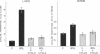Abstract
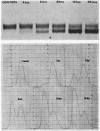
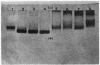
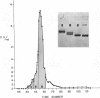
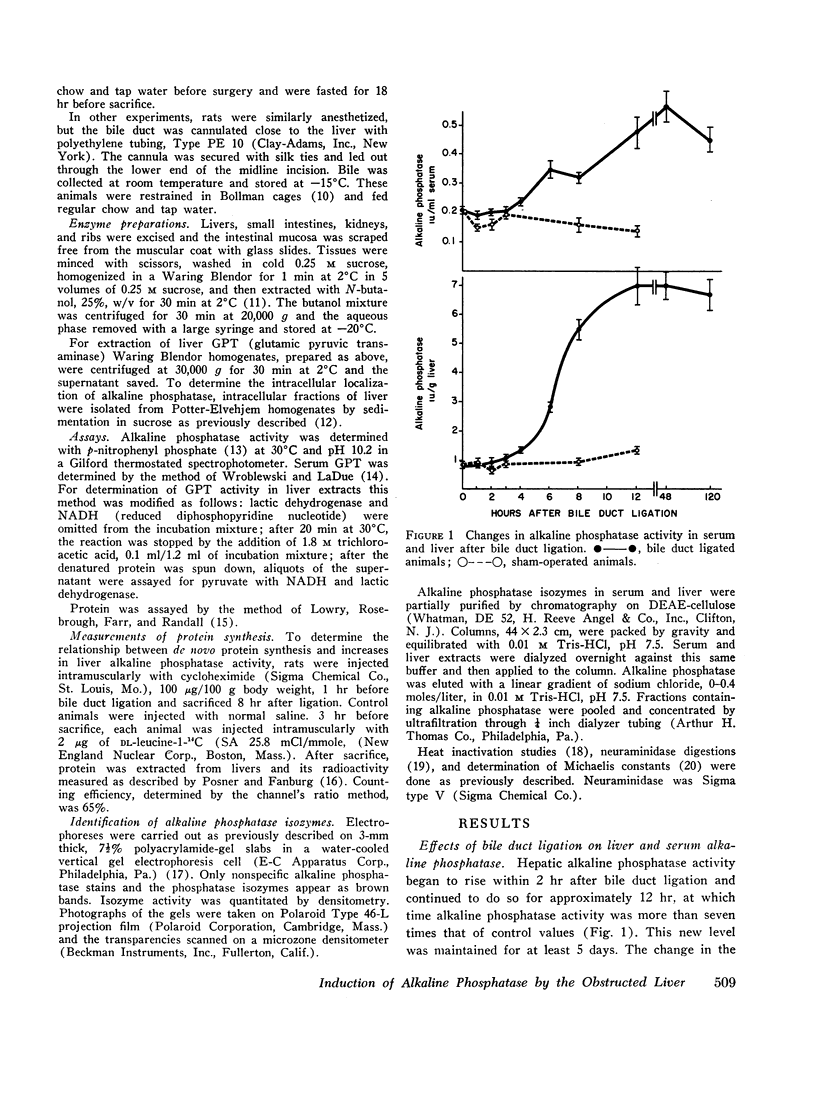
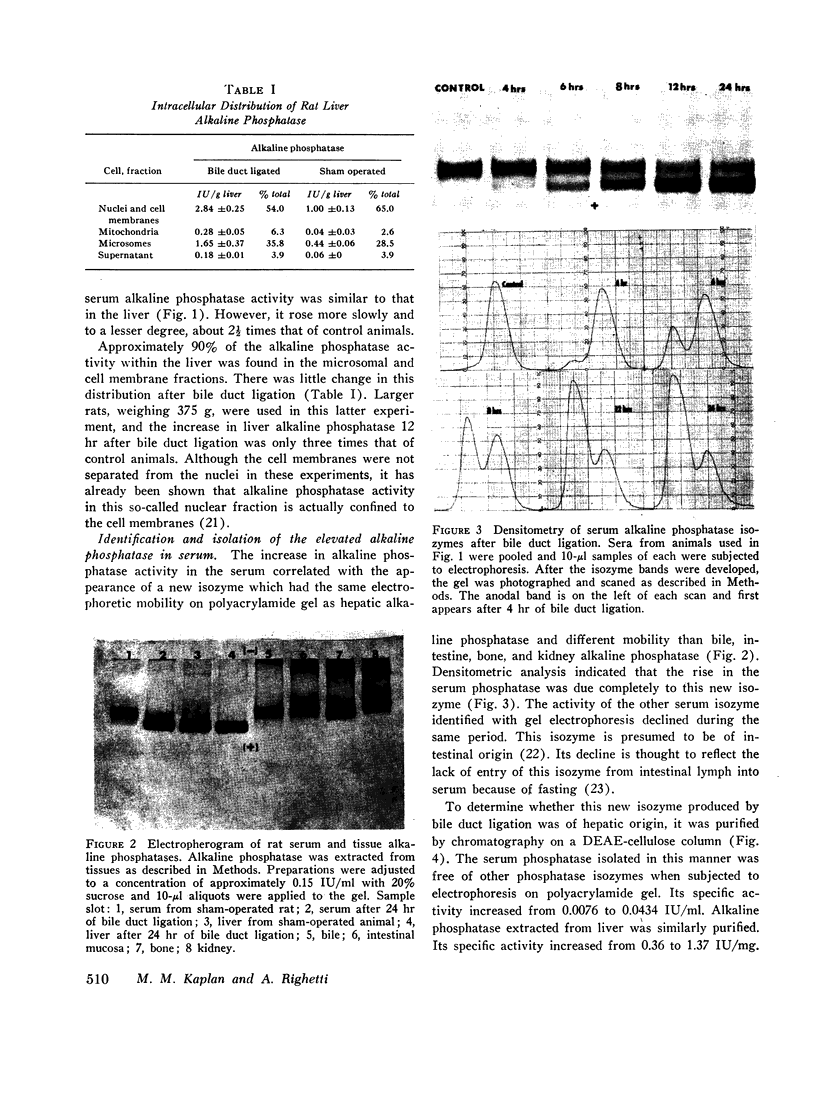
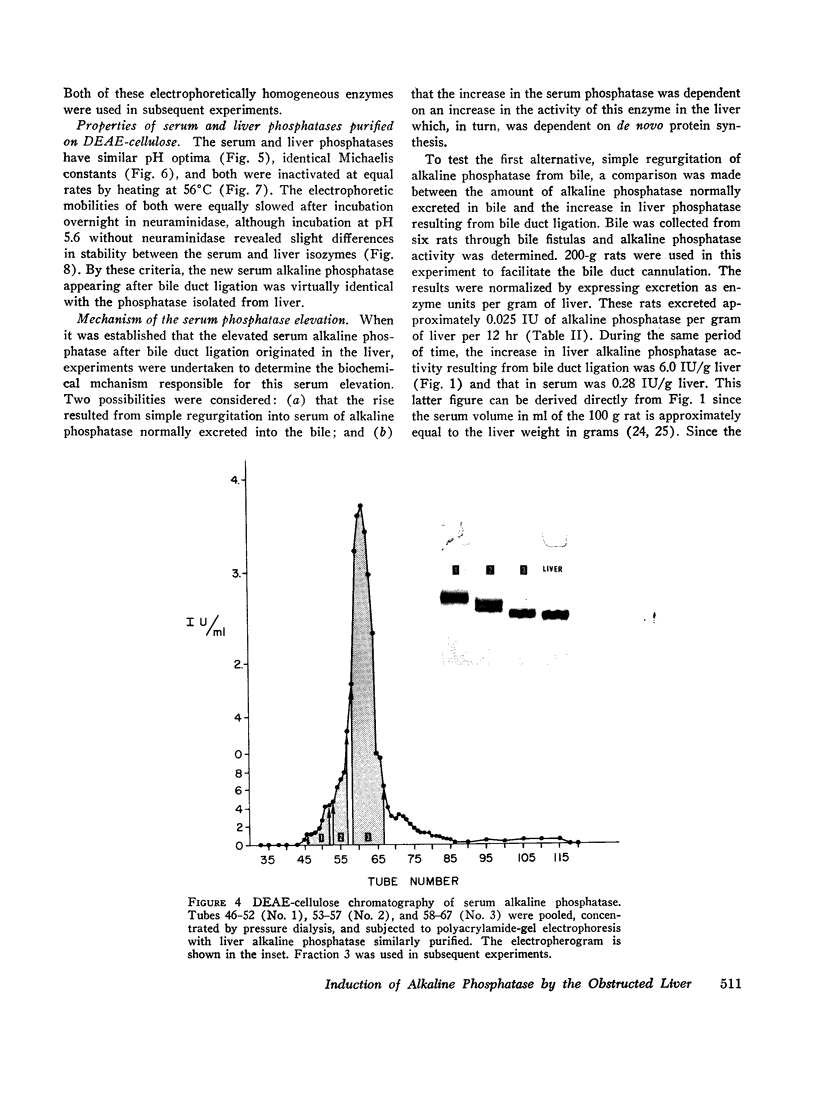
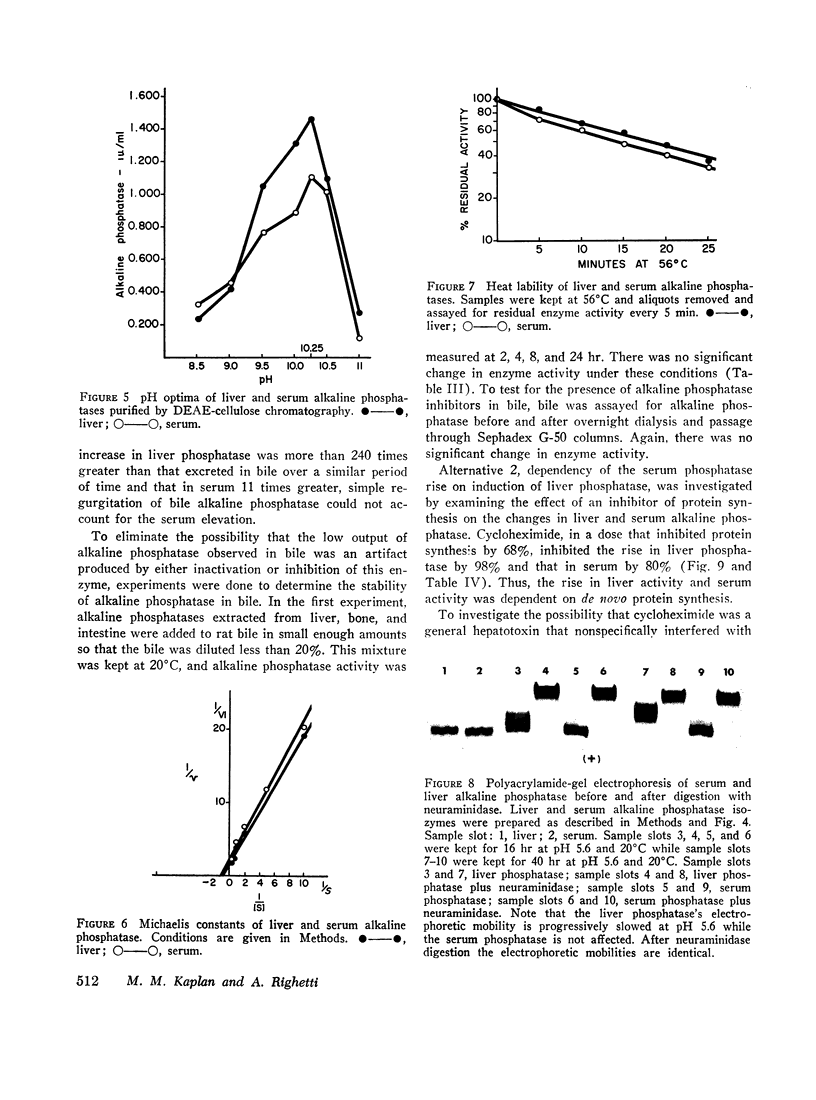
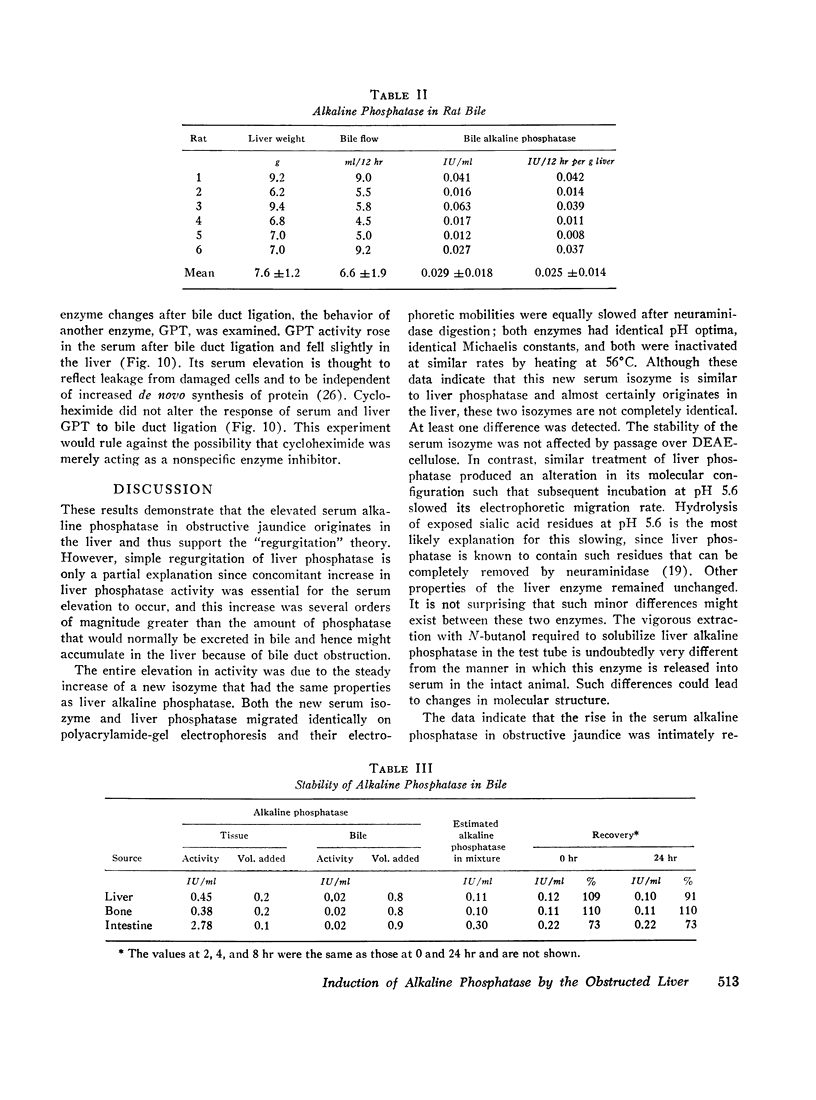
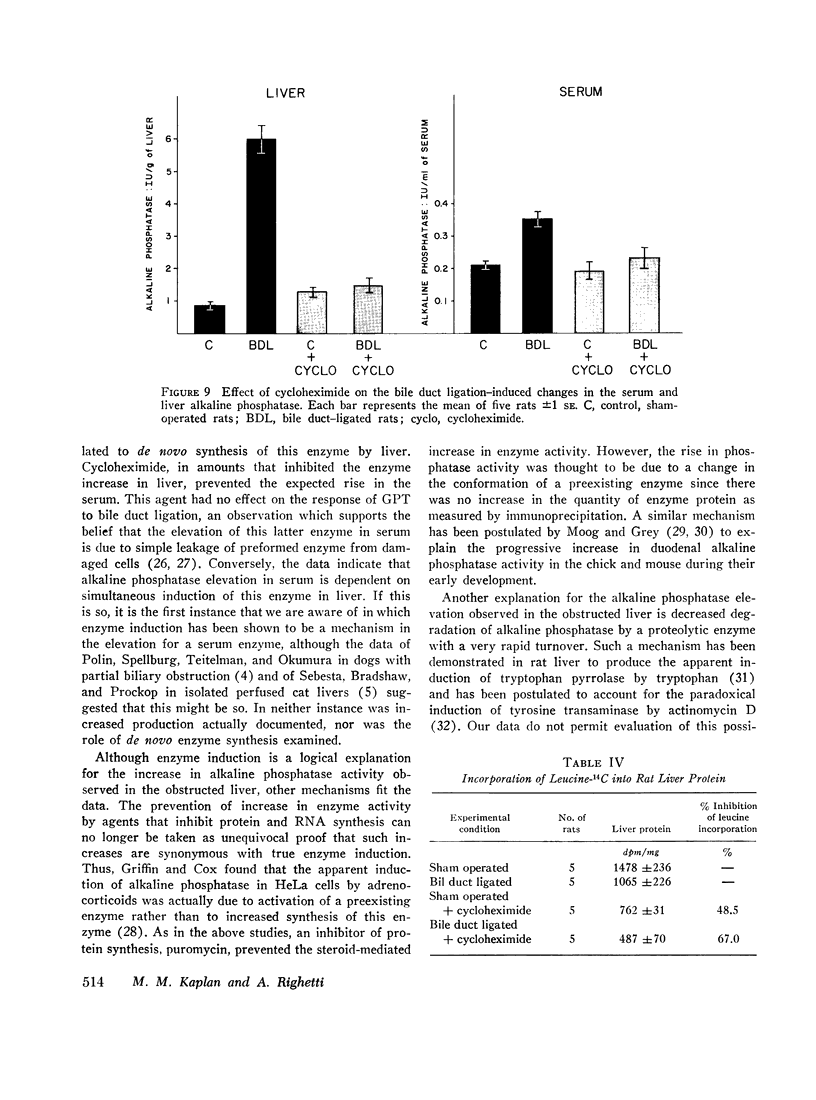
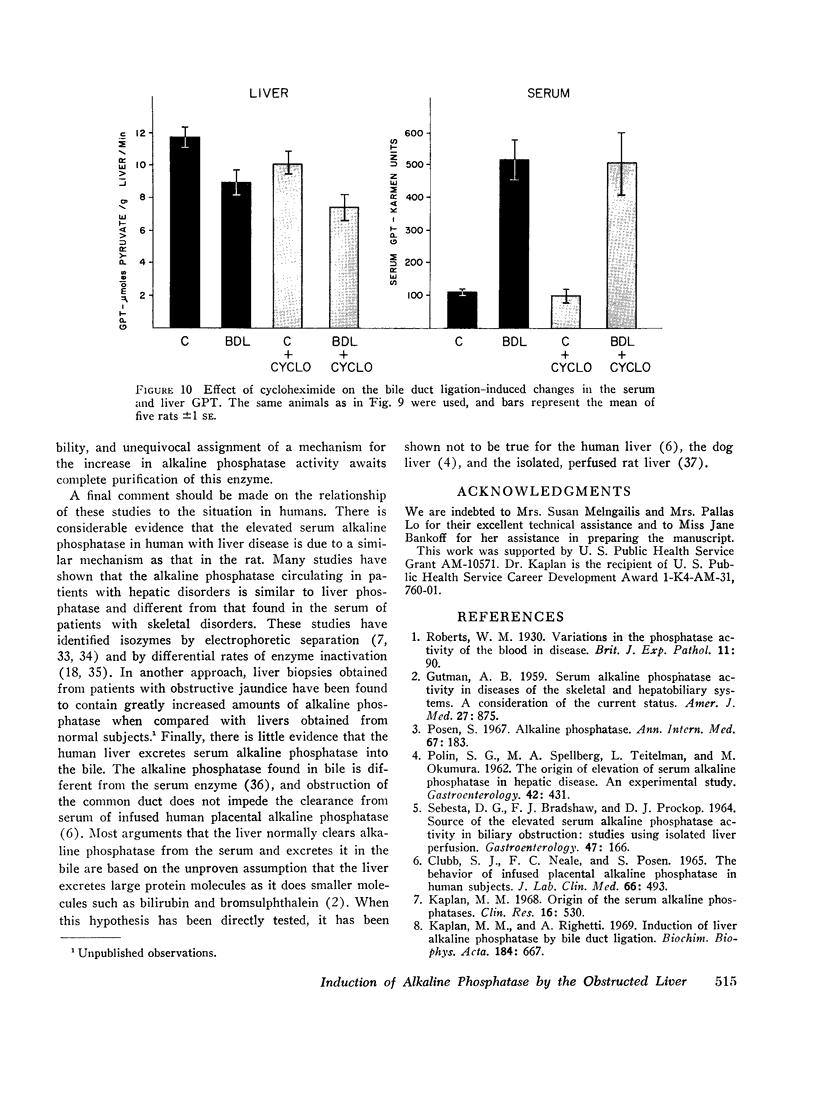
Bile duct ligation in the rat leads to a rapid increase in hepatic and serum alkaline phosphatase activity. Within 12 hr after bile duct ligation, hepatic alkaline phosphatase has increased 7-fold and serum alkaline phosphatase activity 2½-fold. The elevation in the serum activity is completely due to an increase in an isozyme that appears to originate in the liver. This serum isozyme and liver phosphatase, both partially purified by DEAE-cellulose column chromatography, have identical Michaelis constants, pH optima, and rates of heat denaturation. These isozymes migrate identically when subjected to electrophoresis on polyacrylamide gel, and their migration rates are equally slowed after neuraminidase digestion. The data suggest that the rise in hepatic alkaline phosphatase activity is dependent on de novo protein synthesis. Cycloheximide, in a dose that inhibited incorporation of leucine-14C into protein by 68%, inhibited the rise in liver phosphatase by 98% and that in serum by 80%. The rise in liver phosphatase activity could not be accounted for by simple retention of alkaline phosphatase that would normally appear in bile. The rise in liver activity after bile duct ligation was 240 times greater than the amount of phosphatase that normally appears in bile over a similar period of time. Cycloheximide had no effect on the bile duct ligation-induced changes in the serum and liver glutamic pyruvic transaminase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clubb J. S., Neale F. C., Posen S. The behavior of infused human placental alkaline phosphatase in human subjects. J Lab Clin Med. 1965 Sep;66(3):493–507. [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- FISHMAN W. H., GREEN S., INGLIS N. I. Decline in rat-serum alkaline phosphatase following bile-duct ligation. Biochim Biophys Acta. 1962 Aug 13;62:429–431. doi: 10.1016/0006-3002(62)90277-9. [DOI] [PubMed] [Google Scholar]
- GUTMAN A. B. Serum alkaline phosphatase activity in diseases of the skeletal and hepatobiliary systems. A consideration of the current status. Am J Med. 1959 Dec;27:875–901. doi: 10.1016/0002-9343(59)90173-1. [DOI] [PubMed] [Google Scholar]
- Griffin M. J., Cox R. P. Studies on the mechanism of hormone induction of alkaline phosphatase in human cell cultures, II. Rate of enzyme synthesis and properties of base level and induced enzymes. Proc Natl Acad Sci U S A. 1966 Sep;56(3):946–953. doi: 10.1073/pnas.56.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODSON A. W., LATNER A. L., RAINE L. Iso-enzymes of alkaline phosphatase. Clin Chim Acta. 1962 Mar;7:255–261. doi: 10.1016/0009-8981(62)90018-9. [DOI] [PubMed] [Google Scholar]
- Horne M., Cornish C. J., Posen S. Use of urea denaturation in the identification of human alkaline phosphatases. J Lab Clin Med. 1968 Dec;72(6):905–915. [PubMed] [Google Scholar]
- Kaplan M. M., Righetti A. Induction of liver alkaline phosphatase by bile duct ligation. Biochim Biophys Acta. 1969 Sep 2;184(3):667–669. doi: 10.1016/0304-4165(69)90289-x. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Rogers L. Separation of human serum-alkaline-phosphatase isoenzymes by polyacrylamide gel electrophoresis. Lancet. 1969 Nov 15;2(7629):1029–1031. doi: 10.1016/s0140-6736(69)90640-0. [DOI] [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- Krauss S., Sarcione E. J. Role of the liver in the catabolism of serum haptoglobin. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1019–1022. doi: 10.3181/00379727-122-31314. [DOI] [PubMed] [Google Scholar]
- LINDE S. A comparison between the enzyme patterns (GO-T, GP-T, LD) in serum and tissue extracts in cardiac and hepatic diseases. Scand J Clin Lab Invest. 1958;10(3):303–307. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MADSEN N. B., TUBA J. On the source of the alkaline phosphatase in rat serum. J Biol Chem. 1952 Apr;195(2):741–750. [PubMed] [Google Scholar]
- MORTON R. K. The purification of aklaline phosphatases of animal tissues. Biochem J. 1954 Aug;57(4):595–603. doi: 10.1042/bj0570595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog F., Grey R. D. A system regulating alkaline phosphatase activity in the duodenum of the chick embryo and mouse. Biol Neonat. 1965;9(1):10–23. [PubMed] [Google Scholar]
- Moog F., Grey R. D. Alkaline phosphatase isozymes in the duodenum of the mouse: attainment of pattern of spatial distribution in normal development and under the influence of cortisone or actinomycin D. Dev Biol. 1968 Nov;18(5):481–500. doi: 10.1016/0012-1606(68)90053-5. [DOI] [PubMed] [Google Scholar]
- POLIN S. G., SPELLBERG M. A., TEITELMAN L., OKUMURA M. The origin of elevation of serum alkaline phosphatase in hepatic disease. An experimental study. Gastroenterology. 1962 Apr;42:431–438. [PubMed] [Google Scholar]
- POSEN S., NEALE F. C., CLUBB J. S. HEAT INACTIVATION IN THE STUDY OF HUMAN ALKALINE PHOSPHATASES. Ann Intern Med. 1965 Jun;62:1234–1243. doi: 10.7326/0003-4819-62-6-1234. [DOI] [PubMed] [Google Scholar]
- Pope C. E., 2nd, Cooperband S. R. Protein characteristics of serum and bile alkaline phosphatase. Gastroenterology. 1966 May;50(5):631–636. [PubMed] [Google Scholar]
- Posen S. Alkaline phosphatase. Ann Intern Med. 1967 Jul;67(1):183–203. doi: 10.7326/0003-4819-67-1-183. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Fanburg B. L. Ribonucleic acid synthesis in experimental cardiac hypertrophy in rats. II. Aspects of regulation. Circ Res. 1968 Jul;23(1):137–145. doi: 10.1161/01.res.23.1.137. [DOI] [PubMed] [Google Scholar]
- ROBINSON J. C., PIERCE J. E. DIFFERENTIAL ACTION OF NEURAMINIDASE ON HUMAN SERUM ALKALINE PHOSPHATASES. Nature. 1964 Oct 31;204:472–473. doi: 10.1038/204472a0. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- SEBESTA D. G., BRADSHAW F. J., PROCKOP D. J. SOURCE OF THE ELEVATED SERUM ALKALINE PHOSPHATASE ACTIVITY IN BILIARY OBSTRUCTION: STUDIES UTILIZING ISOLATED LIVER PERFUSION. Gastroenterology. 1964 Aug;47:166–170. [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Serum glutamic pyruvic transaminase in cardiac with hepatic disease. Proc Soc Exp Biol Med. 1956 Apr;91(4):569–571. doi: 10.3181/00379727-91-22330. [DOI] [PubMed] [Google Scholar]