyzed ASR.

| Entry | X | y | Yield of 2a[a] | Yield of 3a[a,b] |
|---|---|---|---|---|
| 1 | OMe (1b) | 10 | 91 | 5 |
| 2 | OEt | 10 | 85 | 4 |
| 3[c] | OSiMe3 | 10 | 75 | trace |
| 4[d] | OH | 20 | 56 | <5 |
| 5 | OAc | 10 | 86 | 10 |
| 6 | Cl | 10 | 63 | 20 |
| 7 | OCO2Me | 2.5 | 71 | 26 |
| 8[e] | OCO2Me | 2.5 | 91 | 7 |
Yields were determined by 1H NMR analysis using acetonitrile as an internal standard.
For 3a byproduct E/Z = approx 3:1 in all cases.
Me3SiOTf (1.75 equiv) used in place of Et3SiOTf, 4 h.
Me3SiOTf (3 equiv) used instead of Et3SiOTf.
10 mol% of P(o-anisyl)3 used, 3 h.
Further decreases in the catalyst loading from 10 mol% resulted in low selectivity of 2a over 3a. When 2.5 mol% of Ni(cod)2 and 5.0 mol% of P(o-anisyl)3 were used (entry 7), the formation of desired product 2a (71% yield) was accompanied by a significant amount of 3a (26% yield). Extensive studies to overcome this issue revealed that the use of an excess of P(o-anisyl)3 suppresses the formation of 3a. When 2.5 mol% of Ni(cod)2 and 10 mol% of P(o-anisyl)3 (Ni:phosphine = 1:4) were used (entry 8), 2a was obtained in 91% yield accompanied by a small amount of 3a (7% yield).[27] The reason for the increased selectivity upon using an excess amount of phosphine ligand is as yet unclear.
The scope of Ni-catalyzed ASR of ethylene regarding allylic alcohol derivatives was next examined (Table 3). Both Z-cinnamyl methyl ether (1c) and the corresponding branched isomer 1d provided linear product 2a in good yield with complete E selectivity (entries 2, 3). A broad range of allylic alcohol derivatives functioned well (entries 4-8). When substrates bearing alkyl substituents were used, a small amount of branched products were observed (entries 5-8). To our delight, substituents at any position of the allyl carbonate were tolerated, as demonstrated in entries 9-11.
Table 3.
Ni-Catalyzed Allylic Substitution of CH2=CH2[a]

| Entry | Substrate | Product | Yield[b] | l:b[c] | E : Z[d] |
|---|---|---|---|---|---|
| 1 | 1b | 2a | 75% | >99:1 | >99:1 |
| 2 | 1c | 2a | 83% | >99:1 | >99:1 |
| 3 | 1d | 2a | 74% | >99:1 | >99:1 |
| 4 | 1e | 2b | 84% | >99:1 | 83:17 |
| 5 | 1f | 2c | 97% | 95:5 | 94:6 |
| 6 | 1g | 2d | 73% | 98:2 | 92:8 |
| 7 | 1h | 2d | 82% | 98:2 | 92:8 |
| 8 | 1i | 2d | 76% | 98:2 | 92:8 |
| 9 | 1j | 2e | 57% | >99:1 | 94:6 |
| 10 | 1k | 2f | 81% | >99:1 | 88:12 |
| 11 | 1l | 2g | 71% | >99:1 | >99:1 |
A solution of Ni(cod)2 (10 mol%) and P(o-anisyl)3 (20 mol%) in toluene (0.2 M) was purged with ethylene, and the ethylene atmosphere was maintained with an ethylene balloon. Et3N (6 equiv), allylic alcohol derivative (1 equiv) and Et3SiOTf (1.75 equiv) were then added, and the reaction was stirred at rt.
Isolated yield.
Linear/branched product.
Ratio of geometric isomers of linear products.
Abbreviation: TBS = tert-butyldimethylsilyl; PMB = p-MeOC6H4CH2.

A drawback of the Ni-catalyzed ASR of ethylene was the formation of a small amount of conjugated 1,3-dienes, which as noted above were difficult to separate from the desired 1,4-diene products by standard chromatographic purification. On the expectation that 1,3-dienes would react with dienophiles in a [4+2] cycloaddition to give a separable cycloadduct, a variety of common dienophiles were examined using a mixture of 2a and 3a obtained from Ni-catalyzed ASR of ethylene by 1a. While acrylate, acrolein, acryloyl chloride and maleic anhydride failed to react with 3a at room temperature regardless of the presence of Et3SiOTf, a commercially available dienophile, tetracyanoethylene (TCNE),[28] readily reacted with E-3a selectively and quantitatively at room temperature without Lewis acid, to afford the corresponding cycloadduct 4. The desired product 2a was not affected by TCNE and could be isolated with >98% purity (a tiny amount of Z-3a was included). With a convenient purification method of 2a in hand, Ni-catalyzed ASR of ethylene with 1a was conducted on 10-mmol scale as a demonstration of the scalability of this transformation (Scheme 1). The reaction was conducted under the same conditions as optimized on the small scale reaction. Filtration of the reaction mixture through a pad of silica gel and treatment with TCNE followed by chromatographic purification provided the desired coupling product 2a in 81% yield (1.18 g) with >98% purity.
Scheme 1.
Gram-Scale Allylic Substitution Reaction of Ethylene
2. Nickel-Catalyzed Allylic Substitution of 1-Substituted Simple Alkenes
We next examined the Ni-catalyzed ASR of 1-substituted alkenes, commencing with the gaseous alpha-olefin propylene. It was clear from initial studies that regioselectivity may be a concern when using 1-substituted olefins. Ni-catalyzed ASR of propylene (1 atm) with cinnamyl methyl carbonate (1a) afforded a mixture of 5a, 6 and 7 in favor of 1,1-disubstituted olefin 5a (Table 4). Yield and selectivity of ASR were found to be strongly dependent on phosphine ligand employed. The effect of the phosphine ligand on yield and selectivity is summarized in Table 4, in which the results are arranged in order of a steric bulkiness of phosphine ligand.
Table 4.
Ligand Screening for ASR of Propylene

| Ligand | Cone angle[a] | νCO[b] | Yield (%)[c] | Selectivity (5a:6:7)[d] |
|---|---|---|---|---|
| PMe3 | 118 | 2064.1 | 0 | - |
| PMe2Ph | 122 | 2065.3 | 0 | - |
| P(OEt)Ph2 | 133 | 2071.6 | trace | - |
| PPh3 | 145 | 2068.9 | 0 | - |
| P(p-anisyl)3 | 145 | 2066.1 | 37 | 77:7:16 |
| PCy(p-F-C6H4)2 | (153)[e] | (2066.2)[f] | 64 | 91:1:8 |
| PCyPh2 | 153 | (2064.8)[f] | 65 | 94:1:5 |
| PCy2(p-F-C6H4) | (162)[e] | (2061.3)[f] | 86 | 98:1:1 |
| PCy2Ph | 162 | (2060.6)[f] | 78 | 98:1:1 |
| PCy2(p-anisyl) | (162)[e] | (2059.7)[f] | 87 | 98:1:1 |
| PCy3 | 170 | 2056.4 | 19 | >98:1:1 |
| P(o-tolyl)3 | 194 | 2066.6 | 0 | - |
| P(o-anisyl)3 | (>194)[e] | 2058.3 | 84[g] | 52:20:28 |
The stretching frequencies (νCO) of the terminal CO of Ni(CO)3L in CH2Cl2. Ref. 23.
Yields were determined by 1H NMR analysis using 1,4-dioxane as an internal standard.
Determined by 1H NMR spectroscopy.
Estimated values.
Calculated values using Tolman’s equation. Ref. 23.
Isolated yield.
Product yield is influenced strongly by the steric demand of the phosphine ligand. While relatively large (θ (cone angle) > 170°) and small (θ < 145°) phosphine ligands provided no product, the reactions proceeded well using medium size phosphine ligands (145° < θ < 170°). An exception to this trend was P(o-anisyl)3, which afforded the ASR products in high yield despite its large cone angle (estimated to be θ > 194°).[29] In addition to product yield, regioselectivity for the 1,1-dibstituted olefin 5a also appears to be positively correlated with the steric bulkiness of the phosphine ligand, i.e., the bulkier the phosphine ligand, the higher the selectivity. P(o-anisyl)3 is again an exception to this rule. These observations and trends are discussed in further detail below (Chapter 3). Taking into consideration a balance of yield and selectivity, commercially available PCy2Ph was determined to be the optimal ligand (77% yield, 5a:6:7 = 98:1:1).
Encouraged by the results of propylene, a higher boiling alpha-olefin (i.e., not a gas at STP), 1-octene, was used as a substrate in Ni-catalyzed ASR. The desired product 5b, however, was obtained in low yield under the conditions optimal for a reaction of propylene (Scheme 2). In addition to 5b, several by-products 8-11 were obtained, among which triethylamine adduct 8 was the major by-product (>50% yield). It was found that the formation of major by-product 8 is mediated by Et3SiOTf and that nickel catalyst is not necessary for the formation of 8.
Scheme 2.
Initial Trial of ASR of 1-Octene
In order to suppress by-product formation and improve the yield of 5b, we aimed to accelerate the desired Ni-catalyzed ASR process. Screening of reaction conditions revealed three reaction parameters to be important for high yield of the desired product. First, the initial substrate concentration should be as high as 1 M (previously 0.2 M). Second, a combination of PCy2Ph and P(OPh)3 is necessary. P(OPh)3 is envisioned to accelerate reductive elimination by reducing the electron density of the nickel center, as has been observed previously in the Ni-N-heterocyclic carbene-catalyzed coupling of alkenes and aldehydes.[8c] Third, carbonate 1a must be mixed with the nickel complex prior to the addition of alkene. We have observed that NiL2(η2-1-octene) (L = PCy2Ph) complex 12,[30] generated by mixing Ni(cod)2, PCy2Ph and 1-octene, is slowly converted (over 3 h), upon addition of 1a, into an allyl-Ni complex 13, whereas 13 can be readily formed (<10 min) in the absence of 1-octene (Scheme 3). In the reaction where 1a was mixed with the nickel complex prior to the addition of alkene, rapid generation of a key intermediate 13 should lead to the acceleration of the overall reaction process.[31]
Scheme 3.
Formation of allyl-Ni complex 13 in the presence or absence of 1-octene.
Under these conditions, many simple alkenes gave the coupling products 5 in good yield and with excellent selectivity, including the more sterically demanding vinylcyclohexane[32] (Table 5, entries 2-6). The opposite regioselectivity was observed in the case of styrene, with 14 being the sole coupling product (entry 7). A considerable amount of polystyrene was obtained in the reaction of styrene, probably due to Lewis acidic properties of reaction conditions.
Table 5.
Ni-Catalyzed Allylic Substitution of Alkenes

| Entry | x | y1 | y2 | R1 (Product) | yield (%)[a] |
|---|---|---|---|---|---|
| 1[b] | 10 | 20 | 0 | Me (5a) | 77 (71)[c] |
| 2 | 10 | 10 | 10 | nC6H13 (5b) | 79 |
| 3 | 20 | 20 | 20 | CH2OSiEt3 (5c) | 73 |
| 4 | 10 | 10 | 10 | (CH2)2OTBS (5d) | 83[d] |
| 5 | 20 | 20 | 20 | CH2CHMe2 (5e) | 87 |
| 6[e] | 20 | 40 | 0 | cyclohexyl (5f) | 64[f] |
| 7 | 20 | 40 | 0 | 14 | 25 |
Isolated yield; E/Z selectivity >98:2 in all cases.
Propylene pressure 1 atm (balloon); toluene (0.2 M).
5 mol% Ni(cod)2, 10 mol% PCy2Ph.
Yield of free alcohol after treatment with 1 N HCl.
Et3SiOTf added over 4 h.
Yield includes trace amounts of regioisomers (total <8%).
![[f]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/428d/3224962/91fed810cae9/nihms-308263-f0029.jpg)
Several limitations to this method have been identified. Methyl acrylate and vinyl benzoate failed to react with 1a, probably owing to the catalyst inhibition by an ester group of the substrates. A sterically demanding alpha-olefin, 3,3-dimethyl-1-butene gave the desired product in low yield (<9%). Ethyl vinyl ether provided a complex reaction mixture which was difficult to purify. Cyclic internal alkenes, cyclopentene and cyclohexene, also gave a complex reaction mixture and the coupling products were isolated in low yields. The ASR reaction of vinylbromide with 1a did not provide any of the desired product.
3. Reaction Mechanism of Nickel-Catalyzed Allylic Substitution of Simple Olefins
Ni-catalyzed ASR of simple olefins requires Et3SiOTf, and in order to determine the role of Et3SiOTf, the oxidative addition step was studied by NMR spectroscopy (Scheme 4). When cinnamyl methyl carbonate (1a) was treated with preformed nickel complex, oxidative addition occurred rapidly at room temperature without Et3SiOTf to afford allyl-Ni-complex 13.[33] It is notable that 13 remained intact after addition of an excess amount of 1-octene and that the expected olefin complexes 15 or 16 were not observed. These data suggest that Et3SiOTf-mediated anion exchange is required in order for 1-octene to coordinate to the nickel center and react with an allyl ligand. The necessary cationic property of nickel for subsequent olefin coordination has also been suggested by other groups.[34] On the other hand, mixing cinnamyl methyl ether (1b) with nickel complex did not lead to oxidative addition, but simply resulted in formation of an olefin nickel complex 17.[35] Allyl-nickel complex 13 was not detected. Thus, in the case of Ni-catalyzed ASR of alkenes by allylic alcohol derivatives bearing poor leaving groups such as OMe and OSiMe3, Et3SiOTf was found to be required for oxidative addition to take place.
Scheme 4.
NMR Study for Oxidative Addition Event of Ni-Catalyzed ASR
The proposed reaction mechanism is summarized in Figure 1, in which 1a is used as a representative allylic alcohol derivative. As mentioned above, allyl-Ni complex 13 is generated without assistance of Et3SiOTf. The Ni–O bond of 13 is activated upon addition of Et3SiOTf, forming cationic allyl-Ni complex 18. Subsequent migratory insertion (C–C bond-forming step) and coordination of another phosphine ligand afford 19. Regioselectivity is determined in this olefin migration step. As mentioned above in chapter 2, the bulkier phosphine ligands provided higher selectivity for the 1,1-disubsituted olefin. This observation can be explained by the supposition that as the steric bulkiness of the phosphine ligand becomes larger, allyl-Ni complex 18a would be more favorable than 18b. Although it is still unclear whether olefin migration requires coordination of another phosphine ligand, computational studies of the migration of an olefin ligand to an allyl ligand of Ni or Pd complex have been reported.[34] Those studies suggest that the olefin migration step is thermoneutral or slightly uphill energetically and that concomitant coordination of ligand to the resultant unsaturated metal center after olefin migration makes this process significantly favored. β-Hydride elimination and subsequent reductive elimination provide the 1,4-diene product and regenerate the catalyst.
Figure 1.
Proposed Mechanism of Ni-Catalyzed Allylic Substitution of Olefins (L = organophosphine; triflates (TfO–) omitted for clarity).
4. Applications of Ni-Catalyzed ASR
4.1 Ni-Catalyzed Allylic Substitution of Allyltrimethylsilane
Allyl metal reagents such as allyl-stannanes, -silanes and -boronates are widely used for the introduction of a C3-unit and are much stronger nucleophiles than simple alkenes.[36] Moreover, the fact that the attack of an electrophile on an allyl metal reagent takes place predictably at the terminal position of the double bond (distal from metal) makes allylation using allyl metal reagent important in synthesis. Several allyl–allyl couplings between allylic alcohol derivatives and allyl metal reagents in the presence of various catalysts, providing 1,5-dienes exclusively, have been reported so far.[37]
With the intention of observing which position of the double bond is attacked in the Ni-catalyzed ASR of allyl metal reagent, a reaction of 1a and allyltrimethylsilane under the optimal reaction conditions was conducted (Scheme 5). The reaction proceeded smoothly to afford a mixture of products. It is noteworthy that in this Ni-catalyzed reaction, the 1,1-disubstited olefins 21b and 5a, generated via reaction at the internal position of the double bond, were the major products, overcoming the intrinsic nature of allyltrimethylsilane that generally affords linear 1,5-dienes (such as 7). More surprisingly, the same level of yield and selectivity was observed in the presence of a catalytic amount of Et3SiOTf (20 mol%) and no Et3N. No reaction proceeded in the absence of Et3SiOTf.
Scheme 5.
Ni-Catalyzed ASR of Allyltrimethylsilane
Although the isolation of a considerable amount of undesired protodesilylated product 5a was a matter of concern, the catalytic turnover of silyl triflate is interesting from a mechanistic viewpoint. The proposed mechanism of the Ni-catalyzed ASR of allyltrimethylsilane using a catalytic amount of Et3SiOTf is illustrated in Figure 2. Given the fact that 1,1-disubstituted olefins 21b and 5a are the major products, the reaction pathways prior to the selectivity-determining step (migratory insertion from 17 to 19) are thought to be similar to those described in Figure 1. β-Hydride elimination from intermediate 18 provides 1,4-diene 21b and HNiL2OTf. A possible explanation of the fact that Et3N is not required in this reaction would be that allyltrimethylsilane or silane 21b function as acid scavengers, trapping the HOTf liberated from HNiL2OTf (or more directly from HNiL2OTf) to regenerate both silyl triflate and Ni(0) species. In this transformation, allyltrimethylsilane and silane 21b are converted into propylene and diene 5a, respectively.[38]
Figure 2.
Proposed Mechanism of Ni-Catalyzed Allylic Substitution of Allyltrimethylsilane (L = organophosphine; triflates (TfO–) omitted for clarity).
4.2 Nickel-Catalyzed Reactions of Simple Alkenes with Vinyl Epoxide or Vinyltetrahydrofuran Derivatives
In addition to allylic alcohol derivatives, vinyl epoxide is also known to undergo oxidative addition with Pd(0) to afford allyl-Pd species, which then can be used as key intermediates for catalytic C–C bond-forming processes.[39] We envisioned that vinyl epoxide would react with Ni(0)-phosphine complex to provide an allyl-Ni complex in an analogy to Pd chemistry,[40] and that the resultant allyl-Ni complex could be involved in Ni-catalyzed ASR process of simple alkenes.
As expected, the Ni-catalyzed reaction of ethylene with butadiene monooxide proceeded in the presence of Et3SiOTf in high yield, but the products were a mixture of linear adduct 22a and branched adduct 23a (Scheme 6, equation 7). Similar selectivity was also observed when PCy2Ph was used instead of P(o-anisyl)3. This anomalous low linear-to-branched selectivity, however, was observed only in case of ethylene. A reaction of 1-octene with butadiene monooxide provided the corresponding linear adduct 24 exclusively (Scheme 6, equation 8).
Scheme 6.
Ni-Catalyzed Reactions of Alkenes with Butadiene Monooxide
It is proposed that allyl-Ni complex 25 or its dimer or oligomer is generated in the oxidative addition of Ni(0) into butadiene monooxide (Figure 3). Et3SiOTf readily reacts with this Ni species to form cationic nickel complex 26 bearing triflate as a counter anion. Allyl-Ni complex 26a resembles the Ni-containing species 17 (Figure 1), proposed as the intermediate that leads to linear products in the Ni-catalyzed ASR. Indeed, this pathway was predominant when 1-octene was employed with butadiene monooxide. However, when ethylene was used as a substrate, 26b appears to comparably contribute to the product formation, resulting in branched adduct 23a.
Figure 3.
Proposed Mechanism of Ni-Catalyzed Reactions of Alkenes with Butadiene Monooxide (L = organophosphine; triflates omitted for clarity).
In order to probe the origin of the anomalous low selectivity in the reaction of ethylene with butadiene monooxide, allylic carbonate 1m was employed as a starting material (Scheme 7, equation 9). A reaction of 1m with ethylene proceeded to give a mixture of 22a and 23a with 60:40 selectivity, identical to that observed in a reaction of butadiene monooxide and ethylene. It is likely that a mixture of the intermediates 26a and 26b are generated for both butadiene monooxide and 1m, leading to low selectivity. It should be noted that allylic alcohol derivatives 1f (C1-homologue of 1m), 1g and 1o all gave linear products with high selectivity (equation 11). A reaction of 1m with 1-octene gave linear product 24 exclusively (Scheme 7, equation 10).
Scheme 7.
Ni-Catalyzed ASR of Alkenes with a Methyl Carbonate Derived from 2-Butene-1,4-Diol.
The observation that substrate 1n bearing a sterically demanding triisopropylsilyl group on oxygen also provided low linear-to-branched selectivity (Scheme 7), rules out the possibility that coordination of ethereal oxygen atom to the nickel metal center is causing the anomalous low selectivity. Alternatively, it is possible that the ethereal oxygen atom serves as an electron withdrawing group to electronically dictate the regioselectivity of the reaction. The 3-position of allyl ligand is more electrophilic in nature, and migratory insertion of ethylene from 28b becomes as an operative pathway (Figure 4). On the other hand, in the case of 1-octene, reaction from 29b is still inhibited due to the steric bulk of 1-octene. As a result, in that case, linear product 24 is exclusively obtained (via 29a). This rationalization is controversial because hydroxyl group was reported to be a strong directing group for formation of the new C–C bond distal to the hydroxyl group in Pd-catalyzed ASR leading to regioselective linear product formation.[41]
Figure 4.
Olefin Migration to Allyl Ligand with CH2OSiEt3 Substituent (left ethylene, right 1-octene).
Similarly, a reaction of 2-vinyltetrahydrofuran derivative 30 with ethylene was conducted (Scheme 8). Although a considerable amount of β-hydride elimination by-product 32 was isolated, the desired product 31 was obtained in moderate yield.
Scheme 8.
Ni-Catalyzed Reaction of Ethylene with 2-Vinyltetrahydrofuran 30
4.3 Nickel-Catalyzed Reactions of Ethylene with Aldehydes, Leading to 1,4-Dienes
We previously reported the Ni-catalyzed intermolecular three component-coupling of aldehydes, silyl triflates and alpha-olefins to provide silyl ethers of allylic alcohols 33.[8a,8c] Given that allyl trimethylsilyl ethers can be accommodated in the Ni-catalyzed ASR of ethylene (Table 2), we envisioned a one-step conversion of an aldehyde into a 1,4-diene (equation 12).
Unfortunately, the reaction of benzaldehyde with ethylene at room temperature (Scheme 9) provided the desired 1,4-diene 2a in low yield (4%), along with a considerable amount of initial coupling product 33 (63% yield). The low conversion of 33 suggested that the second reaction was sluggish, probably owing to the steric bulkiness of the R group. Thus, the less sterically demanding cinnamyl aldehyde was chosen as a substrate. Gratifyingly, the corresponding 1,4-diene product 2b was obtained in good yield. Despite the limited substrate scope, this reaction is a useful method that provides easy access to 1,4-dienes from aldehydes.
Scheme 9.
Ni-Catalyzed Reactions of Ethylene with Aldehydes, Leading to 1,4-Dienes
 |
(12) |
4.4 Nickel-Catalyzed Reactions of Ethylene with Aromatic Aldehyde-Derived Dimethyl Acetals
As stated above, the Ni-catalyzed reaction of ethylene with aromatic aldehydes failed to provide 1,4-diene product due to the steric bulkiness of the initially formed product, allyl trimethylsilyl ether 33. We then postulated that dimethyl acetals may react with ethylene and generate allyl methyl ether 39.[42] It was assumed that sterically less hindered 39 thus formed would further react with ethylene in the presence of Et3SiOTf and Ni catalyst, affording the desired 1,4-diene 2.
Our first trial using benzaldehyde dimethyl acetal (34a) failed to afford any desired 1,4-diene 2a or intermediate 39 (Scheme 10). At elevated temperature (60 °C), the desired product 2a was observed, but a considerable amount of 1,3-diene 3a was obtained. To our delight, electron rich p-anisaldehyde dimethyl acetal (34b) was found to be a superior substrate; the desired reaction proceeded at room temperature to afford 1,4-diene 2h in 83% yield.
Scheme 10.
Ni-Catalyzed Reactions of Ethylene with Aromatic Aldehyde-Derived Dimethyl Acetals
It is presumed that the first step of this reaction is Et3SiOTf-activation of 35 then oxidative addition by Ni(0)-phosphine complex to afford 36 (Figure 5). Stabilization of the intermediate oxonium cation may explain why the electron-rich aromatic rings perform better in this reaction. There may be equilibrium between η1-benzyl- and η3-benzyl-Ni complex (36 and 37),[43] the latter of which is similar to allyl-Ni intermediate 17 in the Ni-catalyzed ASR. Migratory insertion then occurs at the benzyl position followed by β-hydride elimination, providing the initial coupling product 39. Allyl methyl ether 39 is a good substrate for the subsequent Ni-catalyzed ASR of ethylene (cf. Table 2) and 1,4-diene 2h is formed via the pathway proposed in Figure 1.
Figure 5.
Proposed Mechanism for Ni-Catalyzed Reaction of Ethylene with Aromatic Aldehyde Dimethyl Acetal (34)
Conclusion
Herein we described the nickel-catalyzed allylic substitution reaction of simple alkenes for formation of 1,4-dienes. Key for the reaction was use of the appropriate Ni-phosphine complex and a stoichiometric amount of silyl triflate. Allylic alcohol derivatives, bearing a variety of leaving groups, can be coupled with a wide range of simple alkenes, including gaseous ethylene and propylene. Reactions of 1-alkyl-substituted alkenes consistently provided 1,1-disubstituted alkenes with high selectivity. Silyl triflate is proposed to activate the Ni–O bond generating a cationic allyl-Ni species poised for the subsequent migratory insertion event. In some cases, 1,3-dienes were also obtained as an inseparable by-product. Therefore, a method utilizing TCNE to selectively trap (E)-1,3-dienes has been developed, whereby a convenient gram scale synthesis of pure 1,4-diene was possible.
Nickel-catalyzed allylic substitution of allyltrimethylsilane was also developed, in which a catalytic amount of Et3SiOTf promoted the reaction. Butadiene monooxide and 2-vinyltetrahydrofuran also performed well as a substrate, generating the desired 1,4-dienes. Finally, the nickel-catalyzed allylic substitution technique was extended to novel types of transformations, namely, conversion of aldehydes and dimethyl acetals into 1,4-dienes. Further exploration into new methods utilizing simple alkenes is ongoing in our group.
Experimental Section
General Information
Unless otherwise noted, all reactions were performed under an oxygen-free atmosphere of argon with rigorous exclusion of moisture from reagents and glassware. Toluene, dichloromethane, tetrahydrofuran, triethylamine and diethyl ether were obtained from an SG Water solvent purification system. Bis(cyclooctadienyl)nickel(0) (Ni(cod)2) and phosphine ligands were purchased from Strem Chemicals, Inc. or Aldrich, stored under nitrogen atmosphere and used without further purification. Ethylene and propylene were purchased from BOC Gases and Aldrich, respectively, and used as received. 1-Octene, vinylcyclohexane and styrene were distilled from CaH2 prior to use. All other reagents and solvents were used as obtained, without further purification. Analytical and preparative thin-layer chromatography were performed using EM Science silica gel 60 F254 plates. The developed chromatogram was visualized by UV lamp or stained using one of the following: aqueous potassium permanganate (KMnO4) and ethanolic phosphomolybdic acid (PMA). Liquid chromatography was performed using a forced flow (flash chromatography) of the indicated solvent system on silica gel (230-400 mesh).
Melting points are uncorrected. 1H and 13C NMR spectra were recorded on a Varian Inova-300 MHz spectrometer, a Bruker AVANCE-400 MHz spectrometer or Varian Inova 500 MHz spectrometers in CDCl3, unless otherwise noted. Chemical shifts in 1H NMR spectra are reported in parts per million (ppm) on the δ scale from an internal standard of tetramethylsilane in CDCl3 (0.00 ppm) or residual benzene in C6D6 (7.16 ppm). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, m = multiplet, and br = broad), coupling constant in hertz (Hz), and integration. Chemical shifts of 13C NMR spectra are reported in ppm from the central peak of CDCl3 (77.00 ppm), or C6D6 (128.00 ppm) on the δ scale. Infrared (IR) spectra were recorded on a Perkin-Elmer 2000 FT-IR. High-resolution mass spectra (HRMS) were obtained on a Bruker Daltonics APEXII 3 Fourier Transform Mass Spectrometer by Ms. Li Li of the Massachusetts Institute of Technology, Department of Chemistry Instrumentation Facility. GCMS spectra were obtained on an Agilent 5973N Gas Chromatograph/Mass Spectrometer and the Restek Rtx-1 GC column (30 m × 250 μm × 1 μm) in the Massachusetts Institute of Technology, Department of Chemistry Instrumentation Facility.
General Procedure for Nickel-Catalyzed Allylic Substitution Reaction Using Ethylene (Table 2)
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (13.8 mg, 0.05 mmol, 10 mol%) and P(o-anisyl)3 (35.2 mg, 0.1 mmol, 20 mol%) were added to the test tube, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (2.5 mL) under argon and stirred for 15-30 min at rt. The reaction mixture was purged with ethylene for 1 min to remove argon, taken care not to introduce oxygen. The ethylene atmosphere was maintained with an ethylene balloon. Triethylamine (418 μL, 3 mmol, 6 equiv), allylalcohol derivative (0.5 mmol, 1 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 15-90 min. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure and to the residue was added a certain amount of CH3CN (10-20 mg) or (PhCH2)2O (15-20 mg) as an internal standard. The mixture was completely dissolved in CDCl3 and analyzed by 1H NMR spectroscopy. The product yield was determined by referring to methyl protons of CH3CN or methylene protons of (PhCH2)2O.
General Procedure for Nickel-Catalyzed Allylic Substitution Reaction Using Ethylene (Table 3)
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (13.8 mg, 0.05 mmol, 10 mol%) and P(o-anisyl)3 (35.2 mg, 0.1 mmol, 20 mol%) were added to the test tube, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (2.5 mL) under argon and stirred for 15-30 min at rt. The reaction mixture was purged with ethylene for 1 min to remove argon, taken care not to introduce oxygen. The ethylene atmosphere was maintained with an ethylene balloon. Triethylamine (418 μL, 3 mmol, 6 equiv), allylalcohol derivative (0.5 mmol, 1 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 20-200 min. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1 v/v). The solvents were removed under reduced pressure and the crude mixture was purified by silica gel column chromatography or preparative thin layer chromatography. The complete results are summarized in the supporting information.
(E)-Penta-1,4-dienylbenzene (2a)
IR (NaCl plate, thin film, cm−1): 3080, 3060, 3026, 2978, 2891, 1944, 1637, 1599, 1495, 1448, 1429, 1305, 992, 965, 914, 742, 692; 1H NMR (500 MHz, CDCl3, δ):7.16-7.36 (m, 5H), 6.41 (d, J = 15.9 Hz, 1H), 6.22 (dt, J = 6.7, 15.9 Hz, 1H), 5.90 (dtt, J = 6.4, 10.1, 17.0 Hz, 1H), 5.11 (dq, J = 1.9, 17.1 Hz, 1H), 5.06 (dq, J = 1.4, 10.1 Hz, 1H), 2.96 (dt, J = 1.5, 6.6 Hz, 2H); 13C NMR (125 MHz, CDCl3, δ): 137.5, 136.4, 130.8, 128.5, 128.1, 127.0, 126.0, 115.6, 37.0.
(1E)-Penta-1,3-dienylbenzene (3a)
1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. (1E, 3E): 6.74 (dd, J = 10.5, 15.7 Hz, 1H), 1.82 (dd, J = 1.1, 6.8 Hz, 3H); (1E, 3Z): 1.86 (dd, J = 1.7, 7.2 Hz, 3H).
(1E)-Hepta-1,3,6-trienylbenzene (2b)
IR (NaCl plate, thin film, cm−1): 3079, 3060, 3023, 2910, 1944, 1844, 1680, 1637, 1596, 1495, 1448, 1428, 1295, 988, 913, 746, 691; 1H NMR (500 MHz, CDCl3, δ): (1E, 3E): 7.16-7.42 (m, 5H), 6.76 (dd, J = 10.5, 15.7 Hz, 1H), 6.46 (d, J = 15.7 Hz, 1H), 6.22 (dd, J = 10.5, 15.2 Hz, 1H), 5.77-5.91 (m, 2H), 5.07 (dq, J = 1.6, 17.2 Hz, 1H), 5.02-5.06 (m, 1H), 2.89 (dt, J = 1.3, 6.7 Hz, 2H); (1E, 3Z): Distinguishable peaks are shown. 7.04 (ddd, J = 1.2, 11.1, 15.6 Hz, 1H), 6.54 (d, J = 15.6 Hz, 1H), 5.10 (dq, J = 1.8, 17.1 Hz, 1H), 5.53 (dt, J = 7.7, 10.7 Hz, 1H); 13C NMR (125 MHz, CDCl3, δ): (1E, 3E):137.5, 136.3, 132.6, 131.5, 130.7, 129.0, 128.5, 127.2, 126.2, 115.6, 36.8; (1E, 3Z): 137.4, 136.3, 132.7, 129.7, 129.4, 128.5, 127.5, 126.3, 124.0, 115.3, 32.1; HRMS-DART (m/z): [M+H]+ calculated for C13H15, 171.1168; found, 171.1171.
Hepta-1,3,5-trienylbenzene (3b)
Distinguishable peaks are shown. 1.79 (dd, J = 1.5, 6.8 Hz, 3H).
tert-Butyl(hepta-3,6-dienyloxy)dimethylsilane (2c-linear)
IR (NaCl plate, thin film, cm−1): 2929, 2858, 1472, 1255, 1102, 968, 912, 836, 775; 1H NMR (500 MHz, CDCl3, δ): (E): 5.82 (ddt, J = 6.4, 10.4, 17.0 Hz, 1H), 5.40-5.55 (m, 2H), 5.02 (dq, J = 1.7, 17.1 Hz, 1H), 4.98 (dd, J = 1.3, 10.1 Hz, 1H), 3.62 (t, J = 6.9 Hz, 2H), 2.75 (t, J = 6.1 Hz, 2H), 2.23 (q, J = 6.7 Hz, 2H), 0.89 (s, 9H), 0.05 (s, 6H); (Z): Distinguishable peaks are shown. 2.81 (t, J = 6.1 Hz, 2H); 13C NMR (125 MHz, CDCl3, δ): (E):137.1, 129.8, 127.8, 114.9, 63.2, 36.8, 36.3, 25.9, 18.4, −5.2; HRMS-DART (m/z): [M+H]+ calculated for C13H27OSi, 227.1826; found, 227.1829.
tert-Butyldimethyl(3-vinylpent-4-enyloxy)silane (2c-branched)
1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. 5.73 (ddd, J = 7.5, 10.5, 17.4 Hz, 2H), 2.89 (quint, J = 7.3 Hz, 1H).
1-((Hepta-3,6-dienyloxy)methyl)-4-methoxybenzene (2d-linear)
IR (NaCl plate, thin film, cm−1): 3076, 3000, 2934, 2906, 2854, 1637, 1613, 1586, 1513, 1464, 1441, 1361, 1302, 1248, 1208, 1172, 1098, 1037, 993, 971, 913, 821, 756; 1H NMR (500 MHz, CDCl3, δ): (E): 7.26 (d, J = 8.8 Hz, 2H), 6.87 (d, J = 8.8 Hz, 2H), 5.81 (ddt, J = 6.4, 10.1, 17.2 Hz, 1H), 5.42-5.55 (m, 2H), 4.96-5.05 (m, 2H), 4.44 (s, 2H), 3.79 (s, 3H), 3.46 (t, J = 6.9 Hz, 2H), 2.75 (t, J = 5.7 Hz, 2H), 2.32 (dq, J = 0.7, 6.9 Hz, 2H); (Z): Distinguishable peaks are shown. 2.81 (t, J = 5.7 Hz, 2H); 13C NMR (125 MHz, CDCl3, δ): (E):159.0, 137.0, 130.5, 129.7, 129.2, 127.6, 114.9, 113.7, 72.4, 69.7, 55.2, 36.7, 33.0; HRMS-DART (m/z): [M+H]+ calculated for C15H21O2, 233.1536; found, 233.1534.
1-Methoxy-4-((3-vinylpent-4-enyloxy)methyl)benzene (2d-branched)
1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. 5.71 (ddd, J = 7.5, 10.4, 17.2 Hz, 2H), 2.90 (quint, J = 7.4 Hz, 1H).
Hexa-2,5-dien-2-ylbenzene (2e)
IR (NaCl plate, thin film, cm−1): 3079, 3058, 3031, 2977, 2922, 1637, 1598, 1493, 1444, 1379, 1026, 993, 910, 756, 695; 1H NMR (500 MHz, CDCl3, δ): (E): 7.38-7.41 (m, 2H), 7.28-7.33 (m, 2H), 7.17-7.26 (m, 1H), 5.89 (ddt, J = 10.1, 17.1, 6.2 Hz, 1H), 5.80 (tq, J = 7.3, 1.4 Hz, 1H), 5.10 (dq, J = 17.1, 1.8 Hz, 1H), 5.02 (dq, J = 10.1, 1.9 Hz, 1H), 2.97 (t, J = 7.0 Hz, 2H), 2.04 (d, J = 1.4 Hz, 3H); (Z): Distinguishable peaks are shown. 5.49 (tq, J = 7.6, 1.5 Hz, 1H), 2.72 (ddq, J = 6.1, 6.1, 1.4 Hz, 2H); 13C NMR (125 MHz, CDCl3, δ): (E):143.7, 136.6, 135.9, 128.1, 126.6, 125.6, 125.2, 114.8, 33.0, 15.8; HRMS-EI (m/z): [M]+ calculated for C12H14, 158.1090; found, 158.1094.
(2E)-Hexa-2,4-dien-2-ylbenzene (3c)
1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. 1.86 (d, J = 6.7 Hz, 3H).
(E)-Hexa-2,5-dien-3-ylbenzene (3d)
1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. 3.26 (d, J = 5.9 Hz, 2H), 1.79 (d, J = 6.9 Hz, 3H).
(2-Methylpenta-1,4-dienyl)benzene (2f)
IR (NaCl plate, thin film, cm−1): 3079, 3023, 2977, 2911, 1653, 1635, 1492, 1441, 1383, 1172, 1073, 993, 915, 830, 742, 698; 1H NMR (500 MHz, CDCl3, δ): (E): 7.27-7.33 (m, 2H), 7.21-7.26 (m, 2H), 7.16-7.20 (m, 1H), 6.30 (s, 1H), 5.88 (ddt, J = 10.1, 17.0, 6.9 Hz, 1H), 5.07-5.15 (m, 2H), 2.90 (d, J = 6.9 Hz, 2H), 1.85 (d, J = 1.3 Hz, 3H); (Z): Distinguishable peaks are shown. 6.39 (s, 1H), 2.96 (d, J = 6.2 Hz, 2H), 1.88 (d, J = 1.5 Hz, 3H); 13C NMR (125 MHz, CDCl3, δ): (E):138.4, 137.2, 136.4, 128.8, 128.0, 125.9, 125.7, 116.3, 45.0, 17.8; (Z): Distinguishable peaks are shown. 115.9, 37.2, 28.0; HRMS-EI (m/z): [M]+ calculated for C12H14, 158.1090; found, 158.1092.
(E)-(3-Methylpenta-1,4-dienyl)benzene (2g)
IR (NaCl plate, thin film, cm−1): 3081, 3060, 3026, 2966, 2927, 2869, 1635, 1599, 1495, 1448, 1411, 1369, 994, 965, 913, 747, 692; 1H NMR (500 MHz, CDCl3, δ): 7.16-7.36 (m, 5H), 6.36 (d, J = 16.0 Hz, 1H), 6.17 (dd, J = 7.1, 16.0 Hz, 1H), 5.86 (ddd, J = 6.6, 10.3, 17.0 Hz, 1H), 5.07 (dt, J = 17.0, 1.5 Hz, 1H), 5.01 (dt, J = 10.3, 1.4 Hz, 1H), 2.97-3.07 (m, 1H), 1.19 (d, J = 6.9 Hz, 3H); 13C NMR (125 MHz, CDCl3, δ): 142.4, 137.6, 134.2, 128.6, 128.4, 127.0, 126.0, 113.3, 40.6, 19.8; HRMS-EI (m/z): [M]+ calculated for C12H14, 158.1090; found, 158.1089.
(E)-Buta-1,3-dienylbenzene (3e) was previously reported.[44]
Gram-Scale Allylic Substitution Reaction of Ethylene (Scheme 1)
A round-bottomed flask and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (68.8 mg, 0.25 mmol, 2.5 mol%) and P(o-anisyl)3 (352 mg, 1 mmol, 10 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (50 mL) under argon and stirred for 15 min at rt. The reaction mixture was purged with ethylene for 1 min to remove argon, taken care not to introduce oxygen. The ethylene atmosphere was maintained with an ethylene balloon. Triethylamine (8.4 mL, 60 mmol, 6 equiv), cinnamyl methylcarbonate (10 mmol, 1 equiv) and Et3SiOTf (4.0 mL, 17.5 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 2 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1 v/v). The solvents were removed under reduced pressure and the crude mixture was dissolved in benzene (50 mL). Tetracyanoethylene (256 mg, 20 mol%) was added to the reaction mixture, and the reaction mixture was kept stirred for 30 min at rt. After evaporation of the solvent, the crude mixture was purified by silica gel column chromatography, to afford 2a (1.18 g, >98% purity, 81% yield) along with 4 (134.8 mg, 5% yield). The inseparable impurity was (3Z)-3a (<2% yield).
3-Methyl-6-phenylcyclohex-4-ene-1,1,2,2-tetracarbonitrile (4)
Mp. 128-129 °C; IR (NaCl plate, thin film, cm−1): 3036, 2098, 2255, 1495, 1455, 1393, 1379, 1219, 1169, 1115, 1031, 911, 823, 753, 735, 702; 1H NMR (400 MHz, CDCl3, δ): 7.44-7.51 (m, 5H), 6.10 (ddd, J = 2.8, 3.6, 10.6 Hz, 1H), 6.00 (dt, J = 10.6, 2.1 Hz, 1H), 4.29 (q, J = 2.5 Hz, 1H), 3.28-3.37 (m, 1H), 1.73 (d, J = 7.5 Hz, 3H); 13C NMR (100 MHz, CDCl3, δ): 132.8, 130.2, 129.8, 129.2, 128.6, 124.5, 111.6, 111.3, 110.0, 109.7, 46.6, 42.9, 42.8, 37.5, 17.8; HRMS-DART (m/z): [M+H]+ calculated for C17H12N4, 273.1135; found, 273.1130.
Experimental Procedure for Nickel-Catalyzed Reaction of Cinnamyl Methyl Carbonate with Propene (Table 4)
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (13.8 mg, 0.05 mmol, 10 mol%) and the phosphine ligand (0.1 mmol, 20 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (2.5 mL) under argon and stirred for 15 min at rt. The reaction mixture was purged with propene for 1 min to remove argon, taken care not to introduce oxygen. The propene atmosphere was maintained with a propene balloon. Triethylamine (418 μL, 3 mmol, 6 equiv), cinnamyl methyl carbonate (96.1 mg, 0.5 mmol, 1 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 3.5 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure, and to the residue was added a certain amount of 1,4-dioxane as an internal standard. The mixture was completely dissolved in CDCl3 and analyzed by 1H NMR spectroscopy. The product yield and selectivity were determined by referring to methylene protons of 1,4-dioxane.
(E)-(4-Methylpenta-1,4-dienyl)benzene (5a)
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (13.8 mg, 0.05 mmol, 10 mol%) and PCy2Ph (27.4 mg, 0.1 mmol, 20 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (2.5 mL) under argon and stirred for 15 min at rt. The reaction mixture was purged with propene for 1 min to remove argon, taken care not to introduce oxygen. The propene atmosphere was maintained with a propene balloon. Triethylamine (418 μL, 3 mmol, 6 equiv), cinnamyl methyl carbonate (96.1 mg, 0.5 mmol, 1 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 3.5 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure and the crude mixture was purified by silica gel column chromatography, to afford 5a (61.1 mg, 77% yield, >98% selectivity). IR (NaCl plate, thin film, cm−1): 3080, 3026, 2970, 2935, 1646, 1495, 1448, 1373, 965, 889, 740, 691; 1H NMR (500 MHz, CDCl3, δ): 7.31-7.38 (m, 2H), 7.25-7.31 (m, 2H), 7.15-7.22 (m, 1H), 6.34-6.44 (m, 1H), 6.16-6.26 (m, 1H), 4.79 (brs, 1H), 4.78 (brs, 1H), 2.89 (d, J = 7.0 Hz, 2H), 1.76 (s, 3H); 13C NMR (125 MHz, CDCl3, δ): 144.5, 137.6, 131.3, 128.5, 128.2, 127.0, 126.0, 111.0, 41.5, 22.5; HRMS-EI (m/z): [M]+ calculated for C12H14, 158.1090; found, 158.1096.
(1E)-Hexa-1,4-dienylbenzene (6)
(A mixture of (1E, 4E) and (1E, 4Z).) 1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. 5.45-5.62 (m, 2H), 1.65-1.72 (m, 3H).
(E)-Hexa-1,5-dienylbenzene (7)
1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. 5.86 (ddt, J = 6.6, 10.2, 17.1 Hz, 1H), 5.06 (dq, J = 1.6, 17.1 Hz, 1H), 4.99 (ddt, J = 1.2, 2.0, 10.2 Hz, 1H), 2.31 (q, J = 6.8 Hz, 2H), 2.23 (q, J = 6.7 Hz, 2H).
(E)-(4-Methylenedec-1-enyl)benzene (5b)
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (13.8 mg, 0.05 mmol, 10 mol%) and PCy2Ph (13.7 mg, 0.05 mmol, 10 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (0.5 mL) under argon and stirred for 15 min at rt. Cinnamyl methyl carbonate (96.1 mg, 0.5 mmol, 1 equiv) was added, and the mixture was stirred for 30 min at rt. P(OPh)3 (13.1 μL, 0.05 mmol, 10 mol%), triethylamine (418 μL, 3 mmol, 6 equiv), 1-octene (393 μL, 2.5 mmol, 5 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 18 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure and the crude mixture was purified by silica gel column chromatography, to afford 5b (90.4 mg, 79% yield, >98% selectivity). IR (NaCl plate, thin film, cm−1): 3081, 3026, 2955, 2927, 2856, 1644, 1495, 1449, 965, 891, 739, 691; 1H NMR (500 MHz, CDCl3, δ): 7.34-7.38 (m, 2H), 7.27-7.32 (m, 2H), 7.17-7.22 (m, 1H), 6.40 (d, J = 15.8 Hz, 1H), 6.23 (dt, J = 15.8, 7.1 Hz, 1H), 4.77-4.81 (m, 2H), 2.90 (d, J = 7.1 Hz, 2H), 2.05 (t, J = 7.6 Hz, 2H), 1.40-1.50 (m, 2H), 1.25-1.35 (m, 8H), 0.88 (t, J = 7.2 Hz, 3H); 13C NMR (125 MHz, CDCl3, δ): 148.7, 137.6, 131.2, 128.5, 127.0, 126.0, 110.0, 39.9, 36.1, 31.8, 29.0, 27.6, 22.6, 14.1; HRMS-EI (m/z): [M]+ calculated for C17H24, 228.1873; found, 228.1875.
(E)-Triethyl(2-methylene-5-phenylpent-4-enyloxy)silane (5c)
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (27.5 mg, 0.1 mmol, 20 mol%) and PCy2Ph (27.4 mg, 0.1 mmol, 20 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (0.5 mL) under argon and stirred for 15 min at rt. Cinnamyl methyl carbonate (96.1 mg, 0.5 mmol, 1 equiv) was added, and the mixture was stirred for 30 min at rt. P(OPh)3 (26.2 μL, 0.1 mmol, 20 mol%), triethylamine (418 μL, 3 mmol, 6 equiv), allyloxytriethylsilane[45] (514 μL, 2.5 mmol, 5 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 50 min. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure and the crude mixture was purified by silica gel column chromatography, to afford 5c (104.8 mg, 73% yield, >98% selectivity). IR (NaCl plate, thin film, cm−1): 3026, 2955, 2910, 2876, 1238, 1111, 1078, 1007, 965, 900, 803, 741, 691; 1H NMR (500 MHz, CDCl3, δ): 7.34-7.38 (m, 2H), 7.28-7.32 (m, 2H), 7.19-7.23 (m, 1H), 6.42 (d, J = 15.8 Hz, 1H), 6.23 (dt, J = 15.8, 7.1 Hz, 1H), 5.12 (s, 1H), 4.92 (s, 1H), 4.12 (s, 2H), 2.94 (d, J = 6.6 Hz, 2H), 0.97 (t, J = 8.0 Hz, 9H), 0.63 (q, J = 8.0 Hz, 6H); 13C NMR (125 MHz, CDCl3, δ): 147.1, 137.5, 131.5, 128.5, 127.8, 127.0, 126.0, 110.1, 65.4, 36.4, 6.8, 4.4; HRMS-ESI (m/z): [M+H]+ calculated for C18H29OSi, 311.1802; found, 311.1809.
(E)-3-Methylene-6-phenylhex-5-en-1-ol (5d-alcohol form)
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (13.8 mg, 0.05 mmol, 10 mol%) and PCy2Ph (13.7 mg, 0.05 mmol, 10 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (0.5 mL) under argon and stirred for 15 min at rt. Cinnamyl methyl carbonate (96.1 mg, 0.5 mmol, 1 equiv) was added, and the mixture was stirred for 30 min at rt. P(OPh)3 (13.1 μL, 0.05 mmol, 10 mol%), triethylamine (418 μL, 3 mmol, 6 equiv), 3-buten-1-ol tert-butyldimethylsilyl ether[46] (573 μL, 2.5 mmol, 5 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 16 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure and the crude mixture was purified by silica gel column chromatography, to afford a mixture of 5d and 3-buten-1-ol tert-butyldimethylsilyl ether, which were found to be inseparable from each other. The mixture was dissolved in MeOH(3 mL), and 12N HCl aq. (ca. 100 mg) was added at rt. The mixture was stirred for 10 min at rt, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to afford 5d-alcohol form (78.0 mg, 83% yield in 2 steps). IR (NaCl plate, thin film, cm−1): 3352, 3080, 3026, 2888, 1644, 1598, 1495, 1448, 1046, 967, 896, 741, 692; 1H NMR (500 MHz, CDCl3, δ): 7.34-7.38 (m, 2H), 7.27-7.32 (m, 2H), 7.18-7.23 (m, 1H), 6.43 (d, J = 15.8 Hz, 1H), 6.20 (dt, J = 15.8, 7.1 Hz, 1H), 4.97 (d, J = 1.4 Hz, 1H), 4.91 (s, 1H), 3.75 (t, J = 6.3 Hz, 2H), 2.94 (d, J = 7.0 Hz, 2H), 2.35 (t, J = 6.4 Hz, 2H), 1.55 (s, 1H); 13C NMR (100 MHz, CDCl3, δ): 144.6, 137.3, 131.8, 128.5, 127.6, 127.1, 126.0, 113.0, 60.3, 39.6, 39.0; HRMS-ESI (m/z): [M+Na]+ calculated for C13H16ONa, 211.1093; found, 211.1098.
(E)-(6-Methyl-4-methylenehept-1-enyl)benzene (5e)
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (27.5 mg, 0.1 mmol, 20 mol%) and PCy2Ph (27.4 mg, 0.1 mmol, 20 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (0.5 mL) under argon and stirred for 15 min at rt. Cinnamyl methyl carbonate (96.1 mg, 0.5 mmol, 1 equiv) was added, and the mixture was stirred for 30 min at rt. P(OPh)3 (26.2 μL, 0.1 mmol, 20 mol%), triethylamine (418 μL, 3 mmol, 6 equiv), 4-methyl-1-pentene (316 μL, 2.5 mmol, 5 equiv), and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 21 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure and the crude mixture was purified by silica gel column chromatography, to afford 5e (87.2 mg, 87% yield, >98% selectivity). IR (NaCl plate, thin film, cm−1): 3081, 3026, 2954, 2924, 2868, 1642, 1496, 1464, 1449, 1383, 1366, 966, 894, 741, 691; 1H NMR (500 MHz, CDCl3, δ): 7.34-7.38 (m, 2H), 7.27-7.31 (m, 2H), 7.17-7.22 (m, 1H), 6.40 (d, J = 15.8 Hz, 1H), 6.22 (dt, J = 15.8, 7.1 Hz, 1H), 4.83 (d, J = 1.7 Hz, 1H), 4.77 (s, 1H), 2.88 (d, J = 7.1 Hz, 2H), 1.94 (d, J = 7.3 Hz, 2H), 1.76-1.85 (m, 1H), 0.89 (d, J = 6.6 Hz, 6H); 13C NMR (100 MHz, CDCl3, δ): 147.3, 137.6, 131.3, 128.5, 128.4, 127.0, 126.0, 111.5, 45.9, 39.5, 25.9, 22.5; HRMS-Dart (m/z): [M−H]+ calculated for C15H19, 199.1481; found, 199.1486.
(E)-(4-Cyclohexylpenta-1,4-dienyl)benzene (5f)
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (27.5 mg, 0.1 mmol, 20 mol%) and PCy2Ph (54.9 mg, 0.2 mmol, 40 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (0.5 mL) under argon and stirred for 15 min at rt. Cinnamyl methyl carbonate (96.1 mg, 0.5 mmol, 1 equiv) was added, and the mixture was stirred for 30 min at rt. Triethylamine (418 μL, 3 mmol, 6 equiv) and vinylcyclohexane (342 μL, 2.5 mmol, 5 equiv) were added in the above order. Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) was then added over 4 h by using a syringe pump. The mixture was stirred at rt for another 12 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure and the crude mixture was purified by silica gel column chromatography, to afford 5f (72.4 mg, 64% yield). Inseparable regioisomers are included (<10% yield) in the reported yield. For more details, see supporting information. IR (NaCl plate, thin film, cm−1): 3081, 3025, 2925, 2851, 1639, 1495, 1448, 965, 887, 742, 691; 1H NMR (500 MHz, CDCl3, δ): 7.33-7.38 (m, 2H), 7.27-7.31 (m, 2H), 7.17-7.22 (m, 1H), 6.39 (d, J = 15.8 Hz, 1H), 6.21 (dt, J = 15.8, 7.1 Hz, 1H), 4.80 (s, 1H), 4.76 (d, J = 1.5 Hz, 1H), 2.93 (d, J = 7.0 Hz, 2H), 1.10-1.93 (m, 11H); 13C NMR (125 MHz, CDCl3, δ): 153.8, 137.7, 131.1, 128.9, 128.5, 126.9, 126.0, 108.4, 44.1, 38.6, 32.3, 26.7, 26.4; HRMS-EI (m/z): [M]+ calculated for C17H22, 226.1716; found, 226.1711.
(1E,4E)-1,5-diphenylpenta-1,4-diene (14).[47]
A test tube (borosilicate glass, 16 × 100 mm) and a stir bar were oven-dried and brought into a glove box. Ni(cod)2 (27.5 mg, 0.1 mmol, 20 mol%) and PCy2Ph (54.9 mg, 0.2 mmol, 40 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (0.5 mL) under argon and stirred for 15 min at rt. Cinnamyl methyl carbonate (96.1 mg, 0.5 mmol, 1 equiv) was added, and the mixture was stirred for 30 min at rt. Triethylamine (418 μL, 3 mmol, 6 equiv), styrene (286 μL, 2.5 mmol, 5 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 18 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure and the crude mixture was purified by silica gel column chromatography, to afford 14 (27.7 mg, 25% yield).
Experimental Procedure for Ni-Catalyzed Allylic Substitution Reaction of Allyltrimethylsilane Using a Catalytic Amount of TESOTf (Scheme 5)
A test tube (borosilicate glass, 16 × 100 mm) and a stirrer bar were oven-dried and brought into a glove box. Ni(cod)2 (27.5 mg, 0.1 mmol, 20 mol%) and PCy2Ph (54.9 mg, 0.2 mmol, 40 mol%) were added to the flask, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (0.5 mL) under argon and stirred for 15 min at rt. Cinnamyl methyl carbonate (96.1 mg, 0.5 mmol, 1 equiv) was added, and the mixture was stirred for 30 min at rt. Allyltrimethylsilane (397 μL, 2.5 mmol, 5 equiv) and Et3SiOTf (22.6 μL, 0.1 mmol, 20 mol%) were added in the above order. The mixture was stirred at rt for 18 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure and the crude mixture was purified by silica gel column chromatography, to afford an inseparable mixture of 21b, 5a, 21l and 7 (80.4 mg, 82% yield, 21b:5a:21l:7 = 46:43:5:6).
(E)-Trimethyl(2-methylene-5-phenylpent-4-enyl)silane (21b)
IR (NaCl plate, thin film, cm−1): (A mixture of 21b, 5a, 21l and 7) 3081, 3026, 2954, 2895, 1942, 1872, 1632, 1599, 1578, 1495, 1448, 1422, 1247, 1155, 965, 851, 741, 692; 1H NMR (500 MHz, CDCl3, δ): 7.28-7.40 (m, 4H), 7.18-7.24 (m, 1H), 6.37-6.46 (m, 1H), 6.18-6.28 (m, 1H), 4.70 (d, J = 1.8 Hz, 1H), 4.61 (s, 1H), 2.87 (d, J = 6.9 Hz, 2H), 1.60 (s, 2H), 0.07 (s, 9H); 13C NMR (125 MHz, CDCl3, δ): 146.1, 137.6, 131.4, 128.5, 128.4, 127.0, 126.0, 108.4, 42.0, 26.7, −1.3; HRMS-ESI (m/z): [M+Na]+ calculated for C15H22SiNa, 253.1383; found, 253.1381.
Trimethyl((2E,5E)-6-phenylhexa-2,5-dienyl)silane (21l)
1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. 1.47 (dd, J = 0.7, 8.0 Hz, 2H).
Experimental Procedure for Nickel-Catalyzed Ring Opening Reaction of Butadiene Monoxide by Ethylene (Scheme 6, eq. 7)
A test tube (borosilicate glass, 16 × 100 mm) and a stirrer bar were oven-dried and brought into a glove box. Ni(cod)2 (13.8 mg, 0.05 mmol, 10 mol%) and P(o-anisyl)3 (35.2 mg, 0.1 mmol, 20 mol%) were added to the test tube, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (2.5 mL) under argon and stirred for 15-30 min at rt. The reaction mixture was purged with ethylene for 1 min to remove argon, taken care not to introduce oxygen. The ethylene atmosphere was maintained with an ethylene balloon. Triethylamine (418 μL, 3 mmol, 6 equiv), butadiene monoxide (40.3 μL, 0.5 mmol, 1 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 2 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure. The crude material was purified by silica gel column chromatography, to afford a mixture of 22a and 23a (85.3 mg, 80% yield, 22a : 23a = 62:38).
(E)-Triethyl(hexa-2,5-dienyloxy)silane (22a)
IR (NaCl plate, thin film, cm−1): 2955, 2912, 2877, 1638, 1458, 1414, 1378, 1239, 1105, 1050, 1015, 971, 913, 810, 744, 668; 1H NMR (400 MHz, CDCl3, δ): 5.83 (ddt, J = 10.2, 16.9, 6.4 Hz, 1H), 5.55-5.73 (m, 2H), 4.97-5.07 (m, 2H), 2.79 (tq, J = 6.4, 1.4 Hz, 2H), 0.96 (t, J = 7.8 Hz, 9H), 0.61 (q, J = 7.8 Hz, 6H); 13C NMR (100 MHz, CDCl3, δ): 136.6, 130.2, 128.9, 115.3, 63.5, 36.3, 6.7, 4.5; HRMS-ESI (m/z): [M+Na]+ calculated for C12H24OSiNa, 235.1489; found, 235.1491.
Triethyl(2-vinylbut-3-enyloxy)silane (23a)
IR (NaCl plate, thin film, cm−1): 3081, 2956, 2912, 2877, 1640, 1458, 1415, 1378, 1239, 1175, 1106, 1004, 915, 804, 744; 1H NMR (400 MHz, CDCl3, δ): 5.75-5.88 (m, 2H), 5.10-5.12 (m, 2H), 5.08 (ddd, J = 1.1, 1.8, 5.4 Hz, 2H), 3.59 (d, J = 6.9 Hz, 2H), 2.93 (quint + t, J = 7.1, 1.2 Hz, 1H), 0.95 (t, J = 8.1 Hz, 9H), 0.59 (q, J = 8.1 Hz, 6H); 13C NMR (100 MHz, CDCl3, δ): 138.0, 115.9, 66.0, 50.4, 6.8, 4.4; HRMS-ESI (m/z): [M+Na]+ calculated for C12H24OSiNa, 235.1489; found, 235.1492.
Experimental Procedure for Nickel-Catalyzed Ring Opening Reaction of Butadiene Monoxide by 1-Octene (Scheme 6, eq. 8)
A test tube (borosilicate glass, 16 × 100 mm) and a stirrer bar were oven-dried and brought into a glove box. Ni(cod)2 (27.5 mg, 0.10 mmol, 20 mol%) and PCy2Ph (54.9 mg, 0.2 mmol, 40 mol%) were added to the test tube, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (0.5 mL) under argon and stirred for 15 min at rt. Butadiene monoxide (40.3 μL, 0.5 mmol, 1 equiv) was added to the reaction mixture, and the mixture was stirred for 30 min. Triethylamine (418 μL, 3 mmol, 6 equiv), 1-octene (393 μL, 2.5 mmol, 5 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 15 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure. The crude material was purified by silica gel column chromatography, to afford 24 (77.9 mg, 53% yield).
(E)-Triethyl(5-methyleneundec-2-enyloxy)silane (24)
IR (NaCl plate, thin film, cm−1): 3078, 2956, 2929, 2876, 1645, 1458, 1414, 1378, 1239, 1121, 1050, 1015, 971, 891, 810, 744, 671; 1H NMR (500 MHz, CDCl3, δ): 5.66 (dtt, J = 15.2, 1.3, 6.8 Hz, 1H), 5.60 (dtt, J = 15.2, 5.3, 1.2 Hz, 1H), 4.73 (d, J = 4.8 Hz, 1H), 4.14 (dq, J = 5.2, 1.2 Hz, 1H), 2.73 (d, J = 6.3 Hz, 2H), 2.00 (t, J = 7.4 Hz, 2H), 1.37-1.46 (m, 2H), 1.23-1.35 (m, 6H), 0.96 (t, J = 7.8 Hz, 9H), 0.88 (t, J = 7.1 Hz, 3H), 0.61 (q, J = 7.8 Hz, 6H); 13C NMR (100 MHz, CDCl3, δ): 148.7, 130.8, 129.1, 109.5, 63.5, 39.1, 36.0, 31.8, 29.0, 27.6, 22.6, 14.1, 6.8, 4.5; HRMS-ESI (m/z): [M+Na]+ calculated for C18H36OSiNa, 319.2428; found, 319.2423.
(E)-Triisopropyl(hexa-2,5-dienyloxy)silane (22b)
IR (NaCl plate, thin film, cm−1): 2943, 2892, 2866, 1639, 1463, 1380, 1250, 1132, 1107, 1060, 1013, 994, 970, 914, 882, 800, 750, 682, 658; 1H NMR (500 MHz, CDCl3, δ): 5.83 (ddt, J = 10.2, 17.1, 6.4 Hz, 1H), 5.70 (dtt, J = 15.3, 1.6, 6.5 Hz, 1H), 5.59 (dtt, J = 15.3, 1.4, 4.9 Hz, 1H), 5.04 (dq, J = 17.1, 1.7 Hz, 1H), 4.96-5.02 (m, 1H), 4.22 (dq, J = 4.9, 1.4 Hz, 2H), 2.80 (tq, J = 6.4, 1.4 Hz, 2H), 1.04-1.14 (m, 21H); 13C NMR (125 MHz, CDCl3, δ): 136.8, 130.5, 128.0, 115.2, 63.8, 36.3, 18.0, 12.0; HRMS-ESI (m/z): [M+Na]+ calculated for C15H30OSiNa, 277.1958; found, 277.1954.
Triisopropyl(2-vinylbut-3-enyloxy)silane (23b)
IR (NaCl plate, thin film, cm−1): 3081, 2943, 2894, 2866, 1836, 1640, 1464, 1413, 1383, 1247, 1113, 1069, 995, 916, 882, 789, 682, 659; 1H NMR (500 MHz, CDCl3, δ): 5.81-5.89 (m, 2H), 5.09-5.11 (m, 2H), 5.06-5.08 (m, 2H), 3.68 (d, J = 6.5 Hz, 2H), 2.91-2.96 (m, 1H), 1.02-1.10 (m, 21H); 13C NMR (125 MHz, CDCl3, δ): 138.2, 115.7, 66.6, 50.6, 18.0, 12.0; HRMS-ESI (m/z): [M+Na]+ calculated for C15H30OSiNa, 277.1958; found, 277.1956.
Experimental Procedure for Nickel-Catalyzed Ring Opening Reaction of (E)-2-styryltetrahydrofuran by Ethylene (Scheme 8)
A test tube (borosilicate glass, 16 × 100 mm) and a stirrer bar were oven-dried and brought into a glove box. Ni(cod)2 (13.8 mg, 0.05 mmol, 10 mol%) and P(o-anisyl)3 (35.2 mg, 0.1 mmol, 20 mol%) were added to the test tube, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (2.5 mL) under argon and stirred for 15-30 min at rt. The reaction mixture was purged with ethylene for 1 min to remove argon, taken care not to introduce oxygen. The ethylene atmosphere was maintained with an ethylene balloon. Triethylamine (418 μL, 3 mmol, 6 equiv), (E)-2-styryltetrahydrofuran (30)[48] (84.6 μL, 0.5 mmol, 1 equiv) and Et3SiOTf (198 μL, 0.875 mmol, 1.75 equiv) were added in the above order. The mixture was stirred at rt for 3.5 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure. The crude material was purified by silica gel column chromatography, to afford a mixture (118.8 mg) of 31 (46% yield) and 32 (32% yield, E:Z = 82.18). In order to characterize 31, the mixture thus obtained was treated with tetracyanoethylene (TCNE) in benzene for 30 min at rt to remove 32.
(E)-Triethyl(6-phenyl-4-vinylhex-5-enyloxy)silane (31)
IR (NaCl plate, thin film, cm−1): 2953, 2911, 2876, 1635, 1599, 1494, 1457, 1414, 1385, 1238, 1098, 1006, 965, 913, 799, 745, 693; 1H NMR (500 MHz, CDCl3, δ): 7.32-7.42 (m, 2H), 7.26-7.31 (m, 2H), 7.16-7.22 (m, 1H), 6.37 (d, J = 15.9 Hz, 1H), 6.10 (dd, J = 7.8, 15.9 Hz, 1H), 5.79 (ddd, J = 7.3, 10.3, 17.3 Hz, 1H), 5.07 (d, J = 17.3 Hz, 1H), 5.03 (d, J = 10.3 Hz, 1H), 3.62 (t, J = 6.1 Hz, 1H), 2.86 (quint, J = 7.0 Hz, 1H), 1.51-1.62 (m, 4H), 0.95 (t, J = 7.9 Hz, 9H), 0.59 (q, J = 7.9 Hz, 6H); 13C NMR (125 MHz, CDCl3, δ): 141.2, 137.6, 133.0, 129.7, 128.4, 127.0, 126.0, 114.4, 62.8, 46.9, 30.9, 30.5, 6.8, 4.4; HRMS-ESI (m/z): [M+H]+ calculated for C20H33OSi, 317.2295; found, 317.2289.
Triethyl((6E)-7-phenylhepta-4,6-dienyloxy)silane (32)
1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. (E): 6.74 (dd, J = 10.4, 15.7 Hz, 1H), 6.45 (d, J = 15.7 Hz, 1H), 6.25 (dd, J = 10.4, 15.2 Hz, 1H), 3.68 (t, J = 6.9 Hz, 2H), 2.38 (q, J = 6.9 Hz, 2H), 0.97 (t, J = 7.2 Hz, 9H). (Z): 7.07 (ddd, J = 1.0, 11.1, 15.6 Hz, 1H), 6.53 (d, J = 15.6 Hz, 1H), 5.54 (dt, J = 10.8, 7.7 Hz, 1H), 3.69 (t, J = 7.0 Hz, 2H), 2.54 (dq, J = 1.5, 7.2 Hz, 2H); HRMS-ESI (m/z): [M+H]+ calculated for C18H28OSi, 289.1988; found, 289.1989.
Experimental Procedure for Nickel-Catalyzed Reaction of Ethylene with Cinnamyl Aldehyde (Scheme 9)
A test tube (borosilicate glass, 16 × 100 mm) and a stirrer bar were oven-dried and brought into a glove box. Ni(cod)2 (27.5 mg, 0.10 mmol, 20 mol%) and P(o-anisyl)3 (70.5 mg, 0.20 mmol, 40 mol%) were added to the test tube, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (2.5 mL) under argon and stirred for 15 min at rt. The reaction mixture was purged with ethylene for 1 min to remove argon, taken care not to introduce oxygen. The ethylene atmosphere was maintained with an ethylene balloon. Triethylamine (418 μL, 3 mmol, 6 equiv), cinnamyl aldehyde (62.9 μL, 0.5 mmol, 1 equiv) and Me3SiOTf (249 μL, 1.38 mmol, 2.75 equiv) were added in the above order. The mixture was stirred at rt for 23 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure. The crude material was purified by silica gel column chromatography, to afford 2b (65% yield).
Experimental Procedure for Nickel-Catalyzed Reaction of Ethylene with p-Anisaldehyde Dimethylacetal (Scheme 10)
A test tube (borosilicate glass, 16 × 100 mm) and a stirrer bar were oven-dried and brought into a glove box. Ni(cod)2 (27.5 mg, 0.10 mmol, 20 mol%) and P(o-anisyl)3 (70.5 mg, 0.20 mmol, 40 mol%) were added to the test tube, which was sealed with a septum, and brought out of the glove box and connected to an argon line. The catalyst mixture was dissolved in toluene (2.5 mL) under argon and stirred for 15 min at rt. The reaction mixture was purged with ethylene for 1 min to remove argon, taken care not to introduce oxygen. The ethylene atmosphere was maintained with an ethylene balloon. Triethylamine (418 μL, 3 mmol, 6 equiv), p-anisaldehyde dimethylacetal (34b, 85.1 μL, 0.5 mmol, 1 equiv) and Et3SiOTf (311 μL, 1.38 mmol, 2.75 equiv) were added in the above order. The mixture was stirred at rt for 4 h. The mixture was then filtered through a plug of silica gel, and washed with a mixture of hexane-EtOAc (1/1). The solvents were removed under reduced pressure. The crude material was purified by silica gel column chromatography, to afford a mixture of 2h (83% yield) and 1-Methoxy-4-(penta-1,3-dienyl)benzene (3f, 12% yield).
(E)-1-Methoxy-4-(penta-1,4-dienyl)benzene (2h)
IR (NaCl plate, thin film, cm−1): 3003, 2956, 2934, 2908, 2835, 1637, 1607, 1511, 1462, 1441, 1296, 1248, 1175, 1106, 1035, 991, 967, 914, 839; 1H NMR (500 MHz, CDCl3, δ): 7.27-7.30 (m, 2H), 6.82-6.85 (m, 2H), 6.35 (d, J = 15.8 Hz, 1H), 6.08 (dt, J = 15.8, 6.7 Hz, 1H), 5.90 (ddt, J = 10.1, 17.1, 6.4 Hz, 1H), 5.10 (dq, J = 17.1, 1.7 Hz, 1H), 5.05 (dq, J = 10.1, 1.3 Hz, 1H), 3.79 (s, 3H), 2.94 (tq, J = 6.6, 1.5 Hz, 2H); 13C NMR (125 MHz, CDCl3, δ): 158.7, 136.7, 130.4, 130.2, 127.1, 125.9, 115.4, 113.9, 55.2, 37.0; HRMS-ESI (m/z): [M+H]+ calculated for C12H14O, 175.1117; found, 175.1119.
1-Methoxy-4-(penta-1,3-dienyl)benzene (3f)
1H NMR (500 MHz, CDCl3, δ): Distinguishable peaks are shown. (E): 6.62 (dd, J = 10.4, 15.7 Hz, 1H), 6.37 (d, J = 15.6 Hz, 1H), 5.77 (dq, J = 15.0, 6.8 Hz, 1H), 1.81 (d, J = 6.9 Hz, 3H); (Z): 1.85 (dd, J = 1.8, 7.2 Hz, 3H).
Supplementary Material
Acknowledgements
Support for this work was provided by the NIGMS (GM-63755). We are grateful to Dr. Li Li (MIT, DCIF) for high resolution mass spectrometric analysis. R. M. thanks JSPS Postdoctoral Fellowships for Research Abroad for financial support.
References
- [1].Lappin GR, Sauer JD, editors. Alpha Olefins Applications Handbook. New York; Marcel Dekker: 1989. [Google Scholar]
- [2].a) Gladysz JA, editor. Special Issue ‘Frontiers in Metal-Catalyzed Polymerization’. Chem. Rev. 2000;100:1167–1682. doi: 10.1021/cr000450+. [DOI] [PubMed] [Google Scholar]; b) Blom R, editor. Organometallic Catalysts and Olefin Polymerization. Springer; New York: 2001. [Google Scholar]
- [3].Claver C, van Leeuwen PWNM. Rhodium Catalyzed Hydroformylation. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. [Google Scholar]
- [4].For review, see: Grubbs RH, editor. Handbook of Metathesis. John Wiley & Sons; New York: 2003. Grubbs RH. Tetrahedron. 2004;60:7117. Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem. 2005;117:4564. doi: 10.1002/anie.200500368. Angew. Chem. Int. Ed. 2005;44:4490.
- [5].For reviews, see: Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009. doi: 10.1021/cr9903048.
- [6].Snider B. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 2. Pergamon Press; Oxford: 1991. pp. 527–561. [Google Scholar]
- [7].a) Ogoshi S, Oka M.-a., Kurosawa H. J. Am. Chem. Soc. 2004;126:11802. doi: 10.1021/ja0460716. [DOI] [PubMed] [Google Scholar]; b) Ogoshi S, Ueta M, Arai T, Kurosawa H. J. Am. Chem. Soc. 2005;127:12810. doi: 10.1021/ja0542486. [DOI] [PubMed] [Google Scholar]; c) Ogoshi S, Haba T, Ohashi M. J. Am. Chem. Soc. 2009;131:10350. doi: 10.1021/ja903510u. [DOI] [PubMed] [Google Scholar]; d) Ogoshi S, Nishimura A, Haba T, Ohashi M. Chem. Lett. 2009;38:1166. [Google Scholar]
- [8].a) Ng S-S, Jamison TF. J. Am. Chem. Soc. 2005;127:14194. doi: 10.1021/ja055363j. [DOI] [PubMed] [Google Scholar]; b) Ho C-Y, Ng S-S, Jamison TF. J. Am. Chem. Soc. 2006;128:5362. doi: 10.1021/ja061471+. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ho C-Y, Jamison TF. Angew. Chem. 2007;119:796. doi: 10.1002/anie.200603907. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:782. [Google Scholar]; d) Ho C-Y, Ohmiya H, Jamison TF. Angew. Chem. 2008;120:1919. doi: 10.1002/anie.200705163. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:1893. [Google Scholar]
- [9].For accounts, see: Ng S-S, Ho C-Y, Schleicher KD, Jamison TF. Pure Appl. Chem. 2008;80:929. doi: 10.1351/pac200880050929. Ho C-Y, Schleicher KD, Chan C-W, Jamison TF. Synlett. 2009:2565. doi: 10.1055/s-0029-1217747.
- [10].a) Trost BM, Lee CB. Chapter 8E. In: Ojima I, editor. Catalytic Asymmetric Synthesis II. Wiley-VCH; New York: 2000. pp. 593–650. [Google Scholar]; b) Tsuji J. Acc. Chem. Res. 1969;2:144. [Google Scholar]; c) Trost BM, Van Vranken DL. Chem. Rev. 1996;96:395. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]; d) Lu Z, Ma S. Angew. Chem. 2008;120:264. [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:258. [Google Scholar]
- [11].Trost BM. Tetrahedron. 1977;33:2615. [Google Scholar]
- [12].For example, see: Braun M, Meier T, Laicher F, Meletis P, Fiden M. Adv. Synth. Catal. 2008;350:303.
- [13].For example, see: Zhao X, Liu D, Zhang W. Tetrahedron. 2009;65:512.
- [14].For example, see: Chuit C, Felkin H, Frajerman C, Roussi G, Swierczewski G. J. Organometal. Chem. 1977;127:371. Consiglio G, Morandini F, Piccolo O. J. Chem. Soc. Chem. Commun. 1983:112. Consiglio G, Indolese A. Organometallics. 1991;10:3425. Nomura N, RajanBabu TV. Tetrahedron Lett. 1997;38:1713. Gomez-Bengoa E, Heron NM, Didiuk MT, Luchaco CA, Hoveyda AH. J. Am. Chem. Soc. 1998;120:7649.
- [15].Yasui H, Mizutani K, Yorimitsu H, Oshima K. Tetrahedron. 2006;62:1410. [Google Scholar]
- [16].Trost BM, Spagnol MD. J. Chem. Soc. Perkin Trans. 1. 1995:2083. and references cited therein. [Google Scholar]
- [17].a) Moreno-Manas M, Pajuelo F, Pleixats R. J. Org. Chem. 1995;60:2396. [Google Scholar]; b) Ohmiya H, Makida Y, Tanaka T, Sawamura M. J. Am. Chem. Soc. 2008;130:17276. doi: 10.1021/ja808673n. [DOI] [PubMed] [Google Scholar]; c) Mino T, Kajiwara K, Shirae Y, Sakamoto M, Fujita T. Synlett. 2008:2711. [Google Scholar]; d) Nishikata T, Lipshutz BH. J. Am. Chem. Soc. 2009;131:12103. doi: 10.1021/ja905082c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].There are far fewer ways to make 1,4-dienes with good regio- and stereocontrol than there are to make 1,3- and 1,5-dienes: Wilson SR, Zucker PA. J. Org. Chem. 1988;53:4682. Trost BM, Probst GD, Schoop A. J. Am. Chem. Soc. 1998;120:9228. Hilt G, du Mesnil F-X, Lüers S. Angew. Chem. 2001;113:408. Angew. Chem. Int. Ed. 2001;40:387. Morten CJ, Jamison TF. Tetrahedron. 2009;65:6648. doi: 10.1016/j.tet.2009.05.074. Moreau B, Wu JY, Ritter T. Org. Lett. 2009;11:337. doi: 10.1021/ol802524r.
- [19].a) Tsukada N, Sato T, Inoue Y. Chem. Commun. 2001:237. doi: 10.1039/b307705e. [DOI] [PubMed] [Google Scholar]; b) Tsukada N, Sato T, Inoue Y. Chem. Commun. 2003:2404. doi: 10.1039/b307705e. [DOI] [PubMed] [Google Scholar]
- [20].a) Oppolzer W, Bedoya-Zurita M, Switzer CY. Tetrahedron Lett. 1988;29:6433. [Google Scholar]; b) Oppolzer W. Angew. Chem. 1989;101:39. [Google Scholar]; Angew. Chem. Int. Ed. 1989;28:38. [Google Scholar]
- [21].Under Oppolzer’s reaction conditions (Ni(cod)2 (10 mol%) and dppb (10 mol%) used as a catalyst in THF or toluene at room temperature), no reaction occurred between cinnamyl acetate and ethylene (1 atm) .
- [22].Matsubara R, Jamison TF. J. Am. Chem. Soc. 2010;132:6880. doi: 10.1021/ja101186p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].a) Tolman CA. Chem. Rev. 1977;77:313. [Google Scholar]; b) Rahman MM, Liu H-Y, Eriks K, Prock A, Giering WP. Organometallics. 1989;8:1. [Google Scholar]
- [24].It is known that cationic Ni-H complex catalyzes olefin isomerization. For example, see: O’Connor AR, Urbin SA, Moorhouse RA, White PS, Brookhart M. Organometallics. 2009;28:2372.
- [25].For examples of Ni-catalyzed coupling reaction using allylic ethers, see: Consiglio G, Indolese A. Organometallics. 1991;10:3425. Nomura N, RajanBabu TV. Tetrahedron Lett. 1997;38:1713.
- [26].Cinnamyl alcohol was likely converted into the corresponding allyl trimethylsilyl ether, which then underwent oxidative addition.
- [27].When Ni(cod)2 (2.5 mol%) and P(o-anisyl)3 (20 mol%) were used, the selectivity for 1,4-diene over 1,3-diene formation was further improved (>95:5) albeit with reduction in yield (42%).
- [28].Drexler J, Lindermayer R, Hassan MA, Sauer J. Tetrahedron Lett. 1985;26:2555. [Google Scholar]
- [29].Although no experimental evidence has not been obtained, it is possible that in the selectivity-determining step (olefin migration), P(o-anisyl)3 is dissociated from the nickel center and propylene serves as a ligand instead. It is also possible that P(o-anisyl)3 chelates the nickel center as a bidentate P-O ligand.
- [30].NiL2(olefin) complex has been known for a long time and used as a Ni(0) precursor for many nickel complexes. Dreissig VW, Dietrich H. Acta Cryst. 1968;B24:108.
- [31].Yamamoto T, Ishizu J, Yamamoto A. J. Am. Chem. Soc. 1981;103:6863. [Google Scholar]
- [32].Only in the case of vinylcyclohexane were other regioisomers observed (<8% yield). See Supporting Information for details.
- [33].A sharp singlet peak (δ = 33.7 (s)) was observed in 31P NMR. We cannot rule out the possibility that carbonate is a counter anion instead of methoxide. Ozawa F, Son T, Ebina S, Osakada K, Yamamoto A. Organometallics. 1992;11:171.
- [34].a) DiRenzo GM, White PS, Brookhart M. J. Am. Chem. Soc. 1996;118:6225. [Google Scholar]; b) Mecking S, Keim W. Organometallics. 1996;15:2650. [Google Scholar]; c) Bray KL, Charmant JPH, Fairlamb IJS, Lloyd-Jones GC. Chem. Eur. J. 2001;7:4205. doi: 10.1002/1521-3765(20011001)7:19<4205::aid-chem4205>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; d) Cárdenas DJ, Alcamí M, Cossío F, Méndez M, Echavarren AM. Chem. Eur. J. 2003;9:96. doi: 10.1002/chem.200390035. [DOI] [PubMed] [Google Scholar]; e) Joseph J, RajanBabu TV, Jemmis ED. Organometallics. 2009;28:3552. doi: 10.1021/om900045p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Two doublet peaks (δ = 29.6 (d, JP-P = 35.8 Hz), 33.6 (d, JP-P = 35.8 Hz)) were observed by 31P NMR.
- [36].For reviews, see: Denmark SE, Fu J. Chem. Rev. 2003;103:2763. doi: 10.1021/cr020050h. Kennedy JWJ, Hall DG. Angew. Chem. 2003;115:4880. Angew. Chem. Int. Ed. 2003;42:4732.
- [37].For intermolecular allyl-allyl coupling reactions, see: Godschalx J, Stille JK. Tetrahedron Lett. 1980;21:2599. Trost BM, Keinan E. Tetrahedron Lett. 1980;21:2595. Goliaszewski A, Schwartz J. J. Am. Chem. Soc. 1984;106:5028. Goliaszewski A, Schwartz J. Tetrahedron. 1985;41:5779. Goliaszewski A, Schwartz J. Organometallics. 1985;4:417. Murakami M, Kato T, Mukaiyama T. Chem. Lett. 1987:1167. Nakamura H, Bao M, Yamamoto Y. Angew. Chem. 2001;113:3308. doi: 10.1002/1521-3773(20010903)40:17<3208::AID-ANIE3208>3.0.CO;2-U. Angew. Chem. Int. Ed. 2001;40:3208. Lee PH, Sung S.-y., Lee K, Chang S. Synlett. 2002:146. Lee PH, Shim E, Lee K, Seomoon D, Kim S. Bull. Korean Chem. Soc. 2005;26:157. Flegeau EF, Schneider U, Kobayashi S. Chem. Eur. J. 2009;15:12247. doi: 10.1002/chem.200902221.
- [38].A portion of 5a may be directly generated from reaction of 1a with propylene.
- [39].a) Trost BM, Horne DB, Woltering MJ. Chem. Eur. J. 2006;12:6607. doi: 10.1002/chem.200600202. [DOI] [PubMed] [Google Scholar]; b) Pineschi M, Bertolini F, Bussolo VD, Crotti P. Curr. Org. Synth. 2009;6:290. [Google Scholar]
- [40].For Ni-catalyzed borylative ring opening of vinyl epoxides, see: Crotti S, Bertolini F, Macchia F, Pineschi M. Org. Lett. 2009;11:3762. doi: 10.1021/ol901429g. For Ni-catalyzed borylative ring opening of vinylcyclopropanes, see: Sumida Y, Yorimitsu H, Oshima K. Org. Lett. 2008;10:4677. doi: 10.1021/ol801982d.
- [41].Trost BM, Molander GA. J. Am. Chem. Soc. 1981;103:5969. [Google Scholar]
- [42].Gomez-Bengoa E, Heron NM, Didiuk MT, Luchaco CA, Hoveyda AH. J. Am. Chem. Soc. 1998;120:7649. [Google Scholar]
- [43].Both η1-benzyl- and η3-benzyl-Ni complexes are known and have been crystallographically characterized. Carmona E, Marín JM, Paneque M, Poveda ML. Organometallics. 1987;6:1757. Carmona E, Marín JM, Palma P, Paneque M, Poveda ML. Inorg. Chem. 1989;28:1895. Carmona E, Paneque M, Poveda ML. Polyhedron. 1989;8:285. Anderson TJ, Vicic DA. Organometallics. 2004;23:623. Chen Y, Wu G, Bazan GC. Angew. Chem. 2005;117:1132. doi: 10.1002/anie.200461630. Angew. Chem. Int. Ed. 2005;44:1108. doi: 10.1002/anie.200461630.
- [44].Mundal DA, Lutz KE, Thomson RJ. Org. Lett. 2009;11:465. doi: 10.1021/ol802585r. [DOI] [PubMed] [Google Scholar]
- [45].Velasco-Torrijos T, Murphy PV. Org. Lett. 2004;6:3961. doi: 10.1021/ol0484254. [DOI] [PubMed] [Google Scholar]
- [46].Ferrié L, Reymond S, Capdevielle P, Cossy J. Org. Lett. 2007;9:2461. doi: 10.1021/ol070670a. [DOI] [PubMed] [Google Scholar]
- [47].Matsuhashi H, Asai S, Hirabayashi K, Hatanaka Y, Mori A, Hiyama T. Bull. Chem. Soc. Jpn. 1997;70:1943. [Google Scholar]
- [48].For its synthesis, see Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.

















