Abstract
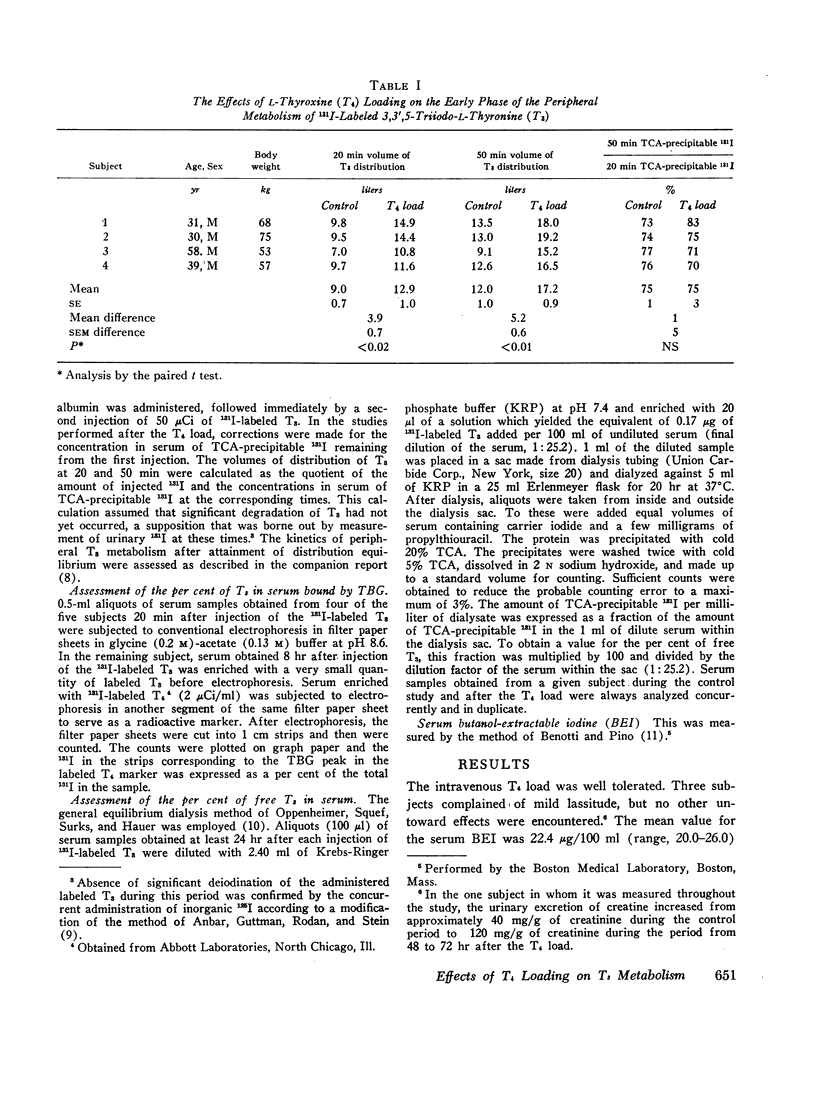
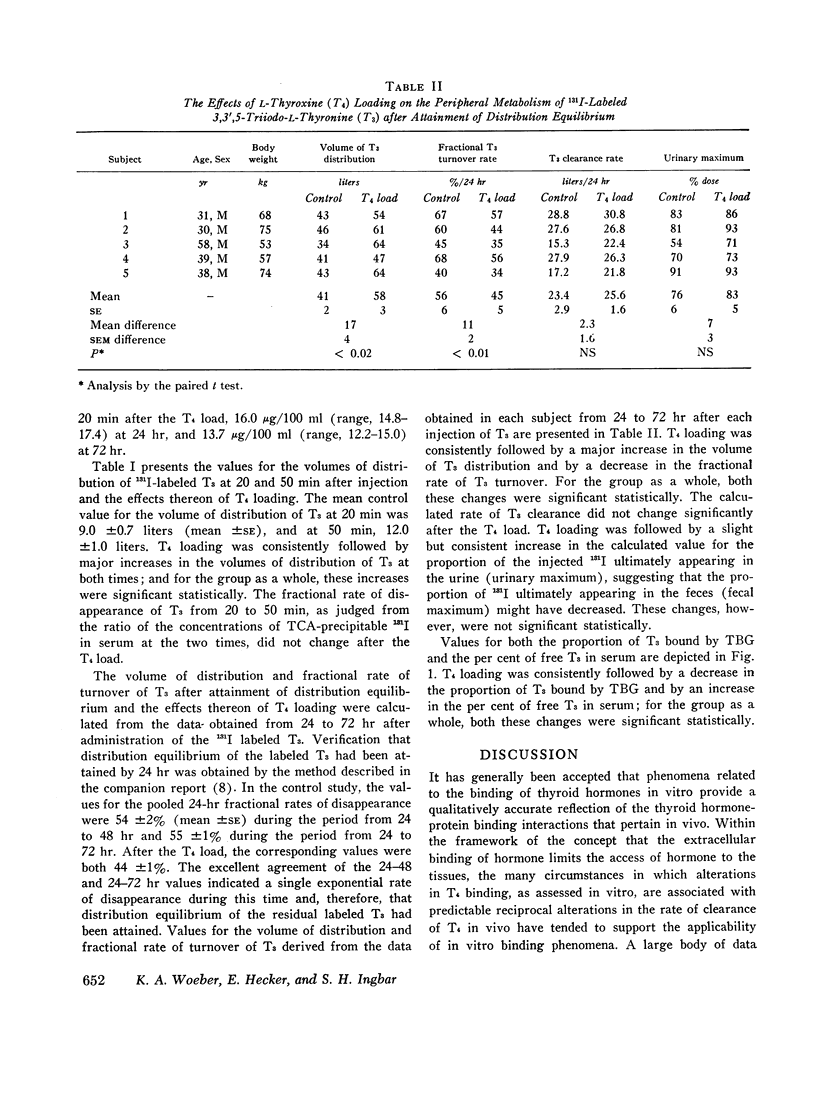
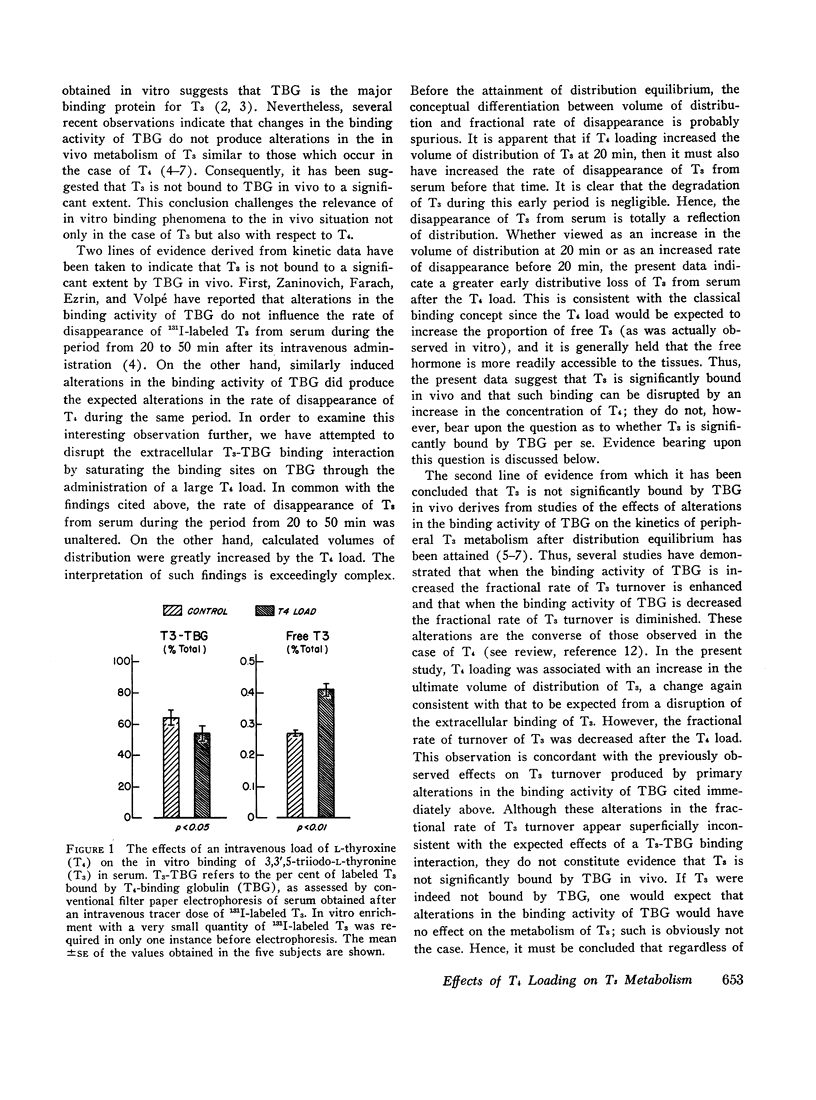
In order to examine the question of whether thyroxine-binding globulin (TBG) influences significantly the peripheral metabolism of 3,3′,5-triiodo-L-thyronine (T3) in vivo, paired studies of the effects of a large intravenous load of L-thyroxine (T4) on the kinetics of 131I-labeled T3 metabolism were carried out in five normal subjects. After the T4 load, both the early distributive loss of labeled T3 from serum and the volume of T3 distribution, observed after distribution equilibrium had been attained, were greatly increased. These alterations were consistent with those to be expected from displacement of T3 from its extracellular binding sites. After the T4 load, however, the fractional rate of T3 turnover was decreased. This finding is ascribed either to competition between T3 and T4 for common intracellular pathways of degradation or excretion or to displacement of T3 from sites of more rapid to sites of less rapid metabolism. These effects of alterations in the binding activity of TBG on the peripheral metabolism of T3, together with those previously reported by others, are consistent with the interpretation that T3 is significantly bound by TBG in vivo. However, it is suggested that the effects of alterations in the T3-TBG binding interaction on the metabolism of T3 are obscured by alterations in the extracellular-cellular partitioning of T4 that would result from concurrent alterations in T4-binding by TBG.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anbar M., Guttmann S., Rodan G., Stein J. A. The determination of the rate of deiodination of thyroxine in human subjects. J Clin Invest. 1965 Dec;44(12):1986–1991. doi: 10.1172/JCI105305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAVERMAN L. E., INGBAR S. H. BINDING OF 3,5,3' -L-TRIIODOTHYRONINE IN HUMAN SERUM DURING AGAR GEL ELECTROPHORESIS AT PH 7.4. Endocrinology. 1965 Mar;76:547–549. doi: 10.1210/endo-76-3-547. [DOI] [PubMed] [Google Scholar]
- Benotti J., Pino S. A simplified method for butanol-extractable iodine and butanol-insoluble iodine. Clin Chem. 1966 Aug;12(8):491–496. [PubMed] [Google Scholar]
- Cavalieri R. R., Searle G. L. The kinetics of distribution between plasma and liver of 131-I-labeled L-thyroxine in man: observations of subjects with normal and decreased serum thyroxine-binding globulin. J Clin Invest. 1966 Jun;45(6):939–949. doi: 10.1172/JCI105409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGBAR S. H., FREINKEL N. Regulation of the peripheral metabolism of the thyroid hormones. Recent Prog Horm Res. 1960;16:353–403. [PubMed] [Google Scholar]
- Ingbar S. H., Braverman L. E., Dawber N. A., Lee G. Y. A new method for measuring the free thyroid hormone in human serum and an analysis of the factors that influence its concentration. J Clin Invest. 1965 Oct;44(10):1679–1689. doi: 10.1172/JCI105275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL M. L., BRADFORD A. H., COLLINS S. DIFFERENCES IN THE INTERACTION OF TRIIODOTHYRONINE-131-I WITH SERUM PROTEINS IN VITRO. J Clin Endocrinol Metab. 1964 Sep;24:867–874. doi: 10.1210/jcem-24-9-867. [DOI] [PubMed] [Google Scholar]
- Nicoloff J. T., Dowling J. T. Studies of peripheral thyroxine distribution in thyrotoxicosis and hypothyroidism. J Clin Invest. 1968 Sep;47(9):2000–2015. doi: 10.1172/JCI105887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SQUEF R., SURKS M. I., HAUER H. BINDING OF THYROXINE BY SERUM PROTEINS EVALUATED BY EQUILIBRUM DIALYSIS AND ELECTROPHORETIC TECHNIQUES. ALTERATIONS IN NONTHYROIDAL ILLNESS. J Clin Invest. 1963 Nov;42:1769–1782. doi: 10.1172/JCI104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Bernstein G., Hasen J. Estimation of rapidly exchangeable cellular thyroxine from the plasma disappearance curves of simultaneously administered thyroxine-131-I and albumin-125-I. J Clin Invest. 1967 May;46(5):762–777. doi: 10.1172/JCI105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H. Role of plasma proteins in the binding, distribution and metabolism of the thyroid hormones. N Engl J Med. 1968 May 23;278(21):1153–1162. doi: 10.1056/NEJM196805232782107. [DOI] [PubMed] [Google Scholar]
- Woeber K. A., Sobel R. J., Ingbar S. H., Sterling K. The peripheral metabolism of triiodothyronine in normal subjects and in patients with hyperthyroidism. J Clin Invest. 1970 Apr;49(4):643–649. doi: 10.1172/JCI106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninovich A. A., Farach H., Ezrin C., Volpé R. Lack of significant binding of L-triiodothyronine by thyroxine-binding globulin in vivo as demonstrated by acute disappearance of 131-I-labeled triiodothyronine. J Clin Invest. 1966 Aug;45(8):1290–1301. doi: 10.1172/JCI105436. [DOI] [PMC free article] [PubMed] [Google Scholar]


