Abstract
We report our experience in managing 13 consecutive clinically suspected cases of Buruli ulcer on the face treated at the hospital of the Institut Médical Evangélique at Kimpese, Democratic Republic of Congo diagnosed during 2003–2007. During specific antibiotherapy, facial edema diminished, thus minimizing the subsequent extent of surgery and severe disfigurations. The following complications were observed: 1) lagophthalmos from scarring in four patients and associated ectropion in three of them; 2) blindness in one eye in one patient; 3) disfiguring exposure of teeth and gums resulting from excision of the left labial commissure that affected speech, drinking, and eating in one patient; and 4) dissemination of Mycobacterium ulcerans infection in three patients. Our study highlights the importance of this clinical presentation of Buruli ulcer, and the need for health workers in disease-endemic areas to be aware of the special challenges management of Buruli ulcer on the face presents.
Introduction
Cutaneous infection by Mycobacterium ulcerans, also known as Buruli ulcer, represents the third most common mycobacterial disease in the world after tuberculosis and leprosy.1 Buruli ulcer has emerged dramatically over the past decade, particularly in western Africa, and has been reported or suspected in 312,3 countries, and confirmed by laboratory tests in 26 countries.4,5
Buruli ulcer is a severely disfiguring and disabling disease (Figure 1), which affects primarily children less than 15 years of age in many tropical and subtropical countries.3–5 Although lesions on the face have been reported in different countries, there is little information on their frequency.2,5–10 The difficulties encountered in treating such cases and the importance of a conservative surgical approach to their management have been emphasized.5,7–10
Figure 1.
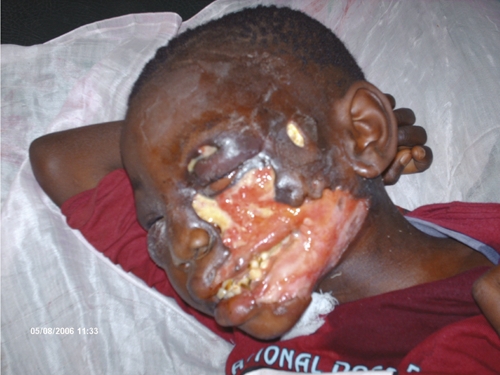
Late diagnosis of Buruli: a large disfiguring ulcer in a nine-year-old girl from Songololo, Democratic Republic of Congo.
The Global Buruli Ulcer Initiative (GBUI) of the World Health Organization has recently recommended the initial use of conservative treatment, particularly when lesions are located on the face, breast, and genitalia. The suggested approach for treatment of such patients is a combination of specific antibiotics, with or without surgery, and to follow an appropriate prevention of disability (POD) program assiduously.11,12
Our first experience was with a four-year-old boy in Angola (not included in the present study) who was treated for edematous M. ulcerans infection on the face and drew our attention to the special features and therapeutic requirements of such cases.10 We report our experience in managing 13 consecutive patients with lesions on the face suspected to be Buruli ulcer at the Institut Médical Evangélique (IME) Hospital in Kimpese, Democratic Republic of Congo, over a five-year period, and compare their clinical presentations and outcomes.
Patients and Methods
The IME Hospital in Kimpese serves an area that contains approximately 150.000 inhabitants, the Kimpese Health Zone in the Songololo Territory, and manages the treatment of most of Buruli ulcer patients in the Bas-Congo Province of the Democratic Republic of Congo. In this prospective descriptive study, we included all consecutive patients with facial lesions suspected to be Burulit ulcer admitted to IME Kimpese Hospital during January 1, 2003–December 31, 2007. All lesions were first suspected to be Buruli ulcer on the basis of clinicoepidemiologic features.2,4 Age, sex, address, date of onset, date of diagnosis, clinical features, hospital stay, type of management, and disease progression were recorded.2
Specimens of exudates and tissues were analyzed by laboratory tests according to World Health Organization recommendations.2,4 Initial direct smear examination by Ziehl-Neelsen (ZN) staining, and histopathologic analysis was performed at the IME/Kimpese Laboratory. Other specimens from the same patient were sent in a transport medium13 to the Mycobacteriology Unit of the Institute of Tropical Medicine in Antwerp, Belgium, where ZN staining, in vitro culture, and an IS2404 polymerase chain reaction (PCR) were performed with appropriate controls.4,14 Data were entered in an Excel database (Microsoft Corporation, Redmond, WA) and analyzed by using Epi-Info version 3.3.2 (Centers for Disease Control and Prevention, Atlanta, GA). As required by the Medical Committee of IME, all patients, or their responsible relative or guardian, provided informed consent for all diagnostic and treatment procedures and publication of any or all images derived from the management of the patient, including clinical photographs, which might reveal patient identity.
Results
During the five-year period of our study, 238 suspected Buruli ulcer patients were admitted to the IME Hospital, of whom 147 (62%) were confirmed by at least one laboratory test.4 Facial lesions were observed among 13 suspected Buruli ulcer patients, of whom 10 (77%) had M. ulcerans disease confirmed by PCR. Thus, the frequency of facial BU was 5% (13 of 238) among suspected Buruli ulcer patients and 7% (10 of 147) among confirmed Buruli ulcer patients. These results are consistent with the frequency of facial Buruli ulcer lesions reported (0.8–16.7%), as shown in Table 1.
Table 1.
Reports of facial Buruli ulcer disease, 1966–2008*
| Year of report | Country | Study design | No. positive | % | Reference |
|---|---|---|---|---|---|
| 1966 | Uganda | Case series | 1/38 | 2.6 | Connor and Lunn24 |
| 1967 | Papua New Guinea | Case report | 2/13 | 15.4 | Reid25 |
| 1970 | DRC | Case series | 19/93 | 20.4† | Smith26 |
| 1971 | Uganda | Case series | 30/164 | 18.3† | Barker27 |
| 1971 | Uganda | Case series | 39/220 | 17.7† | The Uganda Buruli Group28 |
| 1972 | Uganda | Case series | 27/539 | 5.0 | Barker29 |
| 1974 | DRC | Review | 1/14 | 7.1 | Meyers and others30 |
| 1976 | Nigeria | Case report | 1/22 | 4.5 | Oluwasanmi and others31 |
| 1977 | Cameroon | Case report | 4/37 | 10.8† | Ravisse and others32 |
| 1989 | Ghana | Case series | 12/96 | 12.5 | Van der Werf and others33 |
| 1993 | Côte d'Ivoire | Case series | 1/124 | 0.8 | Darie and others34 |
| 1994 | Benin | Case series | 7/227 | 3.1 | Josse and others35 |
| 1995 | Ghana | Case series | 1/26 | 3.8 | Addo36 |
| 1996 | Côte d'Ivoire | Review | 1 | NA | Aujoulat and others37 |
| 1997 | Benin | Case series | 23/867 | 2.7 | Aguiar and others38 |
| 1998 | Ghana | Case series | 1/102 | 1.0 | Asiedu and Etuaful39 |
| 1998 | Burkina Faso | Case report | 1/6 | 16.7 | Ouoba and others40 |
| 2002 | Ghana | Case series | 219/5,770 | 3.8 | Amofah and others41 |
| 2002 | Togo | Case report | 1‡ | NA | Schierle and others42 |
| 2003 | DRC and Angola | Case series | 1/27 | 3.7 | Kibadi and others43 |
| 2003 | Côte d'Ivoire | Case series | 2‡ | NA | Ouatttara and others6 |
| 2003 | Togo | Case series | 7/160 | 4.4 | James and others44 |
| 2004 | Benin | Case series | 58/1,700 | 3.4 | Debacker and others9 |
| 2004 | Cameroon | Cross-sectional | 4/202 | 2.0 | Noeske and others8 |
| 2005 | DRC | Review | 1 | NA | Janssens and others5 |
| 2005 | Ghana | Case series | 32/748 | 4.3 | Hospers and others45 |
| 2005 | Ghana | Case-control | 5/121 | 4.1 | Raghunathan and others46 |
| 2007 | Australia | Case series | 1/79 | 1.3 | Johnson and others47 |
| 2007 | DRC | Case report | 1‡ | NA | Phanzu and others10 |
| 2007 | Benin | Case series | 19/2,598 | 0.7 | Sopoh and others16 |
| 2007 | Cameroon | Case-control | 3/163 | 1.8 | Pouillot and others48 |
| 2008 | Côte d'Ivoire | Case series | 8‡ | NA | Kouame and others7 |
DRC = Democratic Republic of Congo; NA = not applicable.
Head, neck, and trunk combined.
Number in specific or dedicated report of facial Buruli ulcer disease.
Characteristics of the 13 patients are shown in Table 2. Nine (69.2%) of 13 patients had ulcerated lesions, and swab specimens were obtained for ZN staining of smears, followed by swabs for PCR and culture. For patients with preulcerative lesions, punch or incisional skin biopsies were not used to minimize initiating of the ulceration process. In such patients fine needle aspiration (FNA) was used for rapid diagnosis of facial Buruli ulcer. If an ulcer developed later, swabs were taken from the early ulcerative lesion. Specimens of tissue were also obtained during debridements. On the basis of our experience, we recommend ZN staining of swabs from ulcers or FNA from preulcerative forms for rapid diagnosis of facial Burulu ulcer.15 Punch or surgical biopsies from preulcerative forms should be avoided because in our experience biopsies from preulcerative forms became the starting point of extensive ulceration.10
Table 2.
Characteristics of 13 patients with facial BU admitted to Institut Médical Evangélique, Kimpese Hospital, Kimpese, Bas-Congo, Democratic Republic of Congo, 2003–2007*
| Patient | Age, years/sex | Classification | Clinical form | Site | No. lesions | Patient delay, weeks | Hospital stay, days | Treatment | Laboratory confirmation | Physical impairment | Relapse at on-year follow-up | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R + S, days | Surgery | Initial | Final | ||||||||||
| 1 | 18/M | N | U, O | LL, head | 3 | 52 | 80 | No | Yes | PCR | Lagoph | Lagoph | Yes |
| 2 | 68/M | N | E, U | Head | 1 | 3 | 68 | No | Yes | Neg | No | Lagoph | No |
| 3 | 5/M | N | E | Head | 1 | 12 | 213 | Yes (90) | Yes | ZN, PCR | PEC | Lagoph, LOS | No |
| 4 | 11/M | N | E, U | Head | 1 | 8 | 125 | Yes (90) | Yes | ZN, PCR | No | No | No |
| 5 | 17/M | N | U | Head | 1 | 12 | 106 | Yes (60) | Yes | ZN, PCR | No | No | No |
| 6 | 17/M | R | Q, U, No | LL, head | 2 | 12 | 84 | Yes (60) | Yes | PCR | No | No | No |
| 7 | 12/M | N | E | Head | 2 | 4 | 127 | Yes (60) | Yes | PCR | No | No | No |
| 8 | 9/F | N | E, U, O | Head | 1 | 16 | 301 | Yes (60) | Yes | ZN, PCR, HIS | No | Lagoph, ELC | No |
| 9 | 5/F | N | U | Head, neck | 1 | 48 | 32 | No | Yes | Neg | No | No | Yes |
| 10 | 4/F | N | E, P | Head | 2 | 8 | 66 | Yes (60) | No | NT | PEC | No | Yes |
| 11 | 4/F | N | E, U | LL, head | 3 | 24 | 297 | Yes (60) | Yes | ZN, PCR | PEC | No | No |
| 12 | 5/F | N | E | Head | 3 | 6 | 133 | Yes (60) | Yes | ZN, PCR | PEC | No | No |
| 13 | 13/M | N | E, U | Head | 1 | 16 | 151 | Yes (60) | Yes | ZN, PCR | No | No | No |
R + S = rifampicin plus streptomycin; N = new case; U = ulcer; O = osteomyelitis (not laboratory confirmed); LL = lower limb; PCR = polymerase chain reaction; Lagoph = lagophthalmos; E = edema; Neg = negative; ZN = Ziehl-Neelsen staining; PEC = permanent eye closure; LOS = loss of sight (right eye); R = relapse (new diagnosis of Buruli ulcer less than one year after being declared cured with previous antibiotic and/or surgical treatment); Q = plaque; No = nodule; HIS = histopathologic analysis; ELC = excision of labial commissure; P = papule; NT = not tested.
Laboratory test results were negative for two cases (Table 2). One of these patients had ulcerated lesions on the neck and cheek. Conditions other than Buruli ulcer (e.g., cervical mycobacterial lymphadenitis) should not be ruled out. In this case, histopathologic analysis was not performed. The case emphasizes the difficulties in recognizing some Buruli ulcer cases on a clinical basis only, and stresses the importance of additional laboratory tests including histopathologic analysis for the differential diagnosis of Buruli ulcer.
The median age of the 13 patients was 11 years (age range = 4–68 years). Consistent with other studies, the age distribution showed that 9 (69.2%) of 13 patients were less than 15 years of age,7,8,12,16 3 (23.1%) were 15–45 years of age, and one (7.7%) was 68 years of age. There were eight males and five females, giving a sex ratio of 1.6:1, although this difference may not be significant because as in several other studies, numbers of cases of Buruli ulcer were similar in males and females.2,9,12,17,18
Twelve (92.3%) of the patients were from the Cataractes District (11 from Songololo Territory and 1 from the Mbanza-Ngungu Territory). One patient was from the Bas-Fleuve District (Sekebanza Territory). This geographic distribution is consistent with those of other studies in the same area.14,17,18
Median delay in seeking medical attention was 12 weeks (range = 3–52 weeks). This delay seems prolonged compared with that in a report from the same area.14 Despite the extent of necrosis and tissue damage, Buruli ulcers are usually painless. This absence of pain often deters patients from seeking early treatment, resulting in severe sequelae, including amputations of limbs.19,20
The distribution of different clinical forms of Buruli ulcers were mixed forms in 61.5%, single ulcerative lesions in 15.4%, and single edematous forms in 23.1%. There was bone involvement in two (15.4%) patients. Three patients had disseminated lesions affecting the head and lower limbs. Edema was present in 9 (69.2%) patients with tendency to regional infiltration, particularly among children (Figure 2). A similar observation was reported recently in Côte d'Ivoire.7 One question that arises is whether children are more likely to develop extensive facial edema than older persons.
Figure 2.
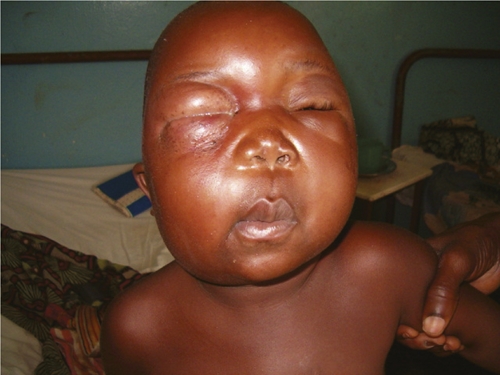
Periorbital advanced edematous lesions on right eye with early extension on left side of a patient, Democratic Republic of Congo.
Extensive edematous forms are considered to be among the category of severe disseminated lesions.1 Why children are prone to have edematous forms develop remains unknown but the cause is probably multifactorial, including immunity. Children may have a less effective protective immune response against M. ulcerans than adults.1 The evidence that mycolactone plays a major role in the pathogenicity of Buruli ulcer has been established.2,19,21 However, factors determining the extent of the lesions are unknown.21 It has been suggested that the cell-mediated immune response of the host is likely to play an important role in Buruli ulcer.2
In Benin, Debacker and others reported that children tended to show development of disease more often on the trunk, head, neck and upper limbs.9 Because of their shorter stature, the entire body of children is nearer the ground, which may explain why children are more likely than adults to develop lesions on the head. Because routine testing for human immunodeficiency virus was not conducted in our series, we cannot exclude that immune suppression could play a role in facial presentations. However, human immunodeficiency virus/acquired immunodeficiency syndrome is infrequent in children in this area of the Democratic Republic of Congo. The periorbital region was the site of predilection among our patients. Similar observations have been reported.7 The location of lesions on the face and on other parts of the body for those with disseminated disease is shown in Table 3.
Table 3.
Site of lesions among 13 patients with facial lesions admitted to Institut Médical Evangélique, Kimpese Hospital, Kimpese, Bas-Congo, Democratic Republic of Congo, 2003–2007
| Site of lesions | Children (n = 9) | % | Adults (n = 4) | % | Total (n = 13) | % |
|---|---|---|---|---|---|---|
| Periorbital | 6* | 66.7 | 1* | 25 | 7 | 53.8 |
| Periorbital and cheek | 1 | 11.1 | 1 | 25 | 2 | 15.4 |
| Cheek | 2† | 22.2 | 2* | 50 | 4 | 30.8 |
One patient with another lesion on the lower limb (disseminated case of Buruli ulcer).
One patient with a lesion reaching the neck.
Among our patients, 12 had new cases (92.3%) and 1 had a relapse (7.7%). Physical impairments were present in 5 patients (38.5%) initially when admitted to the hospital and at the end of treatment. Initial impairments consisted essentially of significant swelling and eye closure (Figure 3) in four patients and limited eyelid closure in one patient.
Figure 3.
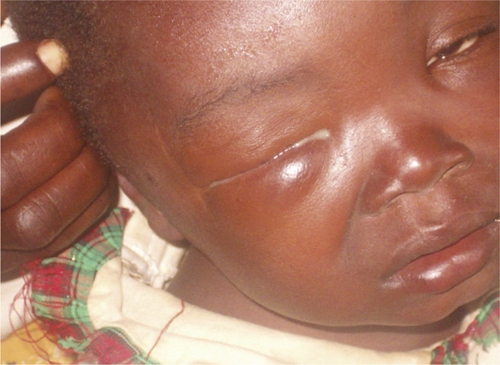
Papula on the temple and painless swelling of the right eyelids (early detection) of a patient, Democratic Republic of Congo.
Nine patients (69.2%) were treated by a combination of antibiotics (rifampicin and streptomycin) and surgery. Three patients (23.1%) were treated only with surgery, and one (7.7%) was treated only with antibiotics (Figure 4). Among patients treated with antibiotics, 8 received 60 days of antibiotic treatment, and it was prolonged to 90 days for 2 patients (Table 2). Surgery was performed at different times (during or after antibiotic treatment) when considered necessary. Seven patients (53.8%) received skin grafts. A total of 12 patients were surgically treated (92.3%). The degree of facial edema, in all patients where it was present, was reduced during antibiotherapy, minimizing the extent of surgery, thus helping to prevent severe disfigurement. We noted in our series that clinical response to specific antibiotic therapy seemed to be related to the stage of the lesion; the earlier the diagnosis of a Buruli ulcer facial lesion, the better the response to antibiotic therapy.
Figure 4.

Patient in Figure 3 one year after specific antibiotic treatment without surgery and no complications, Democratic Republic of Congo.
Complications observed in our study were lagophthalmos in four patients from scarring, with associated ectropion in three of them, loss of sight in one patient (Figure 5), disfigurement with exposure of teeth and gums, excision of left labial commissure in one patient, and dissemination of Buruli ulcer despite specific antibiotherapy in two patients.
Figure 5.
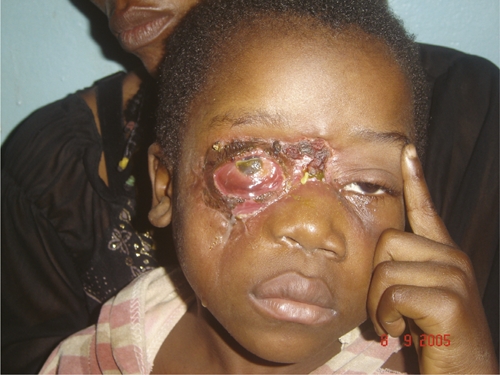
Loss of sight in the right eye and no complications on left side in the patient in Figure 2, Democratic Republic of Congo.
Discussion
In the 1960s, Meyers, who also worked at IME Kimpese Hospital, noted healing of early Buruli ulcer lesions after treatment with rifampicin alone.22 A recent report suggests the possible benefit of specific antibiotherapy to enhance healing and limit the extent of surgical excisions in edematous forms of Buruli ulcer on the face.10
Preservation of vision is of upmost importance and special attention must be given to minimize the risk of corneal dryness, ulcers, and infection. If the eyelids cannot close fully, the cornea needs to be lubricated with artificial tears and with ointment at night. Protection of the eyes during the day with hats and glasses and at night with an eye shield can further reduce dryness from exposure and protect the eye from dust and other foreign objects. Frequent exercise to close the eye helps lubricate the cornea, strengthen weakened muscles, and maintain full movement for eye closure. Gentle massage over the scar area softens it, stretches it, and limits spread of adhesions to adjacent structures, which can limit movement. Taping can also be used to facilitate good eyelid positioning.
Sopoh and others reported difficulties in managing the technical and cosmetic aspects of lesions around the head, neck, and trunk in Benin, where plastic surgeons were not available.16 Our study highlights the importance of this clinical presentation of Burulu ulcer, and the need for health workers in disease-endemic areas to manage Buruli ulcer lesions of the face to be aware of the special challenges such lesions present. Scars can cause the lower eyelid to evert (ectropion), and adhesions to adjacent structures can make it difficult to complete full eye closure. Edema can cause the eyelids to invert (entropion), creating problems with eyelashes turning in and touching the cornea (trichiasis). Special attention to care for the eye is important to preserving vision even when there is an early diagnosis.
Complete eye closure is important; the affected eye should open and close the same as the unaffected side. If not, exercises should be given. The cornea should be lubricated when there is incomplete eye closure with corneal exposure by using artificial tears during the day and ointment at night. An eye shield at night will provide extra protection and prevent foreign matter from falling into the eye that does not completely close. Surgery may be required to correct eyelid and eye lash positions. Excessive edema can cause entropion inverting the eyelash position causing them to touch the cornea during blink. Ectropion involving the lower eyelid may occur as ulcers heal and the strong contracting forces scar tissue limits the eye from closing completely. Scar management and exercises may improve eyelid closure but if the eyelid correction is not obtained with these simple measures, surgical correction of the eyelid is required. If the eye must be removed, special surgical procedures should be given during the initial excision to ensure that a prosthesis can be fit in the future. The faces of children who are not fitted with a prosthesis will not develop normally and their faces will be asymmetrical. The involvement of the maxillar and mandibular areas can create functional problems for speaking, eating, and drinking. Stigma can also be a major problem for persons with Buruli ulcer lesions on the face.23
Thus, community education for early case detection, chemotherapy, POD and rehabilitation, and reconstructive surgery are key components of the current Buruli ulcer control strategy.12
The median hospital stay in this series of patients was 125 days (range = 32–301 days). This range is similar to that of a previous study in the same geographic area.14 Follow-up at one year detected three patients (23%) with relapse, two at the original site (facial) and one at a remote site (lower limb). Among the patients who showed relapse, two had been previously treated by surgery alone and one by antibiotics alone.
Concerning the 10 patients in our study who were treated with antibiotics, we observed only one relapse (10%). Because no samples were taken for culture, we cannot exclude a possible paradoxical reaction to dead bacilli in this patient. The GBUI reported recently that recurrences after surgical treatment alone have decreased to < 2% after introduction of antibiotics. Development of antibiotic treatment is one of many major advances in the 10 years since the creation of the GBUI.12
In conclusion, effective care and support methods to improve the management of Buruli ulcer include community education to improve hygiene and awareness to early detection by a community-based health surveillance system, early antibiotic treatment, with or without surgery, and POD measures. For rapid diagnosis of facial Buruli ulcer, ZN staining of swab specimens from ulcers or FNA material from preulcerative forms is recommended. Our study underscores the importance of prompt referral of suspected Buruli ulcer cases and training of health professionals in areas to which Buruli ulcer is endemic in the early diagnosis of Buruli ulcer, including atypical localizations.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of the IME/Kimpese and the staff of the Mycobacteriology Unit of the Institute of Tropical Medicine ITM (Antwerp) for providing patient care and microbiologic analyses; Bouke de Jong for critically reading the manuscript; and Karin Janssens for outstanding work in preparing the manuscript.
Note: Supplemental figures are available at www.ajtmh.org.
Footnotes
Financial support: This study was supported by the American Leprosy Missions (Greenville, SC) and the European Commission (International Science and Technology Cooperation Development Program), Project No. INCO-CT-2005-05-051476-BURULICO.
Authors' addresses: Delphin M. Phanzu, Institut Médical Evangélique, Kimpese Hospital, Kimpese, Bas-Congo, Democratic Republic of Congo and Epidemiology and Disease Control Unit, Department of Public Health, Institute of Tropical Medicine, Antwerp, Belgium, E-mail: dmavingaphanzu@yahoo.fr. Roger L. Mahema and Désiré-Hubert B. Imposo, Institut Médical Evangélique, Kimpese Hospital, Kimpese, Bas-Congo, Democratic Republic of Congo, E-mails: mahemalut@yahoo.fr and imposodesire@yahoo.fr. Patrick Suykerbuyk, Elie Nduwamahoro, and Françoise Portaels, Mycobacteriology Unit, Department of Microbiology, Institute of Tropical Medicine, Antwerp, Belgium, E-mails: psuykerbuyk.acolson@gmail.com, enduwamahoro@itg.br, and portaels@itg.be. Linda F. Lehman, American Leprosy Missions, Greenville, SC, E-mail: lehman@uaigiga.com.br. Wayne M. Meyers, Department of Environmental and Infectious Disease Sciences, Armed Forces Institute of Pathology, Washington, DC, E-mail: wmekmeyers@comcast.net. Marleen Boelaert, Epidemiology and Disease Control Unit, Department of Public Health, Institute of Tropical Medicine, Antwerp, Belgium, E-mail: mboelaert@itg.be.
References
- 1.Portaels F, Silva MT, Meyers WM. Buruli ulcer. Clin Dermatol. 2009;27:291–305. doi: 10.1016/j.clindermatol.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . In: Buruli Ulcer-Mycobacterium ulcerans Infection. Asiedu K, Sherpbier R, Raviglione M, editors. Geneva: World Health Organization; 2000. /CDS/CPE/GBUI/2001.1. [Google Scholar]
- 3.World Health Organization Buruli ulcer disease. Mycobacterium ulcerans infection: an overview of reported cases globally. Wkly Epidemiol Rec. 2004;79:194–200. [Google Scholar]
- 4.World Health Organization . In: Buruli Ulcer. Diagnosis of Mycobacterium ulcerans Disease. Portaels F, Johnson P, Meyers WM, editors. Geneva: World Health Organization; 2001. WHO/CDS/CPE/GBUI/2001.4. [Google Scholar]
- 5.Janssens P, Pattyn SR, Meyers WM, Portaels F. Buruli ulcer: a historical overview with updating to 2005. Bull Seances Acad R Sci Outre Mer. 2005;51:265–299. [Google Scholar]
- 6.Ouattara D, Aka GK, Meningaud JP, Sica A, Kaba L, Gadegbeku S. Les localisations faciales de l'ulcère de Buruli: à propos de deux cas. Rev Stomatol Chir Maxillofac. 2003;104:231–234. [PubMed] [Google Scholar]
- 7.Kouame K, Ecra E, Cisse M, Gbery I, Kacou E, Kassi K, Kouassi A, Ahogo C, Yoboue P, Kanga JM. Ulcère de Buruli céphalique: huit observations au CHU d'Abidjan, Côte d'Ivoire. Med Trop (Mars) 2008;68:643–644. [PubMed] [Google Scholar]
- 8.Noeske J, Kuaban C, Rondini S, Sorlin P, Ciaffi L, Mbuagbaw J, Portaels F, Pluschke G. Buruli ulcer disease in Cameroon rediscovered. Am J Trop Med Hyg. 2004;70:520–526. [PubMed] [Google Scholar]
- 9.Debacker M, Aguiar J, Stenou C, Zinsou C, Meyers WM, Scott JT, Dramaix M, Portaels F. Mycobacterium ulcerans disease: role of age and gender in incidence and morbidity. Trop Med Int Health. 2004;9:1297–1304. doi: 10.1111/j.1365-3156.2004.01339.x. [DOI] [PubMed] [Google Scholar]
- 10.Phanzu MD, Ablordey A, Imposo BD, Lefevre L, Mahema RL, Suykerbuyk P, Meyers WM, Portaels F. Short report: edematous Mycobacterium ulcerans infection (Buruli ulcer) on the face: a case report. Am J Trop Med Hyg. 2007;77:1099–1102. [PubMed] [Google Scholar]
- 11.World Health Organization . Provisional Guidance on the Role of Specific Antibiotics in the Management of Mycobacterium ulcerans Disease (Buruli Ulcer) Geneva: World Health Organization; 2004. WHO/CDS/CPE/GBUI/2004.10. [Google Scholar]
- 12.World Health Organization Buruli ulcer: progress report, 2004–2008. Wkly Epidemiol Rec. 2008;83:145–156. [PubMed] [Google Scholar]
- 13.Eddyani M, Debacker M, Martin A, Aguiar J, Johnson RC, Uwizeye C, Fissette K, Portaels F. Primary culture of Mycobacterium ulcerans from human tissue specimens after storage in a semi-solid transport medium. J Clin Microbiol. 2008;46:69–72. doi: 10.1128/JCM.00301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phanzu DM, Bafende AE, Dunda BK, Imposo DB, Kibadi KA, Nsiangana ZS, Singa NJ, Meyers WM, Suykerbuyk P, Portaels F. Mycobacterium ulcerans disease (Buruli ulcer) in a rural hospital in Bas-Congo, Democratic Republic of Congo, 2002–2004. Am J Trop Med Hyg. 2006;75:311–314. [PubMed] [Google Scholar]
- 15.Eddyani M, Fraga AG, Schmitt F, Uwizeye C, Fissette K, Johnson C, Aguiar J, Sopoh G, Barogui Y, Meyers WM, Pedrosa J, Portaels F. Fine-needle aspiration, an efficient sampling technique for bacteriological diagnosis of nonulcerative Buruli ulcer. J Clin Microbiol. 2009;47:1700–1704. doi: 10.1128/JCM.00197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sopoh GE, Johnson RC, Chauty A, Dossou AD, Aguiar J, Salmon O, Portaels F, Asiedu K. Buruli ulcer surveillance, Benin, 2003–2005. Emerg Infect Dis. 2007;13:1374–1376. doi: 10.3201/eid1309.061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bafende AE, Phanzu MD, Imposo BB. Buruli ulcer in the Democratic Republic of Congo: epidemiology, presentation and outcome. Trop Doct. 2004;34:82–84. doi: 10.1177/004947550403400207. [DOI] [PubMed] [Google Scholar]
- 18.Portaels F. Epidémiologie des ulcères à Mycobacterium ulcerans. Ann Soc Belg Med Trop. 1989;69:91–103. [PubMed] [Google Scholar]
- 19.En J, Goto M, Nakanaga K, Higashi M, Ishii N, Saito H, Yonezawa S, Hamada H, Small PL. Mycolactone is responsible for the painlessness of Mycobacterium ulcerans infection (Buruli ulcer) in a murine study. Infect Immun. 2008;76:2002–2007. doi: 10.1128/IAI.01588-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kibadi K, Boelaert M, Kayinua M, Minuku JB, Muyembe-Tamfum JJ, Portaels F, Lefèvre P. Therapeutic itineraries of patients with ulcerated forms of Mycobacterium ulcerans (Buruli ulcer) disease in a rural health zone in the Democratic Republic of Congo. Trop Med Int Health. 2009;14:1110–1116. doi: 10.1111/j.1365-3156.2009.02324.x. [DOI] [PubMed] [Google Scholar]
- 21.Silva MT, Portaels F, Pedrosa JR. Pathogenetic mechanisms of the intracellular parasite Mycobacterium ulcerans leading to Buruli ulcer. Lancet Infect Dis. 2009;9:699–710. doi: 10.1016/S1473-3099(09)70234-8. [DOI] [PubMed] [Google Scholar]
- 22.Meyers WM. In: Tropical Pathology. Second edition. Doerr W, Seifert G, editors. Berlin: Springer-Verlag; 1995. pp. 291–377. (Mycobacterial infections of the skin). [Google Scholar]
- 23.World Health Organization . In: Buruli Ulcer: Prevention of Disability. Lehman L, Simonet V, Saunderson P, Agbenorku P, editors. Geneva: World Health Organization; 2006. [Google Scholar]
- 24.Connor DH, Lunn HF. Buruli ulceration. Arch Pathol. 1966;81:183–199. [Google Scholar]
- 25.Reid IS. Mycobacterium ulcerans infection: a report of 13 cases at the Port Moresby General Hospital, Papua. Med J Aust. 1967;1:427–431. doi: 10.5694/j.1326-5377.1967.tb21363.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith JH. Epidemiologic observations on cases of Buruli ulcer seen in a hospital in the Lower Congo. Am J Trop Med Hyg. 1970;19:657–663. doi: 10.4269/ajtmh.1970.19.657. [DOI] [PubMed] [Google Scholar]
- 27.Barker DJ. Buruli disease in a district of Uganda. J Trop Med Hyg. 1971;74:260–264. [PubMed] [Google Scholar]
- 28.The Uganda Buruli Group Epidemiology of Mycobacterium ulcerans infection (Buruli ulcer) at Kinyara, Uganda. Trans R Soc Trop Med Hyg. 1971;65:763–775. doi: 10.1016/0035-9203(71)90090-3. [DOI] [PubMed] [Google Scholar]
- 29.Barker DJ. The distribution of Buruli disease in Uganda. Trans R Soc Trop Med Hyg. 1972;66:867–874. doi: 10.1016/0035-9203(72)90121-6. [DOI] [PubMed] [Google Scholar]
- 30.Meyers WM, Connor DH, McCullough B, Bourland J, Moris R, Proos L. Distribution of Mycobacterium ulcerans infection in Zaïre, including the report of new foci. Ann Soc Belg Med Trop. 1974;54:147–157. [PubMed] [Google Scholar]
- 31.Oluwasanmi JO, Solankee TF, Olurin EO, Itayemi SO, Alabi GO, Lucas AO. Mycobacterium ulcerans (Buruli) skin ulceration in Nigeria. Am J Trop Med Hyg. 1976;25:122–128. doi: 10.4269/ajtmh.1976.25.122. [DOI] [PubMed] [Google Scholar]
- 32.Ravisse P. L'ulcère cutané à Mycobacterium ulcerans au Cameroun. I. Etude clinique, épidémiologique et histologique. Bull Soc Pathol Exot. 1977;70:109–124. [PubMed] [Google Scholar]
- 33.Van der Werf TS, Van der Graaf WT, Groothuis DG, Knell AJ. Mycobacterium ulcerans infection in Ashanti region, Ghana. Trans R Soc Trop Med Hyg. 1989;83:410–413. doi: 10.1016/0035-9203(89)90521-x. [DOI] [PubMed] [Google Scholar]
- 34.Darie H, Le Guyadec T, Touze JE. Aspects épidémiologiques et cliniques de l'ulcère de Buruli en Côte d'Ivoire. A propos de 124 observations récentes. Bull Soc Pathol Exot. 1993;86:272–276. [PubMed] [Google Scholar]
- 35.Josse R, Guédénon A, Aguiar J, Anagonou S, Zinsou C, Prost C, Foundohou J, Touze JE. L'ulcère de Buruli, une pathologie peu connue au Bénin. A propos de 227 cas. Bull Soc Pathol Exot. 1994;87:170–175. [PubMed] [Google Scholar]
- 36.Addo HA. Mycobacterium ulcerans infection (Buruli ulcer) in Ga District of Greater Accra Region. Ghana Med J. 1995;29:595–602. [Google Scholar]
- 37.Aujoulat I, Huguet-Ribas MP, Koïta Y. L'ulcère de Buruli: un problème de santé publique méconnu, appelant une mobilisation internationale. Dev Sante. 1996;125:22–30. [Google Scholar]
- 38.Aguiar J, Domingo MC, Guédénon A, Meyers WM, Stenou C, Portaels F. L'ulcère de Buruli, une maladie mycobactérienne importante et en recrudescence au Bénin. Bull Seances Acad R Sci Outre Mer. 1997;3:325–356. [Google Scholar]
- 39.Asiedu K, Etuaful S. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am J Trop Med Hyg. 1998;59:1015–1022. doi: 10.4269/ajtmh.1998.59.1015. [DOI] [PubMed] [Google Scholar]
- 40.Ouoba K, Sano D, Traoré A, Ouédraogo R, Sakandé B, Sanou A. Les ulcères cutanés à Mycobacterium ulcerans au Burkina Faso: à propos de six observations et revue de la littérature. Nouv Dermatol. 1998;17:358–362. [Google Scholar]
- 41.Amofah G, Bonsu F, Tetteh C, Okrah J, Asamoa K, Asiedu K, Addy J. Buruli ulcer in Ghana: results of a national case search. Emerg Infect Dis. 2002;8:167–170. doi: 10.3201/eid0802.010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schierle HP, Lemperle G, Erdmann D. The Buruli type ulcer. Plast Reconstr Surg. 2002;109:2608. doi: 10.1097/00006534-200206000-00088. [DOI] [PubMed] [Google Scholar]
- 43.Kibadi K, Tsakala M, Mputu-Yamba JB, Muyembe T, Kashongwe M, Imposo B, Nsiala A. L'ulcère de Buruli chez les réfugiés angolais des sites de Kimpese, Bas-Congo, RD Congo. Sante. 2003;13:39–41. [PubMed] [Google Scholar]
- 44.James K, Attipou KK, James YE, Blakime M, Tignokpa N, Abete B. L'ulcère de Buruli au Togo: à propos d'une enquête hospitalière. Cah Santé. 2003;13:43–47. [PubMed] [Google Scholar]
- 45.Hospers IC, Wiersma IC, Dijkstra PU, Stienstra Y, Etuaful S, Ampadu EO, van der Graaf WT, van der Werf TS. Buruli ulcer in Ghana: results of a national case search. Distribution of Buruli ulcer lesions over body surface area in a large case series in Ghana: uncovering clues for mode of transmission. Trans R Soc Trop Med Hyg. 2005;3:196–201. doi: 10.1016/j.trstmh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Raghunathan PL, Whitney EA, Asamoa K, Stienstra Y, Taylor TH, Jr, Amofah GK, Ofori-Adjei D, Dobos K, Guarner J, Martin S, Pathak S, Klutse E, Etuaful S, van der Graaf WT, van der Werf TS, King CH, Tappero JW, Ashford DA. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case-control study in Ghana. Clin Infect Dis. 2005;40:1445–1453. doi: 10.1086/429623. [DOI] [PubMed] [Google Scholar]
- 47.Johnson PD, Azuolas J, Lavender CJ, Wishart E, Stinear TP, Hayman JA, Brown L, Jenkin GA, Fyfe JA. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis. 2007;11:1653–1660. doi: 10.3201/eid1311.061369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pouillot R, Matias G, Wondje CM, Portaels F, Valin N, Ngos F, Njikap A, Marsollier L, Fontanet A, Eyangoh S. Risk factors for Buruli ulcer: a case-control study in Cameroon. PLoS Negl Trop Dis. 2007;1:e101. doi: 10.1371/journal.pntd.0000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


