Abstract
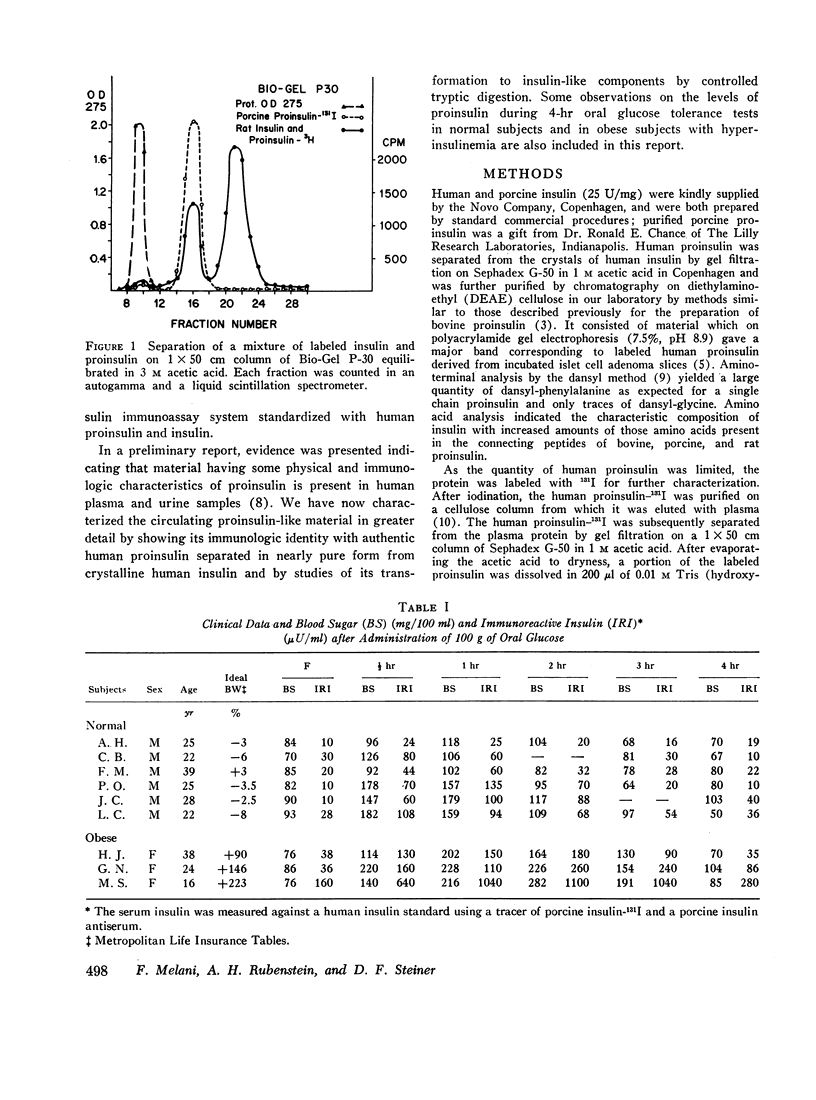
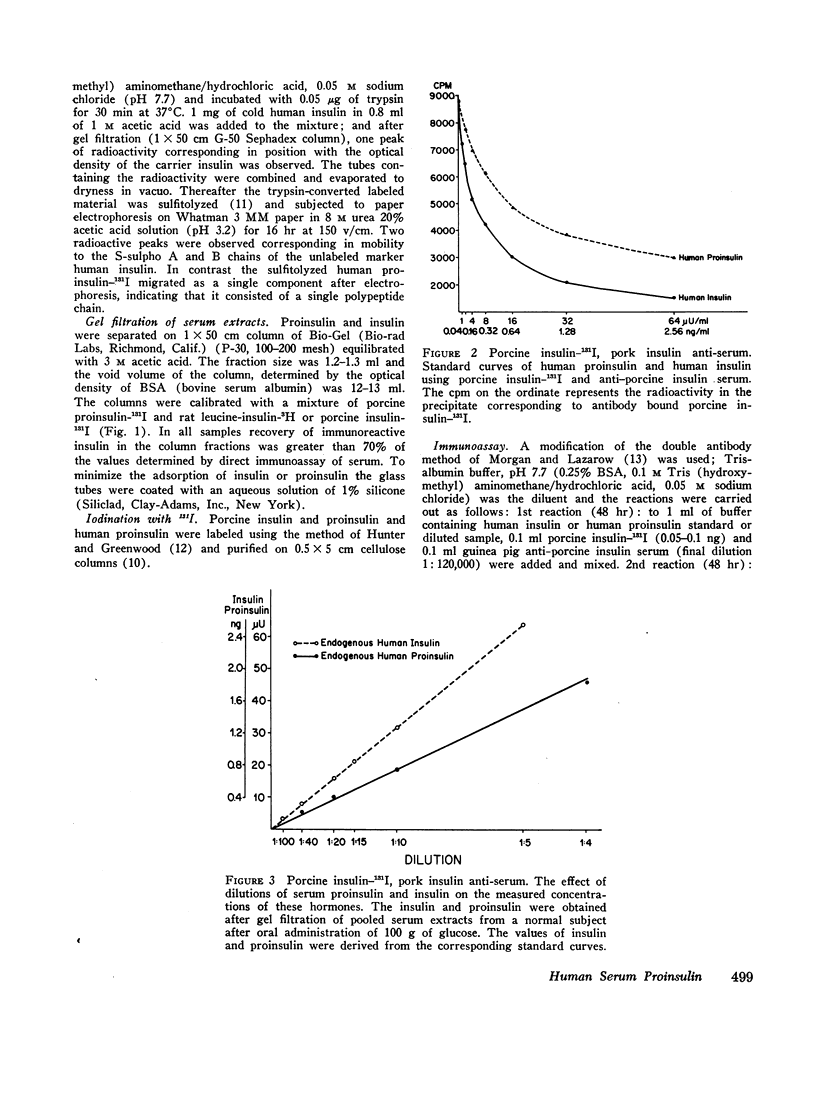
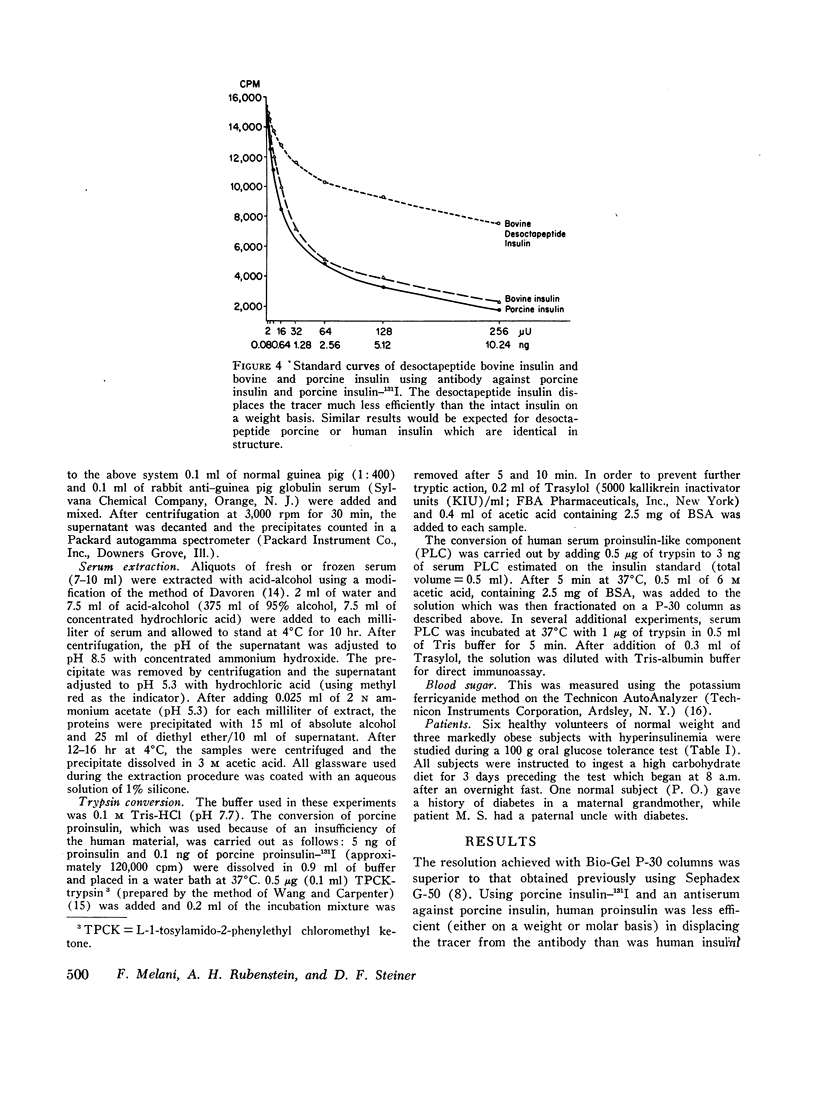
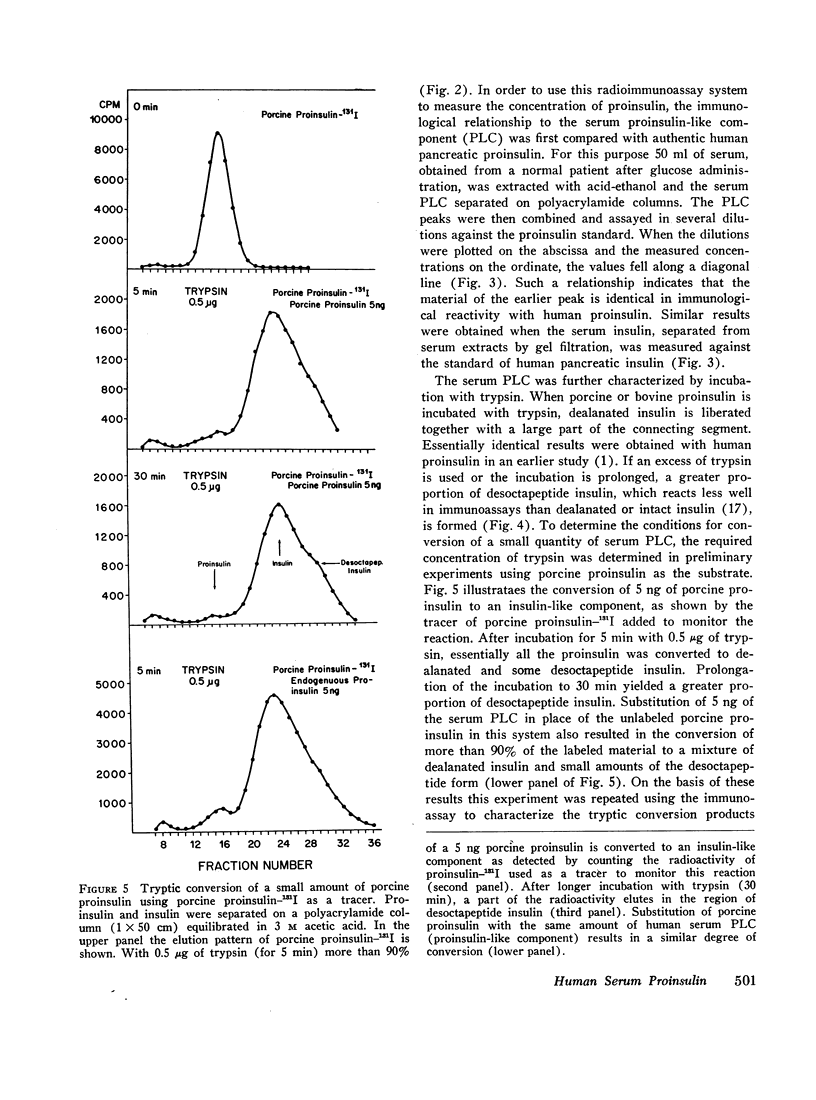
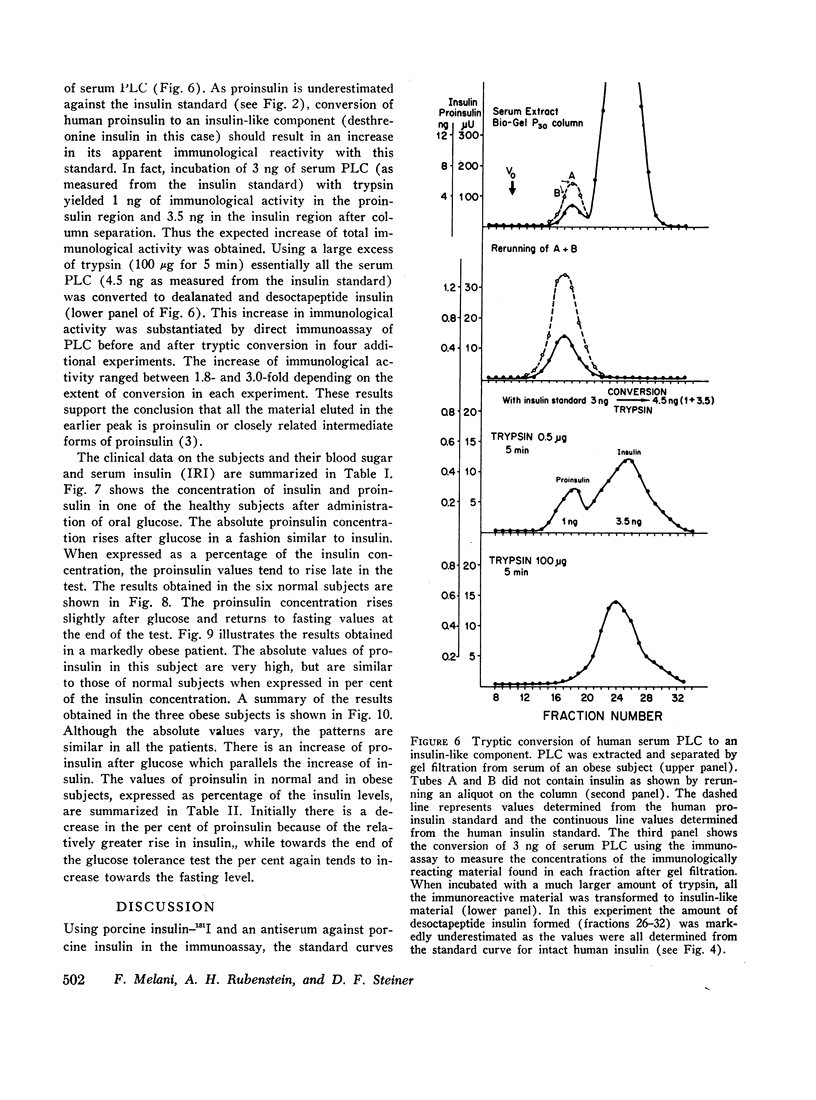
Gel filtration of human serum extracts on Bio-Gel P-30 columns produced two peaks of material reactive with insulin antisera. The earlier eluting fraction appeared at the elution position of proinsulin (serum proinsulin-like component, PLC) while the second fraction corresponded in elution volume to insulin. In assays using porcine insulin-131I and an antiserum against porcine insulin, human pancreatic proinsulin was less reactive than human insulin. Serial dilutions of the serum PLC in the immunoassay showed immunological identity with the human proinsulin standard. Partial tryptic digestion of the serum PLC yielded products with increased immunological reactivity as estimated with insulin as the standard. With larger amounts of trypsin, all the serum PLC was converted to insulin-like components (desthreonine and desoctapeptide insulin). On the basis of these results we conclude that the earlier eluting fraction of human serum extracts is proinsulin.
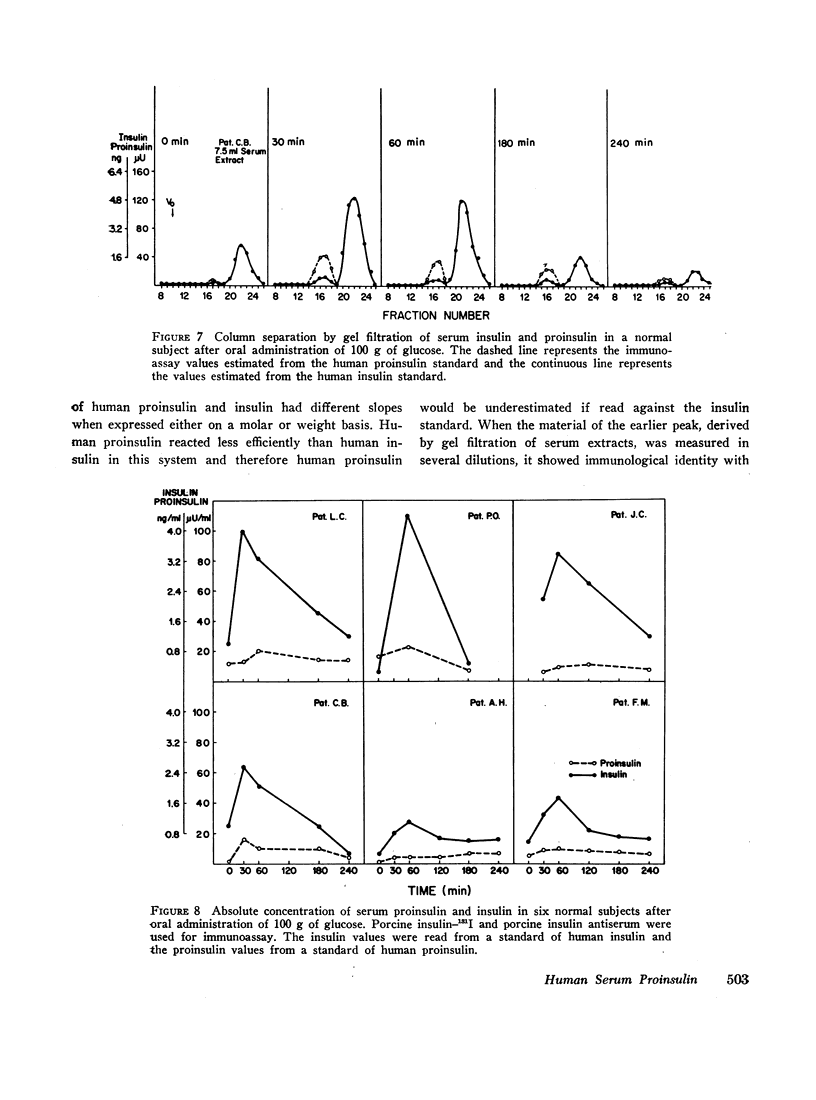
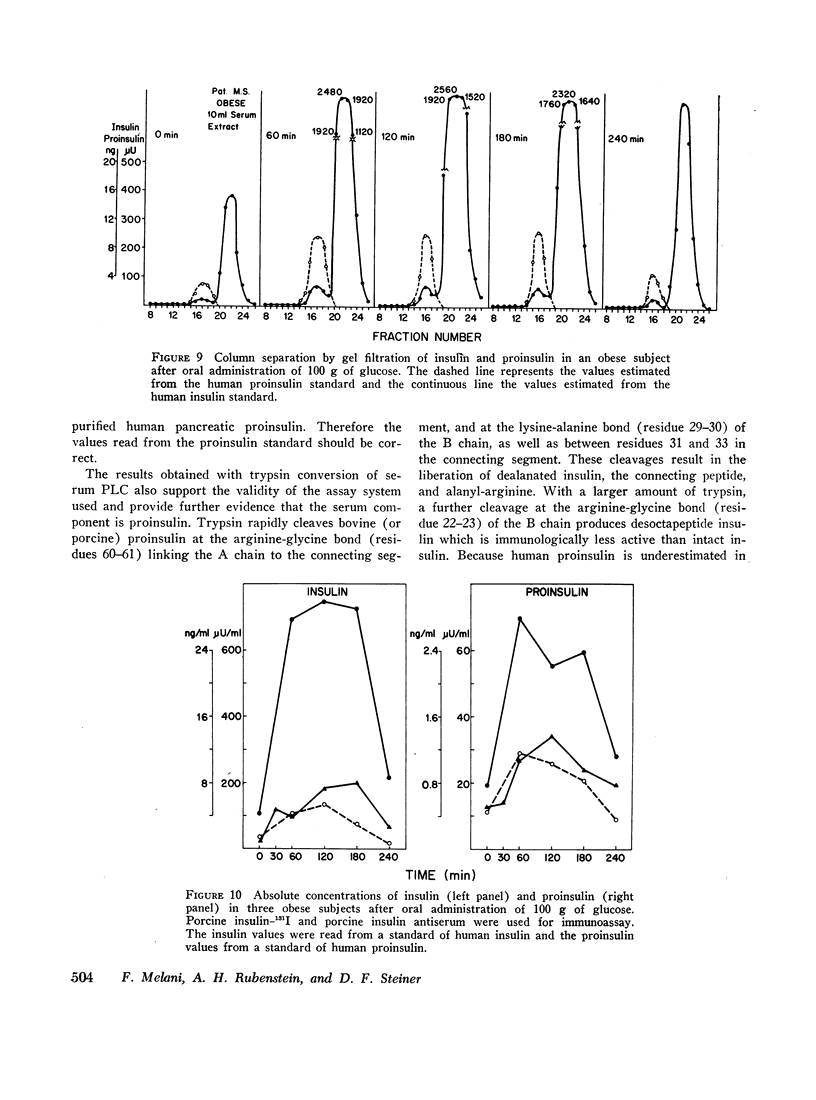
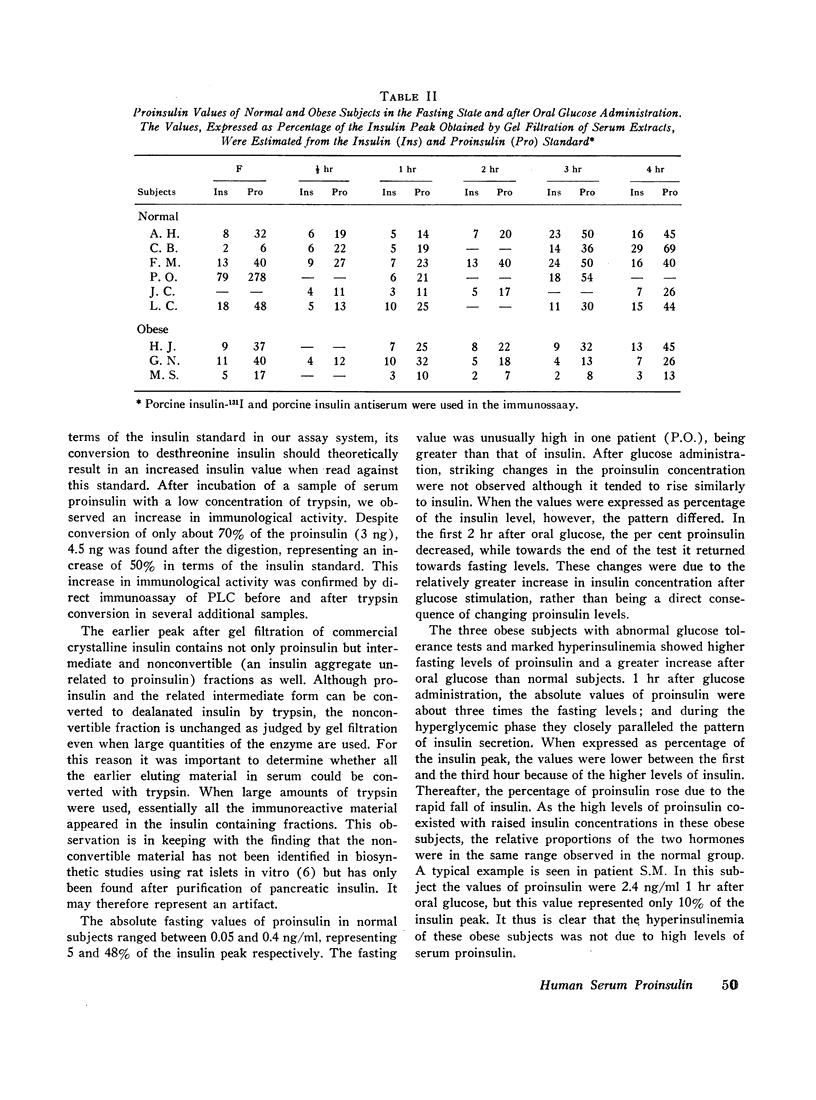
The fasting values of proinsulin in normal subjects ranged between 0.05 and 0.4 ng/ml, representing from 5 to 48% of the insulin concentration. In one subject the values of proinsulin were higher than those of insulin. After oral administration of 100 g of glucose, the proinsulin levels tended to rise similarly to insulin. Three obese patients with hyperinsulinemia had higher fasting levels of proinsulin and a greater increase after glucose than the normal subjects. As the high levels of proinsulin coexisted with raised insulin concentration in these obese subjects, the relative proportions of the two hormones were in the same range observed in the normal group. Thus hyperinsulinemia in these obese subjects was not accompanied by an increase in the fraction of serum proinsulin. When the values for serum proinsulin were expressed as percentage of the insulin levels, there was a decrease in the per cent proinsulin in the first hour of the glucose tolerance test. After the second hour, the per cent tended to rise towards the fasting levels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W., Creutzfeldt C., Frerichs H., Perings E., Sicklinger K. The morphological substrate of the inhibition of insulin secretion by diazoxide. Horm Metab Res. 1969 Mar;1(2):53–64. doi: 10.1055/s-0028-1095173. [DOI] [PubMed] [Google Scholar]
- DAVOREN P. R. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962 Sep 10;63:150–153. doi: 10.1016/0006-3002(62)90347-5. [DOI] [PubMed] [Google Scholar]
- Frank B. H., Veros A. J. Physical studies on proinsulin-association behavior and conformation in solution. Biochem Biophys Res Commun. 1968 Jul 26;32(2):155–160. doi: 10.1016/0006-291x(68)90362-8. [DOI] [PubMed] [Google Scholar]
- Gorden P., Roth J. Circulating insulins. "Big" and "little". Arch Intern Med. 1969 Mar;123(3):237–247. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Lacy P. E. [Ultrastructural changes of the beta-cells during insulin secretion]. Acta Diabetol Lat. 1968 Oct;5 (Suppl 1):436–445. [PubMed] [Google Scholar]
- Logothetopoulos J. Electron microscopy of the pancreatic islets of the rat. Effects of prolonged insulin injections. Diabetes. 1966 Nov;15(11):823–829. doi: 10.2337/diab.15.11.823. [DOI] [PubMed] [Google Scholar]
- Roth J., Gorden P., Pastan I. "Big insulin": a new component of plasma insulin detected by immunoassay. Proc Natl Acad Sci U S A. 1968 Sep;61(1):138–145. doi: 10.1073/pnas.61.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. H., Cho S., Steiner D. F. Evidence for proinsulin in human urine and serum. Lancet. 1968 Jun 22;1(7556):1353–1355. doi: 10.1016/s0140-6736(68)92040-0. [DOI] [PubMed] [Google Scholar]
- Shaw W. N., Chance R. E. Effect of porcine proinsulin in vitro on adipose tissue and diaphragm of the normal rat. Diabetes. 1968 Dec;17(12):737–745. doi: 10.2337/diab.17.12.737. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L., Nolan C., Rubenstein A. H., Margoliash E., Aten B., Oyer P. E. Proinsulin and the biosynthesis of insulin. Recent Prog Horm Res. 1969;25:207–282. doi: 10.1016/b978-0-12-571125-8.50008-9. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Hallund O., Rubenstein A., Cho S., Bayliss C. Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes. 1968 Dec;17(12):725–736. doi: 10.2337/diab.17.12.725. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG S. S., CARPENTER F. H. A COMPOSITIONAL ASSAY FOR INSULIN APPLIED TO A SEARCH FOR "PROINSULIN". J Biol Chem. 1965 Apr;240:1619–1625. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunologic aspects of insulin. Am J Med. 1961 Dec;31:882–891. doi: 10.1016/0002-9343(61)90030-4. [DOI] [PubMed] [Google Scholar]


