Abstract
The major antigenic component of licensed influenza vaccines, hemagglutinin (HA), elicits predominantly type-specific antibody responses, thus necessitating frequent antigenic updates to the annual vaccine. However, accumulating evidence suggests that influenza vaccines can also induce significant cross-reactive T-cell responses to highly divergent, heterosubtypic HA antigens not included in the vaccine. Influenza vaccines are less effective among the elderly and studies that characterize cross-reactive T-cell immunity in this vulnerable population are much needed. Here, we systematically compare the ex vivo frequency, cytokine profile and phenotype of vaccine-elicited HA-specific T-cell responses among a cohort of young (18–49 years old) and elderly (≥70 years old) vaccinees, as well as the maturation and activation phenotype of total CD4+ and CD8+ T-cells. IFN-γ production after in vitro expansion and HA-specific Ab titers were also determined. We find that vaccine-elicited ex vivo frequencies of CD4+ T-cells elicited by vaccination reactive to any given homo- or heterosubtypic Ag were comparable across the two age groups. While, no differences were observed between age groups in the phenotype of Ag-specific or total CD4+ T-cells, PBMC from young adults were superior at producing IFN-γ after short-term Ag-specific culture. Significantly, while vaccine-elicited T cell responses were durable among the younger vaccinees, they were short-lived among the elderly. These results have important ramifications for our understanding of vaccine-induced changes in the magnitude and functionality of HA-specific CD4+ T-cells, as well as age-related alterations in response kinetics.
Keywords: Hemagglutinin, Durability, T-cell Quality, Proliferation
Introduction
The influenza pandemic of 1918 saw the introduction of an avian-origin H1N1 that is estimated to have caused over 40 million deaths worldwide (1). The influenza pandemic of 2009 similarly saw the introduction of an antigenically novel H1N1 into the global population and is estimated to have caused over 18,449 deaths globally (2). In spite of the dominant emergence of this novel swine-origin H1N1 lineage in 2009, multiple instances of lethal avian influenza infections among humans have also occurred over the past decade. While divergent influenza subtypes including H7N7, H9N2, and H7N3 have circulated sporadically, recurrent outbreaks of the highly pathogenic H5N1 have been documented consistently (3–5). Though these outbreaks have primarily occurred in south-east Asia, avian influenza infections among humans and birds have also been reported from Central and Western European nations thus increasing the likelihood of a potential H5N1 pandemic emerging in the future (6).
A number of studies in humans (7–10) as well as in animal model systems (11, 12) suggest that protective immunity against influenza correlates with the prevalence of influenza-specific antibodies, primarily those directed against the viral hemagglutinin (HA). However, humoral immunity to influenza is predominantly type-specific and affords limited protection against heterosubtypic strains of circulating viruses (13). Thus, the continual drift- and periodic antigenic shift-events that introduce antigenically novel influenza viruses into the global population have required annual changes in influenza vaccines to achieve reliable vaccine-induced protection (14). However, instances of mismatches between seasonal influenza Ags found in the vaccine and circulating influenza viruses have generally lead to limited vaccine efficacy in some years (13, 15–19). Equally, with subtypes such as H5N1 that show future pandemic potential, several distinct heterotypic strains have been involved in the outbreaks to date, thus increasing uncertainty as to which strain or strains may emerge as a potential avian pandemic strain.
With such antigenic plasticity as a backdrop, cellular immune responses are being increasingly recognized as an alternate correlate of immune protection that could provide protection from influenza viruses of disparate antigenic lineages (20). A number of studies have recently characterized the existence of subtype-independent, cross-reactive cellular immune responses against diverse influenza strains (21–24), which may provide protection from influenza infection or reduce severity of influenza disease (24, 25). Importantly, recent studies clearly suggest that among the elderly, a population that bears the brunt of influenza morbidity and mortality, cell mediated immunity provides a better correlate of protection than do serum antibody responses (20). As with antibody responses, HA is a major component of protective cellular immunity against influenza (26, 27), including heterosubtypic immunity (28).
Accumulating evidence suggests that cross-reactive T-cell responses can be induced de novo or boosted over pre-existing levels following seasonal vaccination with an unmatched HA antigen (23). Though our earlier study utilizing a cohort of 18–49-year-olds was unable to assess cross-reactive T-cell responses among the truly elderly (29), we found that ageing reduced the magnitude of T-cells cross-reactive to Pandemic H1 even in this relatively young cohort. Also, our use of IFN-γ ELISPOT following expansion in vitro precluded the assessment of HA-specific T-cell frequencies, as well as their memory and activation status directly ex vivo. In the present study, polychromatic flow cytometry was used to compare the ex vivo frequency, maturation and activation phenotypes of HA-specific CD4+ T-cells elicited following seasonal influenza vaccine administration among young (18–49 years of age) and elderly (≥70 years of age) adults. As our trial was conducted in 2007, we also address the ability of seasonal vaccination to induce cross-reactive T-cells against antigens that vaccinees had not yet been exposed to, such as the Avian H5 that has only seen sporadic outbreaks in Asia, and the novel Pandemic swine-origin H1 of 2009 that emerged two seasons following sample collection.
Materials and Methods
Human subjects and sample collection
This study was performed at Cincinnati Children’s Hospital Medical Center in the fall of 2007 before seasonal influenza circulation in the community and two seasons prior to the emergence of the novel pandemic 2009 H1N1 strain globally. A total of 30 subjects were recruited into the study and provided informed consent following appropriate Institutional Review Board (IRB) and Institutional Biosafety Committee evaluation, and included 15 subjects each in the “young” (18–49 years old) and elderly (≥70 years of age) age groups. All subjects received the trivalent inactivated influenza vaccine (TIV) containing the 2007–2008 seasonal vaccine antigenic components intramuscularly (Fluarix™; GlaxoSmithKline). This incorporated an A/H1N1/Solomon Islands/3/2006-like virus. Subjects who had an acute influenza like illness (ILI) or laboratory-diagnosed influenza (LDI) within the preceding 3 months were excluded. Individuals who had received any other live or inactivated vaccine in the preceding 30 days, were pregnant or had given birth within the preceding two months, were diagnosed with cancer, receiving immunomodulatory therapy, or otherwise suffering from a chronic disease were also excluded. Other exclusion criteria included known allergy to any component of the vaccine and a history of Guillain-Barré syndrome. Blood specimens were collected pre-vaccination on the day of vaccination (pre) and at 14 (2 weeks) and 42 days (memory) post vaccination in Vaccutainer heparin tubes for peripheral blood mononuclear cell (PBMC) isolation and parallel SST tubes for serum collection (Becton Dickinson). One subject from the elderly group was lost to follow-up, resulting in 29 subjects completing and being evaluated in the study (Table 1). This study was performed in accordance with The Code of Ethics of the World Medical Association.
Table 1.
Cohort information
| Young | Elderly | |
|---|---|---|
| n | 15* | 14 |
| age [range] | 20–50 | 71–94 |
| age [median (IQR)] | 38 (32–44) | 79 (73.5–83) |
| gender [% female] | 60 | 71.4 |
The pre-vaccination time-point was not available for one subject.
Sample preparation and Ag-stimulation
Peptide pools were composed of 16–17mer peptides overlapping by 11–12 amino acids and spanning the whole HA protein of A/H1N1/New Caledonia/20/99 (hereafter referred to as Seasonal H1), A/H5N1/Thailand/4/Sp-528/2004 (hereafter referred to as Avian H5) (BEI Resources, Manassas, VA), or A/H1N1/California/04/2009 (hereafter referred to as Pandemic H1) (29) (Mimotopes, Melbourne, Australia).
Cryopreserved PBMC were thawed in pre-warmed RPMI 1640, 10% FCS, 2mM L-glutamine, 100 U/ml penicillin and 100µg/ml streptomycin (all from Gibco; this medium will hereafter be referred to as RPMI complete), in the presence of 20µg/ml benzonase nuclease (Novagen). Cells were rested in RPMI complete for 3–6 hours at 37°C, 5% CO2 and then stimulated overnight in 200µl RPMI complete with 1.25µg/ml peptide pools for Seasonal H1, Pandemic H1 or Avian H5, or left unstimulated (costimulation only control). All stimulation cultures and controls contained monensin, Brefeldin A, anti-CD49d mAb and anti-CD28Cy5-PE mAb (all from BD Biosciences). Healthy donor PBMC were stimulated with SEB (Sigma) to serve as a positive control.
Flow cytometry
The reagent panel used in the present study is given in Supplementary Table 1. The following reagents were used: anti-CD3APC-Cy7 (clone SK7), anti-CD14PacBlu (clone M5E2), anti-CD28PE-Cy5 (clone CD28.2), anti-IL-2APC (clone MQ1-17H12), anti-Ki67FITC clone B56), anti-TNFPE-Cy7 (clone MAb11) (BD Pharmingen), anti-CD127PE-Cy5.5 (clone R34.34; Immunotech Coulter), anti-Granzyme BPE (clone GB12; Caltag) and streptavidinQD605 (Invitrogen). Anti-CD4 (clone M-T477), anti-CD8 (clone RPA-T8), anti-CD19 (clone HIB19), anti-CD27 (clone M-T271) anti-CD45RO (clone UCHL1), anti-CD57 (clone NK-1), anti-IFN-γ (clone 4S.B3) (BD Pharmingen) and anti-CCR7 (clone 150503; R&D Systems) were conjugated in-house to QD655, QD585, pacific blue, biotin, QD545, QD705, Ax594 and Ax680 (Invitrogen), respectively. Dead cells were detected with the Violet Amine Reactive Viability Dye (Molecular Probes, Invitrogen). For intracellular staining, cells were treated with BD Cytofix/Cytoperm Permeabilization Solution (BD Biosciences). Data were acquired on an LSR II (BD Biosciences) and analyzed using FlowJo version 9.2 (Tree Star, Inc., Ashland, OR), Pestle version 1.6.2 (from MR), SPICE version 5.1 (30), JMP (SAS Institute, Cary, NC) and Prism (GraphPad, Inc.) software. All primary data analysis was performed prior to unblinding of analysts with respect to patient group.
Hemagglutination inhibition assay (HAI)
Donor sera were treated overnight at 37°C with Vibrio cholerae-derived receptor-destroying enzyme (RDE; Denka-Seiken, CA) and heat-inactivated at 56°C before testing in a standard HAI assay beginning with a dilution of 1:4. Seasonal H1 [A/New Caledonia/20/99 (H1N1)] and Avian H5 [A/Vietnam/1203/2004 (H5N1)]; and Pandemic H1 [A/California/04/2009 (H1N1)] antigens were acquired from Centers for Disease Control and Prevention (CDC), courtesy of Dr. Alex Klimov, Dr. Xian Xu; and Protein Sciences. Four units of HA were incubated with serially diluted RDE-treated sera for 30 minutes at room temperature, before adding turkey (H1N1 antigens) or horse (H5N1) red blood cells (Viromed, CT) to a final concentration of 0.5%. Hemagglutination titers were determined as described earlier and sera with titers <4 were assigned a titer of 2 (29).
T-cell expansion and IFN-γ ELISPOT assay
Cryopreserved PBMC were thawed, washed thrice in complete RPMI media and stimulated for seven days in the presence of media or HA peptide pools used earlier in ex vivo stimulation, namely seasonal H1, pandemic H1 or avian H5. Peptide pool specific interferon gamma (IFN-γ) secretion was detected by ELISPOT assays. Briefly, multiscreen 96-well plates (Millipore) were coated overnight at 4°C with 1µg/ml anti-human IFN-γ capture antibody (Mabtech), washed with PBS and blocked for 4 hours with complete RPMI medium (Invitrogen). Lymphocytes were added at 5×104 cells/well in triplicate and stimulated with peptide pools or media for 18 hrs at 37°C. Phytohemagglutinin, PHA (Sigma) at 5 µg/ml was used as a positive control. Plates were washed with PBS and incubated with alkaline phosphatase-conjugated anti-human IFN-γ mAb (clone 7B6-1, Mabtech) for 2 hrs at 37°C and washed again with PBS before chromogenic development with NBT/BCIP (Pierce, Thermo Scientific). Spot-forming units (SFU) were enumerated using an automated ELISPOT reader (CTL Technologies) and mean SFU from triplicate wells was calculated for each stimulating condition after subtracting values from negative control wells. Data is presented as mean SFU per 106 PBMC using standard procedures (29, 31).
Statistical analysis
Statistical comparisons of pies were performed in SPICE software using 10,000 permutations (30). For single measurements, donor groups were compared using the Student’s t-test. The Wilcoxon matched pairs signed rank test was applied for comparisons of donor-matched samples. Data correlations were performed in JMP software using linear least-squares regression.
Results
Age related changes in global CD4+ T-cell differentiation and activation status
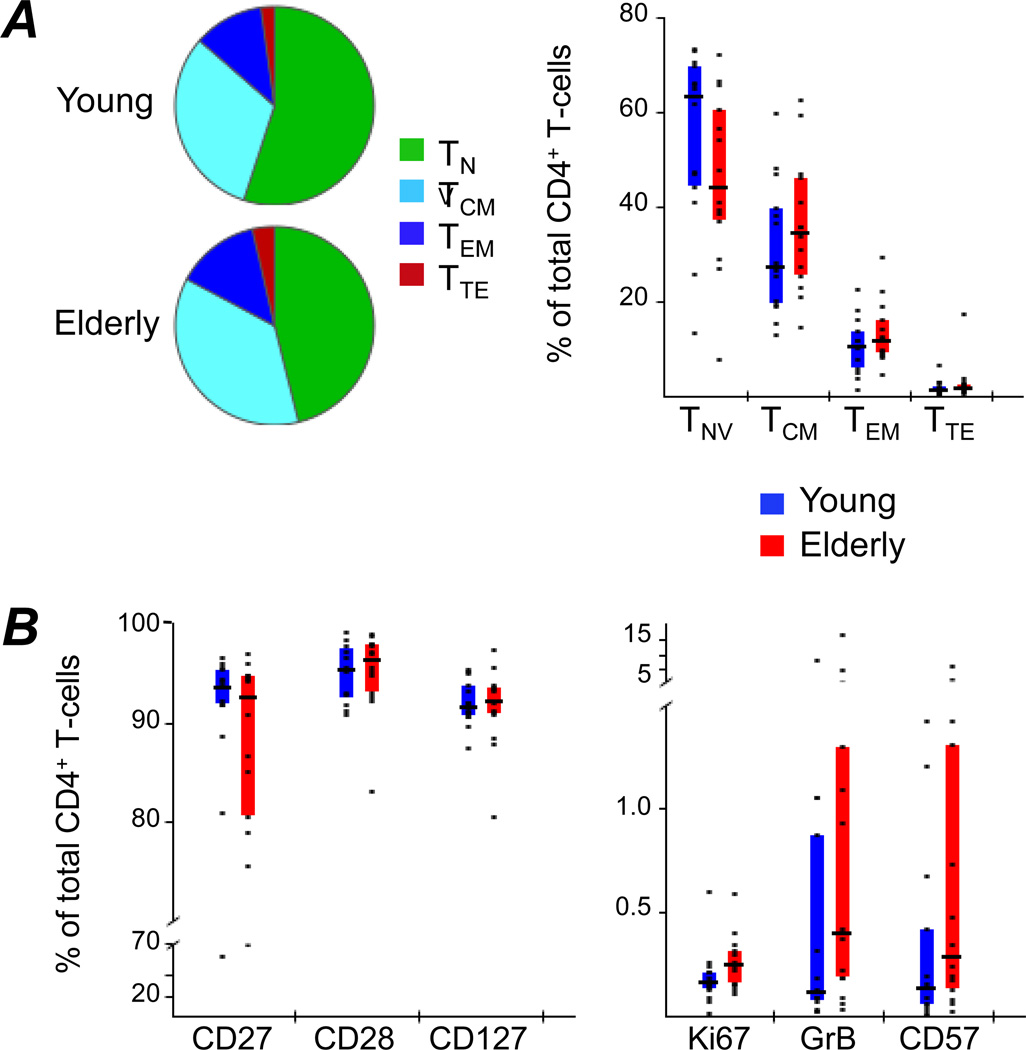
While deficits in both magnitude and functionality of influenza-specific T-cells have previously been documented in relation to ageing of the host (32–34), the mechanisms underlying these deficits are currently not well understood. It is conceivable that this is a result of global changes in the maturation and activation status of T-cell subsets among the elderly that is not specific to influenza. To address this, total T-cell populations of young and elderly vaccinees were assessed by flow cytometry, investigating their differentiation stage using CCR7 and CD45RO (Fig.1A), as well as phenotyping them according to expression of the following differentiation and activation markers: CD27, CD28, CD127, Ki67, GrB and CD57 (Fig.1B). To this end, PBMC samples were labeled with the reagent panel described in Supplementary Table 1 and flow cytometry results gated as illustrated in Supplementary Figure 1. No significant differences were observed between age groups in CD4+ T-cells discriminated by differentiation or activation markers. In contrast, the CD8+ T-cell phenotypes were highly biased towards a more mature (Supplementary Fig.2A) and activated state (Supplementary Fig.2B) in elderly adults. This was evidenced by significantly lower levels of naïve, along with higher frequencies of effector memory (TEM) and terminal effector (TTE) cells among the elderly. Also, CD8+ T-cells from the elderly were characterized by reduced CD27, CD28 and CD127 expression, with a concomitant increase of Ki67, GrB and CD57. These findings are in agreement with other studies documenting drastic changes in the CD8+ T-cell compartment during aging and less pronounced, though similar, changes in the CD4+ T-cell compartment (35)–(36).
Figure 1. Differentiation and activation of total CD4+ T-cells.
(A) The differentiation status of total CD4+ T-cells from PBMC of young and elderly adults was compared by flow cytometric analysis. CD45RO and CCR7 were used to define Naïve (TNV: CD45RO− CCR7+), Central Memory (TCM: CD45RO+ CCR7+), Effector Memory (TEM: CD45RO+ CCR7−) and Terminal Effector (TTE: CD45RO− CCR7−) cells. Pies represent relative proportions of the four subpopulations in each donor group, while the bar chart directly compares individual subpopulations between groups. (B) The activation state was investigated by measuring the frequency of total CD4+ T-cells expressing CD27, CD28, CD127, Ki67, Granzyme B (GrB) or CD57, respectively. Bars represent inter-quartile ranges, horizontal bars show medians and dots illustrate individual donor data.
Magnitude of ex vivo CD4+ T-cell responses against vaccine-matched and -unmatched HA antigens among the young and elderly
Though no significant differences were observed in maturation or activation phenotypes of total CD4+ T-cells between young and elderly adults in our cohort, this did not preclude deficits confined to HA-specific CD4+ T-cells among the elderly. To address this, the total frequency of HA-specific CD4+ T-cells (positive for IFN-γ and/or IL-2 and/or TNF) and their corresponding cytokine profiles were compared between young and elderly subjects prior to receiving the vaccine (pre), or 2 weeks and 6 weeks (memory) post vaccination. This was done after stimulation with Seasonal H1, which was a component of the vaccine received, or with Pandemic H1 and Avian H5, which were not present in the vaccine (Fig.2A).
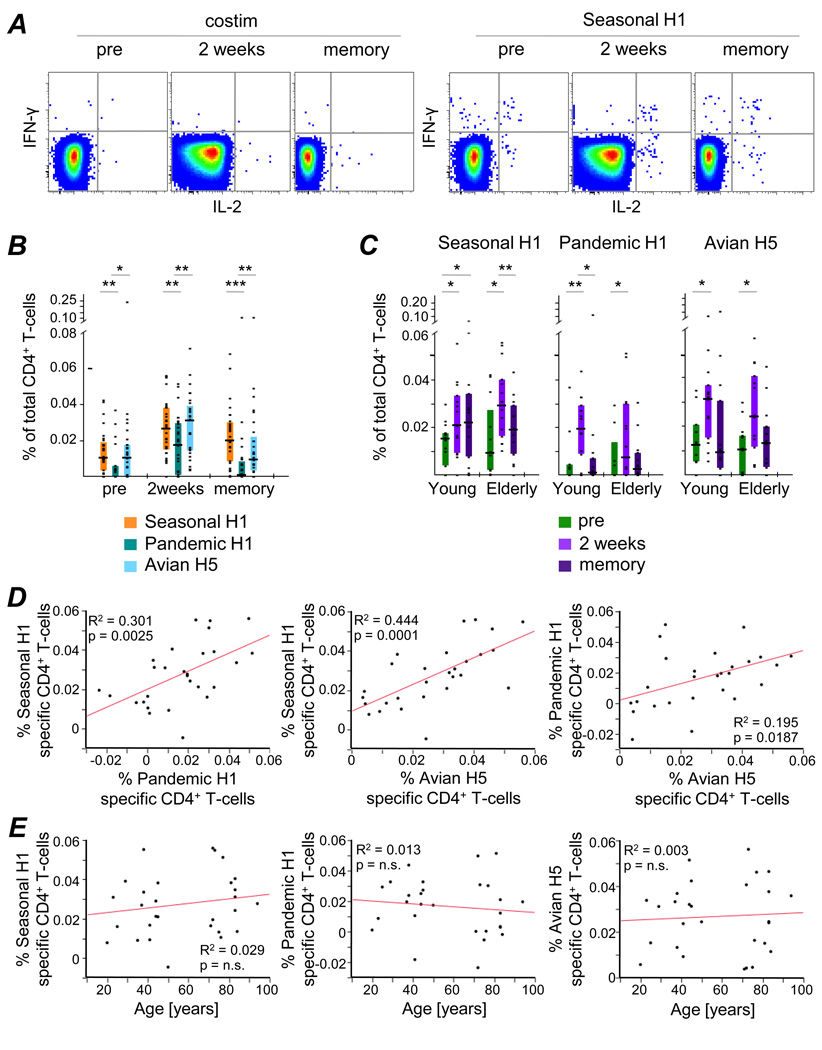
Figure 2. Cross-reactivity of Influenza HA-specific CD4+ T-cells.
(A) IFN-γ and IL-2 production by CD4+ T-cells of a 2 week PBMC sample after costimulation only (costim) or Seasonal H1 stimulation are shown for a representative donor. TNF was also measured but is not shown here. (B) Total cytokine-production by CD4+ T-cells after stimulation with Seasonal H1, Pandemic H1 or Avian H5 peptide pools was determined by measuring production of IFN-γ, IL2 and/or TNF and compared at individual time-points, independent of age. (C) The frequencies of CD4+ T-cells reactive to the three HA variants were compared between young and elderly adults. Correlation analyses were performed to test for relationships between the frequency of HA-responsive CD4+ T-cells and (D) cross-reactivity or (E) age at 2 weeks post vaccination. Statistically significant differences between responses to disparate HA variants (B) or within age group (C) are indicated above the graphs: * p<0.05, ** p<0.005, *** p<0.0005.
Initially, T-cell responses to the three HA variants were compared independent of age (Fig.2B). Prior to vaccination, very low levels of CD4+ T-cells reactive to Seasonal H1, Pandemic H1 or Avian H5 were observed. Responses to the vaccine-targeted and heterosubtypic HA peptide pools were significantly boosted by vaccination (2 weeks) and waned again thereafter (memory), indicating significant cross-reactivity of HA-specific CD4+ T-cells. Interestingly, stimulation with Avian H5 generated comparable response magnitudes as Seasonal H1 stimulation, while frequencies of Pandemic H1-reactive cells were significantly lower (pre: p=0.0047, 2 weeks: p=0.0033, memory: p=0.0003; Wilcoxon signed rank test; Fig 2B).
CD4+ T-cell responses were not statistically different between age groups at any of the sampling time-points analyzed, nor after stimulation with either the vaccine-matched or heterosubtypic HA variants (Fig.2C). However, only the Seasonal H1-specific CD4+ T-cell response was maintained to 6 weeks post vaccination in young adults, while cross-reactive responses to the heterosubtypic HA variants reverted back to pre-immunization levels in most individuals tested, independent of age (Fig.2C).
2 weeks after TIV administration, cross-reactive responses (to Pandemic H1 and Avian H5) were found to correlate well with Seasonal H1-induced responses, though demonstrating a weaker correlation among each other (Fig.2D). In all cases, the magnitude of the CD4+ HA-specific T-cell response 2 weeks after vaccination was independent of age (Fig.2E).
Finally, CD8+ T-cell responses were of much lower magnitude (Supplementary Fig.3), which is consistent with previous findings that influenza HA-specific responses are predominantly generated by CD4+ T-cells (37). However, 2 weeks post-immunization the CD8+ T-cell responses to Seasonal H1 (p=0.017) and Pandemic H1 (p=0.029) were significantly higher in young adults compared to the elderly cohort (Supplementary Fig.3).
Cytokine signatures of influenza HA-specific CD4+ T-cells are unique at different sampling time-points
IFN-γ is the sole cytokine usually investigated in influenza-related T-cell studies. Here we independently measured the production of three effector cytokines (IFN-γ, IL-2 and TNF) on a cell-by-cell basis. When each cytokine was quantified in isolation (singly), we found that none of the cytokines were statistically significantly changed throughout the study, albeit with a trend observed for TNF+ cells (Supplementary Fig.4). In contrast, T-cell quality, as reflected by the cytokine pattern (i.e., the relative proportion of cells producing any of the possible cytokine combinations), showed significant differences between individual sampling time-points (Fig.3A). Notably, no statistically significant differences were observed between young and elderly adults (not shown), nor between the influenza HA peptide pools. Since the different influenza HA variants induced very similar cytokine patterns, data from Seasonal H1 and Avian H5 stimulated samples were combined in order to obtain a more robust inter-time-point comparison (Fig.3B). At each sampling time-point, a unique cytokine pattern was detected. Most notably, influenza immunization induced an elevated fraction of cells producing only TNF 2 weeks post-vaccination, while after another 4 weeks (memory) the proportion of single-cytokine producing cells was markedly reduced, mainly in favor of triple-positive cells.
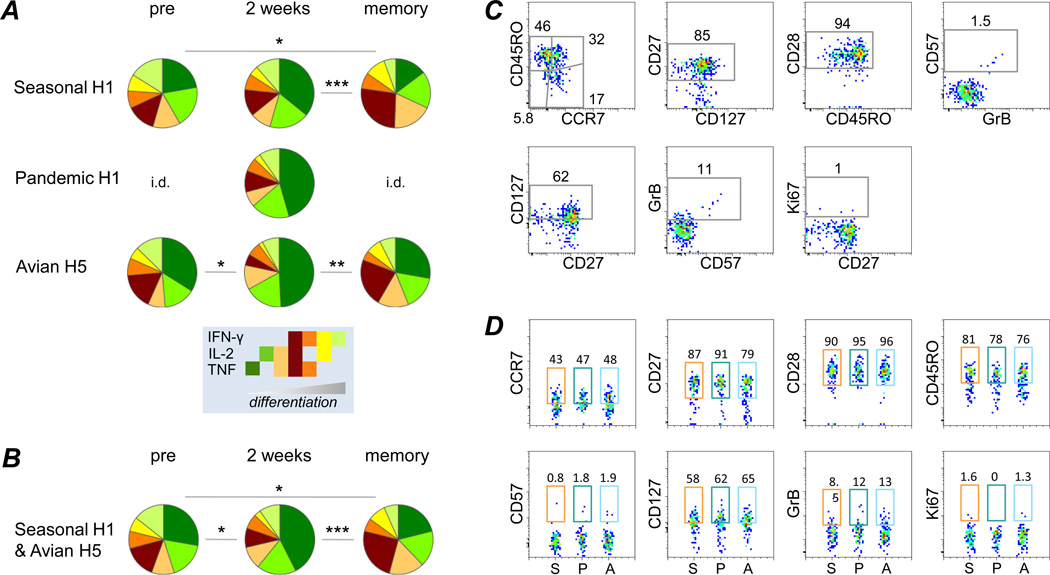
Figure 3. Quality and Phenotype of HA-specific CD4+ T-cells.
(A) The proportion of cells within total cytokine producing CD4+ T-cells generating IFN-γ, IL-2, TNF or any combination thereof was investigated after stimulating with a peptide pool for Seasonal H1, Pandemic H1 or Avian H5. Pie segments are arranged in order of progressive CD4+ T-cell differentiation, as proposed by Seder et al (38). (B) Since no statistically significant differences were observed between the different Ag-stimulations, all samples were combined per time-point, with the exclusion of Pandemic H1-stimulated samples, as there were not enough data for all time-points. (C) Cytokine+ cells from all 2 week samples were concatenated, independent of stimulating Ag, in order to investigate their T-cell subset distribution and activation phenotype. Gates were set on total CD4+ T-cells (see Supplementary Fig.1) and then applied to cytokine+ cells. Only samples with ≥10 cytokine+ events and a frequency >3x background (as measured in mock-stimulated samples) were included. (D) Phenotypes of cytokine+ cells were compared after stimulations with different HA peptide pools. Numbers indicate the fraction of positive cells after stimulating with the respective Ag. Statistically significant differences between time-points are indicated: * p<0.05, ** p<0.005, *** p<0.0005. i.d. – insufficient data (less than 3 samples fit the inclusion criteria; summary analysis is not possible).
Influenza vaccination predominantly elicits Central Memory and Effector Memory CD4+ T-cells
To investigate the phenotype of cytokine-producing cells, we included only those samples with ≥10 cytokine+ events and cytokine+ frequencies >3x over the background (30). Eight samples fulfilled these stringent inclusion criteria, all of which were sampled 2 weeks post-vaccination. Hypothesizing that the phenotypes would be comparable between samples, we combined all cytokine-positive events from these eight samples to analyze the phenotype. HA-specific CD4 T cells were found to be mostly Central Memory (TCM) and TEM, cells largely expressing CD27, CD28 and CD127, but negative for CD57, GrB and Ki67, consistent with cells having strong homeostatic potential. Among the eight samples (2 Seasonal H1; 3 Pandemic H1; 3 Avian H5), there were no striking differences in the phenotype of the specific CD4+ T-cell responses (Fig.3D).
Interestingly, a sizeable proportion of cytokine+ cells were found to have a naïve-like phenotype (16.8% were CD45RO− CCR7+). It has previously been proposed that throughout their differentiation CD4+ T-cells first gain and then lose effector functions, with certain cytokine signatures being produced predominantly by specific phenotypic subsets (38). In agreement with this, the naïve-like influenza HA reactive cells were overwhelmingly single-positive for TNF (data not shown), pointing to an early differentiation stage of these cells. Thus, these data suggest that in addition to boosting existing Ag-experienced cells, the seasonal influenza vaccination induces de novo recruitment of HA-reactive CD4+ T-cells.
Relationship between HA-specific CD4+ T-cell frequencies ex vivo and humoral immunity
A preponderance of evidence suggests that protective immunity to influenza is associated with a strong HA-specific Ab response (10, 39–42) and hence influenza vaccines are designed to boost influenza-specific Ab responses. In our cohort, seasonal H1-specific HAI titers were significantly increased 2 weeks following TIV administration and were still elevated at 6 weeks compared to pre vaccination titers (Fig.4A). In contrast, Pandemic H1- and Avian H5-specific Abs were absent in pre-vaccine samples, all of which were collected in the Fall 2007, indicating that study subjects were naïve to these novel Ags prior to vaccination. While seasonal vaccination induced and/or expanded cross-reactive T-cells, it failed to elicit significant levels of Abs cross-reactive to the novel Pandemic H1 (with the exception of two elderly subjects who achieved titers of ≤1:64 post-vaccination) or Avian H5 Ags (Fig 4A).
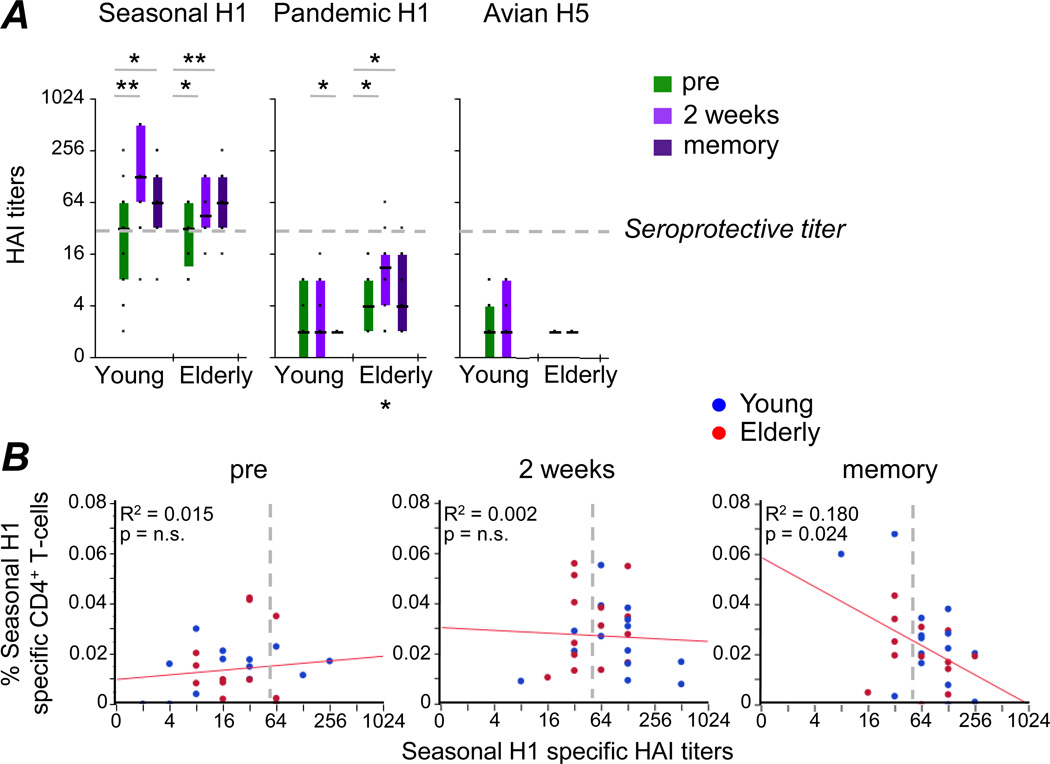
Figure 4. Ex vivo Influenza Ha-specific CD4+ T-cell responses do not correlate with corresponding HAI titers.
(A) HAI titers were determined in young and elderly adults at all three sampling time-points in assays using Seasonal H1, Pandemic H1 and Avian H5 proteins. (B) Cytokine production of CD4+ T-cells after stimulation with Seasonal H1 was correlated with corresponding HAI titers at the three sampling time-points. Data for young (blue) and elderly adults (red) are indicated. Lines of best fit for all data are highlighted in red. Broken lines indicate the threshold for what is considered protective seropositivity. Statistically significant differences within age group are indicated above the graphs, while differences between age groups are indicated below (color coded): * p<0.05, ** p<0.005.
Yet, the relationship between HA-specific Ab levels and HA-specific T-cell responses within individuals is less well understood. While no relationship between HAI titers and HA-specific IFN-γ production after in vitro expansion could be discerned previously (23), it is conceivable that Ab levels correlate with HA-specific T-cell frequencies measured directly ex vivo, without prior in vitro culture. However, no significant correlation was found between these two parameters either pre or post vaccination (Fig. 4B), even though vaccination boosted both the Seasonal H1-specific Ab and T-cell levels. No correlation analyses were performed for either Pandemic H1 or Avian H5, since no significant HAI titers were detected to these proteins.
Relationship between HA-specific CD4+ T-cell frequencies ex vivo and their ability to produce IFN-γ in an Ag-specific manner following in vitro expansion
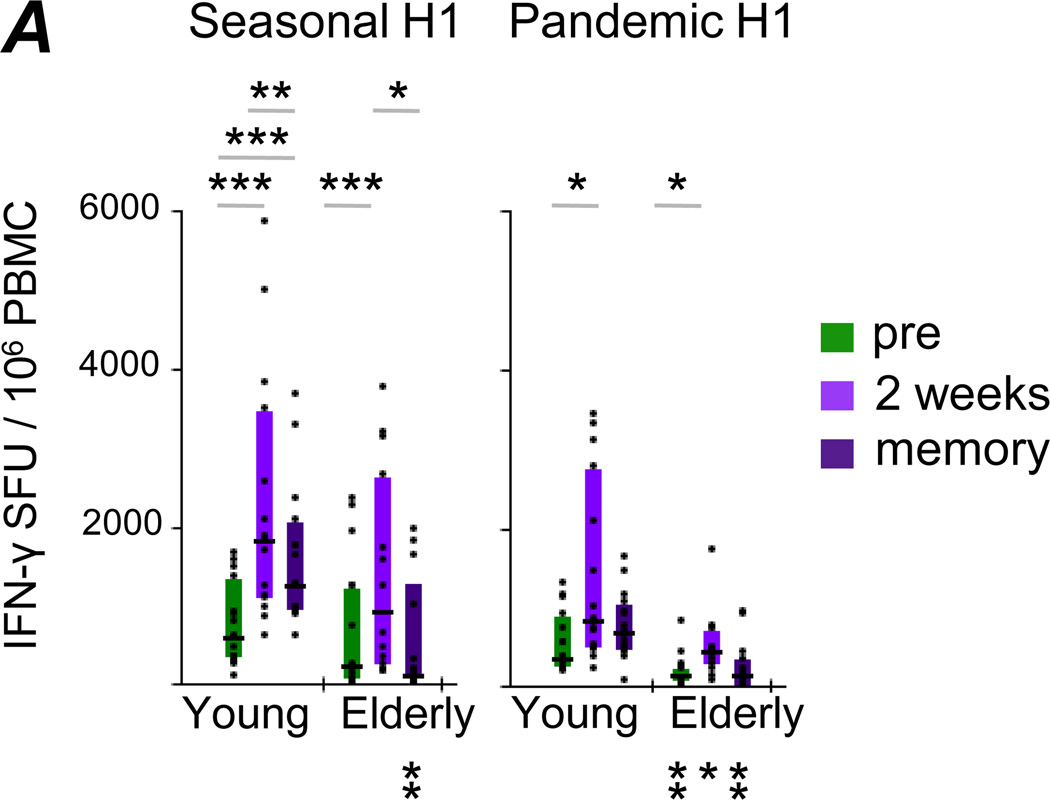
Using an IFN-γ ELISPOT assay after Ag-specific in vitro expansion, we have previously reported age-dependent functional deficits among Pandemic H1-specific T-cells expanded in vitro (29). We assessed how the capacity of T-cells to produce IFN-γ after a 7 day in vitro expansion and subsequent stimulation corresponded to frequencies of HA-reactive CD4+ T-cells measured directly ex vivo (Fig.5). Similar to the ex vivo observations (Fig.2C), Seasonal H1-specific IFN-γ production following in vitro expansion was significantly boosted by TIV administration in both young and elderly adults, and such responses were better maintained in younger individuals (Fig.5A, left panel). As well, boosted Pandemic H1-specific responses were observed in both age groups following TIV administration (Fig.5A, right panel). In contrast to the ex vivo data, elderly adults demonstrated lower responses following Ag-specific expansion at all time-points compared to younger individuals. Furthermore, we found no correlation between the frequency of HA-reactive CD4+ T-cells ex vivo and corresponding IFN-γ production following in vitro expansion for both Seasonal H1 and Pandemic H1. This indicates that these two assays provide related, yet independent measures of HA-specific cellular immunity.
Figure 5. Cytokine production by Influenza HA-specific CD4+ T-cells after in vitro expansion.
Correlation analyses of ex vivo cytokine production and IFN-γ ELISPOT data following in vitro T-cell expansion after stimulation with Seasonal H1 or Pandemic H1 were performed at the three sampling time-points. SFU – spot-forming units. Statistically significant differences within age groups across time-points are indicated above the graphs, while differences between age groups at the same time-point are indicated below (color coded): * p<0.05, ** p<0.005, *** p<0.0005.
Discussion
While the preponderance of influenza specific humoral responses is type-specific, a number of studies have now established the existence of robust cross-reactive T-cell responses against heterosubtypic antigens of influenza (21–24). We have previously shown that such heterosubtypic T-cell responses can be boosted by vaccination with unmatched antigens among human vaccinees (23). Importantly, the major antigenic component of all licensed influenza vaccines, HA, has been identified as a significant component of protective T-cell immunity against influenza, including heterosubtypic immunity (26, 27, 43, 44). T-cell immunity to influenza, including heterosubtypic immunity, is impaired with advancing age (29, 45), though the mechanistic details of such deficits remain unclear. Careful analysis of T-cell phenotypic markers, including those associated with maturation, activation, and functionality can provide much needed clues into the mechanistic aspects of such age-related deficits.
A large number of studies have characterized phenotypic markers that correlate with ageing and immunosenescence of T-cells in general (46–51). In particular, CD57 expression on T-cells has been linked to their functional senescence (52–55), though, Appay and coworkers found that diminution of the naïve T-cell population rather than accumulation of CD57+ cells best identifies immunosenescence in HIV+ individuals (56). In our cohort, the representation of CD8+ T-cell subsets defined by differentiation markers was strongly associated with age; however, neither the relative representation of differentiation subsets, nor expression of the differentiation and activation markers CD27, CD28, CD57, CD127, Granzyme B or Ki67 on CD4+ T-cells was significantly altered among CD4+ T-cells in elderly adults.
Though T-cells have not been formally shown to prevent the establishment of influenza infection, they can provide partial protection by promoting viral clearance and reducing severity of symptoms (57). Due to the low frequency of HA-specific T-cells, previous studies relied on IFN-γ ELISPOT measurement after several days of Ag-specific in vitro expansion. Using an optimized flow cytometry approach, we were able to measure such responses directly ex vivo in PBMC samples from healthy donors sampled both before and after receiving a seasonal influenza vaccination. Currently available inactivated subunit vaccines only inefficiently boost CTL responses due to the lack of cross-presentation (37). This is confirmed by our ex vivo data, in which we found essentially no CD8+ T-cell response. In contrast, low but consistently detectable HA-specific CD4+ T-cell responses were prevalent among vaccinees and boosted by such inactivated vaccines.
Strong evidence suggests that B-cell dependent immunity plays a key role in heterosubtypic immunity against influenza (58, 59). In the present cohort, HA-specific Abs were found not to be cross-reactive to heterosubtypic HA variants, while HA-specific CD4+ T-cells, whether measured directly ex vivo or after Ag-specific in vitro expansion, did display cross-reactivity. Even though TIV administration boosted both humoral and ex vivo cellular HA-specific responses, no correlation was found between the two, either pre- or post-vaccination. This is in agreement with published observations that found no significant correlation between HAI titers and IFN-γ production by HA-specific T-cells after Ag-specific in vitro expansion (23, 29).
It has been suggested that influenza-specific T-cell responses may be significantly impaired with ageing (20, 45) and that this may be particularly pronounced in their ability to target highly shifted, novel HA antigens (29). As our earlier study relied on HA-specific in vitro expansion to compare vaccine elicited T-cell responses to Seasonal and Pandemic H1 antigens (23), it was not clear if differences detected between young and elderly subjects were already present ex vivo, that is to say prior to in vitro culture. In the present study cells from elderly adults clearly demonstrate reduced proliferation and/or IFN-γ production following expansion in the presence of Pandemic H1, but no equivalent impairment in their CD4+ T-cells’ ex vivo reactivity to the same Pandemic H1. This points to a functional deficit among HA-specific CD4+ T-cells derived from the elderly to either expand in vitro when cultured with a heterosubtypic HA variant and/or to secrete IFN-γ upon stimulation with that same HA variant following in vitro expansion.
Importantly, data from this study suggests that the in vivo kinetics of HA-specific CD4+ T-cell responses elicited by influenza vaccination differ between the young and elderly, and that such differences are restricted to the matched HA antigen found in the vaccine: while younger individuals maintained vaccine-elicited CD4+ T-cell responses through to 6 weeks post vaccination, such responses returned to pre vaccination levels among the elderly during the same period of time. Hence, in vivo contraction of Ag-specific CD4+ T-cells appears to be more rapid in the elderly. These results are in agreement with a prior study that observed comparable levels of CD4+ T cell responses among the young and the elderly, but notable impairment among the elderly in their ability to expand such T cell responses (45). It is not clear whether this is due to a longer survival of Ag-stimulated CD4+ T-cells in younger subject, or a more active contraction of such cells in the elderly. Careful studies are needed to dissect mechanistic aspects of these observations both in vivo and in vitro. Also, further studies with larger cohorts are warranted to ascribe significant statistical power to the observations from this study: given the data-distribution measured, a cohort size of 56 subjects per age-group would be needed to determine at 80% power whether the responses to TIV vaccination are significantly different between age groups.
Given our inability to predict future candidate pandemic strains or their drift during an actual pandemic, understanding how T-cells respond when primed with one Ag and are then boosted by a heterosubtypic Ag is important to improve the design of an effective vaccine. In fact, from 1991 to 2007, only a 55–69% match was achieved by licensed vaccine components that target the rapidly drifting seasonal H3N2 Ags (60).
Given the annual epidemics across the globe and significant influenza disease burden among children (61, 62), vaccination of adults, both young and old, invariably functions in the ambit of previous exposure and primed immune responses against this ever-evolving pathogen. While data presented here suggest that both naïve and Ag-experienced memory CD4+ T-cells specific for HA are stimulated by influenza vaccination, this study did not address whether there might be alterations at the epitope level between HA variants investigated. Future studies are required to dissect T-cell responses at the epitope level in order to identify potential variations in heterosubtypic peptides that can still stimulate T-cells previously primed on closely related peptides. Since T-cell responses are inherently more cross-reactive than Ab responses, future vaccines to this constantly changing virus may benefit by targeting the T-cell arm of the immune system.
Highlights.
>Influenza vaccine (TIV) immunogenicity among young (18–49 years old) and elderly (≥70 years old) >Vaccine elicits predominantly central memory and effector memory CD4+ T-cells >Vaccine-elicited T cell responses are short-lived among the elderly >PBMC from younger subjects are superior at producing IFN-γ after short-term Ag-specific culture.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Alex Klimov and Dr. Xiyan Xu at the Center for Disease Control and Prevention (CDC), as well as BEI Resources for the influenza reagents. We thank Dr. David Bernstein, Tarek Shata and Saleem Basha for discussions; Tara Foltz for clinical study coordination; and Joanne Yu for preparation of Ab-conjugates. We also thank Dr. Martha Nason for help with statistical analysis and power calculations. This work was supported by the Intramural Research Program of the National Institute for Allergy and Infectious Diseases, NIH. The funding bodies did not have any involvement in the actual research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 2.WHO, W.H.O. Global Alert and Response (GAR): Pandemic (H1N1) 2009 - update 112. 2010
- 3.Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, Meijer A, van Steenbergen J, Fouchier R, Osterhaus A, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 4.Webster RG, Hulse DJ. Microbial adaptation and change: avian influenza. Rev Sci Tech. 2004;23:453–465. doi: 10.20506/rst.23.2.1493. [DOI] [PubMed] [Google Scholar]
- 5.Subbarao K, Katz J. Avian influenza viruses infecting humans. Cell Mol Life Sci. 2000;57:1770–1784. doi: 10.1007/PL00000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Normile D. Infectious diseases. Genetic analyses suggest bird flu virus is evolving. Science. 2005;308:1234–1235. doi: 10.1126/science.308.5726.1234a. [DOI] [PubMed] [Google Scholar]
- 7.Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 8.Delem A. Protective efficacy of RIT 4025, a live attenuated influenza vaccine strain, and evaluation of heterotypic immunity to influenza A viruses in ferrets. J Hyg (Lond) 1977;79:203–208. doi: 10.1017/s0022172400053006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodeve A, Potter CW, Clark A, Jennings R, Schild GC, Yetts R. A graded-dose study of inactivated, surface antigen influenza B vaccine in volunteers: reactogenicity, antibody response and protection to challenge virus infection. J Hyg (Lond) 1983;90:107–115. doi: 10.1017/s0022172400063907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies JR, Grilli EA. Natural or vaccine-induced antibody as a predictor of immunity in the face of natural challenge with influenza viruses. Epidemiol Infect. 1989;102:325–333. doi: 10.1017/s0950268800030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virelizier JL. Host defenses against influenza virus: the role of anti-hemagglutinin antibody. J Immunol. 1975;115:434–439. [PubMed] [Google Scholar]
- 12.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 13.de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000;61:94–99. [PubMed] [Google Scholar]
- 14.Couch RB. Seasonal inactivated influenza virus vaccines. Vaccine. 2008;26 Suppl 4:D5–D9. doi: 10.1016/j.vaccine.2008.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrose CS, Wu X, Belshe RB. The Efficacy of Live Attenuated and Inactivated Influenza Vaccines in Children as a Function of Time Postvaccination. Pediatr Infect Dis J. 2010 doi: 10.1097/INF.0b013e3181e2872f. [DOI] [PubMed] [Google Scholar]
- 16.David S, Skowronski D, Tweed S, Tuk T, Danderfer G, Li Y, Krajden M, Petric M, McNabb G, Gillies R. Epidemiologic profile of a new H3N2 variant of influenza A mismatched to vaccine, 2003–2004 influenza season. Can Commun Dis Rep. 2005;31:21–31. [PubMed] [Google Scholar]
- 17.Baldo V, Baldovin T, Pellegrini M, Angiolelli G, Majori S, Floreani A, Busana MC, Bertoncello C, Trivello R. Immunogenicity of three different influenza vaccines against homologous and heterologous strains in nursing home elderly residents. Clin Dev Immunol. 2010;2010 doi: 10.1155/2010/517198. 517198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson LA, Gaglani MJ, Keyserling HL, Balser J, Bouveret N, Fries L, Treanor JJ. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infect Dis. 2010;10:71. doi: 10.1186/1471-2334-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25:6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 20.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 21.Roti M, Yang J, Berger D, Huston L, James EA, Kwok WW. Healthy human subjects have CD4+ T cells directed against H5N1 influenza virus. J Immunol. 2008;180:1758–1768. doi: 10.4049/jimmunol.180.3.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge X, Tan V, Bollyky PL, Standifer NE, James EA, Kwok WW. Assessment of seasonal influenza A virus-specific CD4 T-cell responses to 2009 pandemic H1N1 swine-origin influenza A virus. J Virol. 2010;84:3312–3319. doi: 10.1128/JVI.02226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subbramanian RA, Basha S, Shata MT, Brady RC, Bernstein DI. Pandemic and seasonal H1N1 influenza hemagglutinin-specific T cell responses elicited by seasonal influenza vaccination. Vaccine. 2010;28:8258–8267. doi: 10.1016/j.vaccine.2010.10.077. [DOI] [PubMed] [Google Scholar]
- 24.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84:9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topham DJ, Doherty PC. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J Virol. 1998;72:882–885. doi: 10.1128/jvi.72.1.882-885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel A, Tran K, Gray M, Li Y, Ao Z, Yao X, Kobasa D, Kobinger GP. Evaluation of conserved and variable influenza antigens for immunization against different isolates of H5N1 viruses. Vaccine. 2009;27:3083–3089. doi: 10.1016/j.vaccine.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Rao SS, Kong WP, Wei CJ, Van Hoeven N, Gorres JP, Nason M, Andersen H, Tumpey TM, Nabel GJ. Comparative efficacy of hemagglutinin, nucleoprotein, and matrix 2 protein gene-based vaccination against H5N1 influenza in mouse and ferret. PLoS One. 2010;5:e9812. doi: 10.1371/journal.pone.0009812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Reeth K, Braeckmans D, Cox E, Van Borm S, van den Berg T, Goddeeris B, De Vleeschauwer A. Prior infection with an H1N1 swine influenza virus partially protects pigs against a low pathogenic H5N1 avian influenza virus. Vaccine. 2009;27:6330–6339. doi: 10.1016/j.vaccine.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Subbramanian RA, Basha S, Brady RC, Hazenfeld S, Shata MT, Bernstein DI. Age-related changes in magnitude and diversity of cross-reactive CD4+ T-cell responses to the novel pandemic H1N1 influenza hemagglutinin. Hum Immunol. 2010;71:957–963. doi: 10.1016/j.humimm.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of postcytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiecker F, Streitz M, Ay B, Cherepnev G, Volk HD, Volkmer-Engert R, Kern F. Analysis of antigen-specific T-cell responses with synthetic peptides--what kind of peptide for which purpose? Hum Immunol. 2004;65:523–536. doi: 10.1016/j.humimm.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ely KH, Roberts AD, Kohlmeier JE, Blackman MA, Woodland DL. Aging and CD8+ T cell immunity to respiratory virus infections. Exp Gerontol. 2007;42:427–431. doi: 10.1016/j.exger.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 35.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee LY, Ha do LA, Simmons C, de Jong MD, Chau NV, Schumacher R, Peng YC, McMichael AJ, Farrar JJ, Smith GL, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 39.Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis. 2005;58:195–207. [PubMed] [Google Scholar]
- 40.Treanor J, Wright PF. Immune correlates of protection against influenza in the human challenge model. Dev Biol (Basel) 2003;115:97–104. [PubMed] [Google Scholar]
- 41.Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, Abrutyn E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37:427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 42.Renegar KB. Influenza virus infections and immunity: a review of human and animal models. Lab Anim Sci. 1992;42:222–232. [PubMed] [Google Scholar]
- 43.Jeon SH, Arnon R. Immunization with influenza virus hemagglutinin globular region containing the receptor-binding pocket. Viral Immunol. 2002;15:165–176. doi: 10.1089/088282402317340314. [DOI] [PubMed] [Google Scholar]
- 44.Gogolak P, Simon A, Horvath A, Rethi B, Simon I, Berkics K, Rajnavolgyi E, Toth GK. Mapping of a protective helper T cell epitope of human influenza A virus hemagglutinin. Biochem Biophys Res Commun. 2000;270:190–198. doi: 10.1006/bbrc.2000.2384. [DOI] [PubMed] [Google Scholar]
- 45.Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Pan XD, Xie Y, Zhang GB, Jiang M, Zheng L, Wang JH, Shi JF, Zhang XG. Altered CD28 and CD95 mRNA expression in peripheral blood mononuclear cells from elderly patients with primary non-small cell lung cancer. Chin Med J (Engl) 2010;123:51–56. [PubMed] [Google Scholar]
- 47.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 48.Effros RB. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol. 1997;21:471–478. doi: 10.1016/s0145-305x(97)00027-x. [DOI] [PubMed] [Google Scholar]
- 49.Saba I, Kosan C, Vassen L, Moroy T. IL-7R-dependent survival and differentiation of early T-lineage progenitors is regulated by the BTB/POZ domain transcription factor Miz-1. Blood. 2011;117:3370–3381. doi: 10.1182/blood-2010-09-310680. [DOI] [PubMed] [Google Scholar]
- 50.Shen CH, Ge Q, Talay O, Eisen HN, Garcia-Sastre A, Chen J. Loss of IL-7R and IL-15R expression is associated with disappearance of memory T cells in respiratory tract following influenza infection. J Immunol. 2008;180:171–178. doi: 10.4049/jimmunol.180.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech Ageing Dev. 2009;130:709–712. doi: 10.1016/j.mad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–116. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 53.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175:8415–8423. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 54.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt P, Karim R, Kern DM, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Appay V, Fastenackels S, Katlama C, Ait-Mohand H, Schneider L, Guihot A, Keller M, Grubeck-Loebenstein B, Simon A, Lambotte O, et al. Old age and anti-CMV immunity are associated with altered T cell reconstitution in HIV-1 infected patients. Aids. 2011 doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- 57.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen HH, Zemlin M, Ivanov II, Andrasi J, Zemlin C, Vu HL, Schelonka R, Schroeder HW, Jr, Mestecky J. Heterosubtypic immunity to influenza A virus infection requires a properly diversified antibody repertoire. J Virol. 2007;81:9331–9338. doi: 10.1128/JVI.00751-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001;75:5141–5150. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richard SA, Viboud C, Miller MA. Evaluation of Southern Hemisphere influenza vaccine recommendations. Vaccine. 2010;28:2693–2699. doi: 10.1016/j.vaccine.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinhoff MC. Influenza in young children: burden, immunisation, and policy. Lancet Infect Dis. 2011;11:2–3. doi: 10.1016/S1473-3099(10)70263-2. [DOI] [PubMed] [Google Scholar]
- 62.Henkle E, Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, McNeal M, Breiman RF, Moss WJ, Zaman K. Incidence of influenza virus infection in early infancy: a prospective study in South Asia. Pediatr Infect Dis J. 2011;30:170–173. doi: 10.1097/INF.0b013e3181f63c39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.