Abstract
Exercise has been shown to impact brain plasticity and function by involving the action of BDNF; however, mechanisms involved are poorly understood. Two types of BDNF coexist in the brain, the precursor (proBDNF) and its mature product (mBDNF), which preferentially bind specific receptors and exert distinct functions. It is crucial to understand how exercise affects crucial steps in the BDNF processing and signaling to evaluate therapeutic applications. We found that 7 days of voluntary exercise increased both pro and mature BDNF in the rat hippocampus. Exercise also increased the activity of tissue-type plasminogen activator (tPA), a serine proteinase shown to facilitate proBDNF cleavage into mBDNF. The blockade of tPA activity reduced the exercise effects on proBDNF and mBDNF. The tPA blocking also inhibited the activation of TrkB receptor, and the TrkB signaling downstream effectors phospho-ERK, phospho-Akt, and phospho-CaMKII. The blocking of tPA also counteracted the effects of exercise on the plasticity markers phospho-synapsin I and GAP-43. These results indicate that the effects of exercise on hippocampal plasticity are dependent on BDNF processing and subsequent TrkB signaling, with important implications for neuronal function.
Keywords: Brain-derived neurotrophic factor, hippocampus, rat, signaling, synaptic plasticity
Introduction
The ability of voluntary exercise and brain-derived neurotrophic factor (BDNF) to enhance brain plasticity and function has received abundant experimental support (Vaynman et al., 2004, Soya et al., 2007, Chytrova et al., 2008). The synthesis of the mature form of BDNF (mBDNF) involves proteolytic cleavage of a precursor stage or proBDNF (Pang and Lu, 2004). Recent evidence indicates that the pro and mature forms of BDNF bind separate receptors and play distinctive roles in the brain (Lee et al., 2001, Lu, 2003, Pang et al., 2004, Teng et al., 2005, Woo et al., 2005). Mature BDNF activates TrkB receptor to promote survival, growth and differentiation of select neuronal types. In addition, mBDNF is a strong facilitator of the synapse, acting on systems involved with intracellular signaling and synaptic transmission (Pang et al., 2004, Keifer et al., 2009). In contrast, proBDNF binds p75NTR receptor and its action has been related to neuronal cell death and synaptic withdrawal (Lee et al., 2001, Lu, 2003, Teng et al., 2005, Woo et al., 2005). Although the action of exercise on BDNF-related synaptic plasticity is well established, the influence of BDNF processing and signaling is poorly understood. This information is crucial to properly understand how the action of a multiple signal task such as exercise can influence specific aspects of brain plasticity and function.
The serine protease tissue-type plasminogen activator (tPA) is widely distributed in the CNS, including the hippocampus (Qian et al., 1993, Salles and Strickland, 2002), and its action is fundamental for BDNF processing. The enzymatic action of tPA is effective for converting the inactive precursor plasminogen to plasmin (Plow et al., 1995). In turn, plasmin cleaves the proBDNF to mBDNF (Pang and Lu, 2004, Pang et al., 2004). Neuronal activity stimulates the release of tPA from axonal terminals (Gualandris et al., 1996), and tPA has been shown to be crucial for supporting hippocampal synaptic plasticity (Lu, 2003). The overall information implies that tPA critically contributes to the modulation of BDNF function on synaptic plasticity. Accordingly, the purpose of this study is to determine whether exercise can use tPA to regulate the processing of BDNF in the hippocampus. Given the preponderant role of BDNF as a modulator of synaptic plasticity, we have also evaluated the possibility that tPA action can serve to modulate BDNF-related plasticity during exercise.
Exercise has been shown to affect the expression of select molecular system related to the action of BDNF on synaptic plasticity. Synapsin I is a nerve terminal protein involved in neurotransmitter release, axonal elongation and maintenance of synaptic contacts (Brock and O'Callaghan, 1987, Wang et al., 1995). Its synthesis (Wang et al., 1995) and phosphorylation (Jovanovic et al., 2000) are affected by BDNF. Growth associated protein 43 (GAP-43) is present in growing axon terminals and has also been implicated in learning and memory (Routtenberg et al., 2000). The Akt (Yoshii and Constantine-Paton, 2007) and CaMKII signaling systems (Vaynman et al., 2007) are involved in BDNF-related hippocampal synaptic plasticity with important effects on learning and memory. Accordingly, the current study designed to assess the effects of exercise on BDNF processing integrates the action of BDNF on the synaptic plasticity markers synapsin I, GAP-43, and CaMKII. Results support an involvement of tPA in the effects of exercise on BDNF processing and synthesis, and suggest that tPA action is important for regulation of synaptic plasticity.
Materials and methods
The study was designed to assess the effects of the tPA activity inhibitor tPA-STOP on BDNF processing in the hippocampus of rats exposed to exercise. Twenty-eight adult male Sprague–Dawley rats (200–220g) (Charles River laboratories, Inc, Wilmington, MA, USA) were randomly assigned into four groups, i.e., sedentary/vehicle control (SC), exercise/vehicle control (EC), sedentary/tPA-STOP (ST) and exercise/tPA-STOP (ET). All animals were housed individually in standard polyethylene cages in a 12/12 h light/dark cycle at 22–24 °C, with food and water available ad libitum. The experiments were performed in accordance with the United States National Institutes of Health Guild for the Care and Use of Laboratory Animals. All animals were continually monitored, and the experimental procedures were approved by the UCLA Chancellor’s Animal Research Committee.
Exercise paradigm
The exercise rats were given access to a wheel (diameter=31.8cm, width=10 cm) that freely rotated against a resistance of 100 g, attached to a receiver that monitored revolutions (VitalViewer Data Acquisition System software (MiniMitter Inc., Sunriver, OR, USA). The sedentary rats were confined to a cage with no access to a running wheel. After 7 days of exercise, animals were killed by decapitation the morning following the last treatment day and their hippocampi were rapidly dissected out, immediately placed on dry ice, and stored at −70 °C for protein measurements.
Injection of tPA-STOP into the hippocampus
We used tPA-STOP to block tPA activity as described in previous studies (Pawlak et al., 2002, Liot et al., 2004, Pawlak et al., 2005, Kozai et al., 2007). According to Pawlak et al’s study (2002), 6.3 nmole of tPA-STOP infused into the hippocampus (within 2 hours) could affect mouse learning ability as early as 90 min and such effect lasted as long as 7 days. In this study, tPA-STOP was slowly injected into the rat hippocampus at a single dose of 7.5 nmole. All surgeries and injections were performed under aseptic conditions. Animals were anaesthetized with isoflurane (2–2.5%, Mobile Laboratory Animal), and positioned in a stereotaxic apparatus. tPA-STOP (3.75 nmole/µl, 2µl each side; American Diagnositca Inc. Stamford, CT, USA) or physiological saline (vehicle control) was bilaterally injected into the hippocampus (3.8 mm posterior to Bregma, 2.6 mm from the midline, and 3.7 mm vertically) using a Hamilton syringe over a 8 min period for each side (Ding et al., 2006). Animals were returned to the cages with running wheels the same day after the injection.
Protein preparation
The right hippocampus was homogenized in lysis buffer A (100mM Tris, 2mM EDTA, 1% TritonX-100, pH7.6) for tPA activity measurement, The left hippocampus was homogenized in lysis buffer B (137 mM NaCl, 20mM Tris, 1% NP40, 10% glycerol, 1mM PMSF, 10 µg/ml aprotinin, 1 µg/ml leupeptin, 0.5mM sodium vanadate, pH8.0) for western blot analysis. Tissue was homogenized in freshly prepared lysis buffer (1:10 w/v) and centrifuged at 12,000g for 20 min. The supernatants were collected, aliquoted and stored in −70°C. The total protein concentration of hippocampal homogenates was determined with MicroBCA kit (Pierce, Rockford, IL, USA), using BSA as a standard.
tPA activity determination
We measured tPA activity in brain extracts with an amidolytic assay that detects the activation of plasminogen to plasmin, by assessing plasmin activity on the synthetic substrate suc-Val-Leu-Lys-pNA (S-2251) (Festoff et al., 1994). To 50 µl of brain extracts, we added 50 µl of S-2251 (150 µg/ml) and 50 µl of Glu-plasminogen (45 µg/ml) which were diluted in 0.1 M sodium phosphate (10 mM EDTA, 0.01% NaN3, 0.01% Triton X-100, pH 7.3). The samples were incubated at 37°C in flat-bottomed microtiter plates (Imulon II; Dynatech, Arlington, VA, USA) and monitored the release of pNA in each well at 405 nm with a micro-ELISA auto reader (HTS 7000 Plus, Perkin Elmer). Each sample was measured in duplicate. tPA enzymatic activity was measured based on the initial change rate of OD then normalized by protein concentration.
Western blot
Aliquots containing an equal amount of hippocampal protein extracts (25 µg) were mixed with gel loading buffer and separated on 10% SDS-polyacrylamide gels. After electrophoresis, the proteins were transferred onto PVDF membranes and nonspecific binding was blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween-20 (TBST). Membranes were incubated with the following primary antibodies. Anti-BDNF, anti-tPA, anti-pAkt, anti-pERK, anti-pCaMKII and anti-GAP-43 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-pTrkB (PY20) was from BD Biosciences (San Diego, CA, USA). Anti-pSynapsin I was from Cell Signaling (Danvers, MA, USA). After incubation with the primary antibodies, membranes were washed with TBST and incubated with appropriate horse radish peroxidase (HRP)-conjugated secondary antibodies (1:4000), Immunocomplexes were visualized by chemiluminescence using the ECL kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) according to the manufacturer’s instructions. The film signals were digitally scanned and then quantified using NIH imageJ software.
Statistical analysis
The mean values for protein/activity levels between different treated groups were compared with using two-way analysis of variance (ANOVA) (exercise and drug). A Tukey’s range test was used for between-group comparisons. The correlation between tPA/mBDNF and running distance was analyzed by linear regression analysis. Results were expressed as the mean percent of control values for graphic clarity and represent the mean ± standard error of the mean (SEM). Statistical differences were considered significant where p< 0.05(*), p<0.01(**), or p<0.001(***), n=7/group for tPA activity measurement and n=6/group for protein measurement.
Results
tPA function blocking counteracts the exercise effects on pro and mature BDNF (Figure 1)
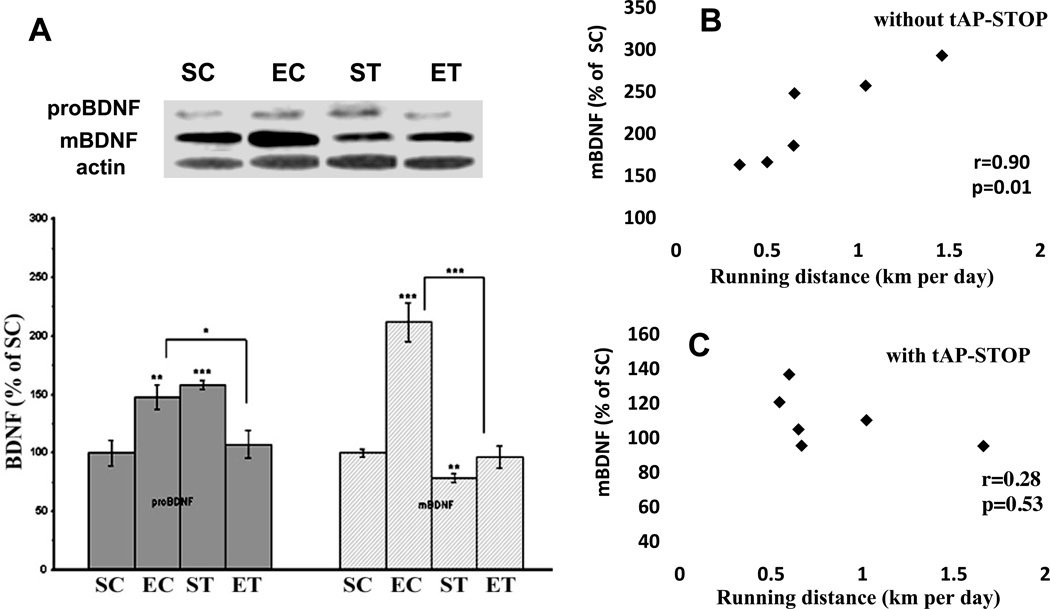
Figure 1.
(A) Effects of exercise and tPA-STOP on the levels of proBDNF and mature BDNF in the rat hippocampus. Exercise increased both pro BDNF and mature BDNF (EC group). These increases were abolished by tPA-STOP (ET group). In the sedentary rats, tPA-STOP increased proBDNF and decreased mature BDNF (ST group). B. There was a positive correlation between running distance and mBDNF (r=0.90, p=0.01). This correction was disrupted after tPA-STOP administration (r=0.28, p=0.53) (C).
SC: Sedentary/Control; EC: Exercise/Control; ST: Sedentary/tPA-STOP; ET: Exercise/tPA-STOP. Results are expressed as means ± S.E.M. *p<0.05, **p<0.01, ***p<0.001; two-way ANOVA (exercise and drug) followed by Tukey’s range test, n=6/group.
Animals were randomly divided into four groups: sedentary/control (SC), exercise/control (EC), sedentary plus tPA-STOP (ST) and exercise plus tPA-STOP (ET). Two-way ANOVA analysis indicated that there was a significant interaction between drug and exercise in terms of proBNDF (32kDa) level (F(1,20)=22.7; p=1.1E-4) and mBDNF(14kDa) level (F(1,20)=29.0, p=2.9E-04). A Tukey’s range test showed that exercise (EC) increased levels of proBDNF to 148%, (p=0.005) and mBDNF to 211% (p=1.2E-05) compared to the SC group (Fig 1A). Also, a positive correlation was observed between mature BDNF and running distance (Fig 1B). These changes were abolished by the hippocampal infusion of tPA-STOP, such that proBDNF levels were reduced from 148% to 107% (p=0.02), and mBDNF levels from 211% to 96% (mBDNF, p=3.2E-05) (Fig.1A), and the correlation between mature BDNF and running distance was also disrupted (Fig. 1C). In the sedentary rats, tPA-STOP treatment decreased mBDNF levels to 79% (p=0.002), but increased proBDNF levels to 158% (p=5.6E-04) of untreated SC animals (ST vs SC, Fig. 1A). The levels of Truncated BDNF (28kDa) were also analyzed and no significant differences were found between any groups (data not shown).
tPA function blocking abolished the exercise-induced increase in tPA activity (Figure 2)
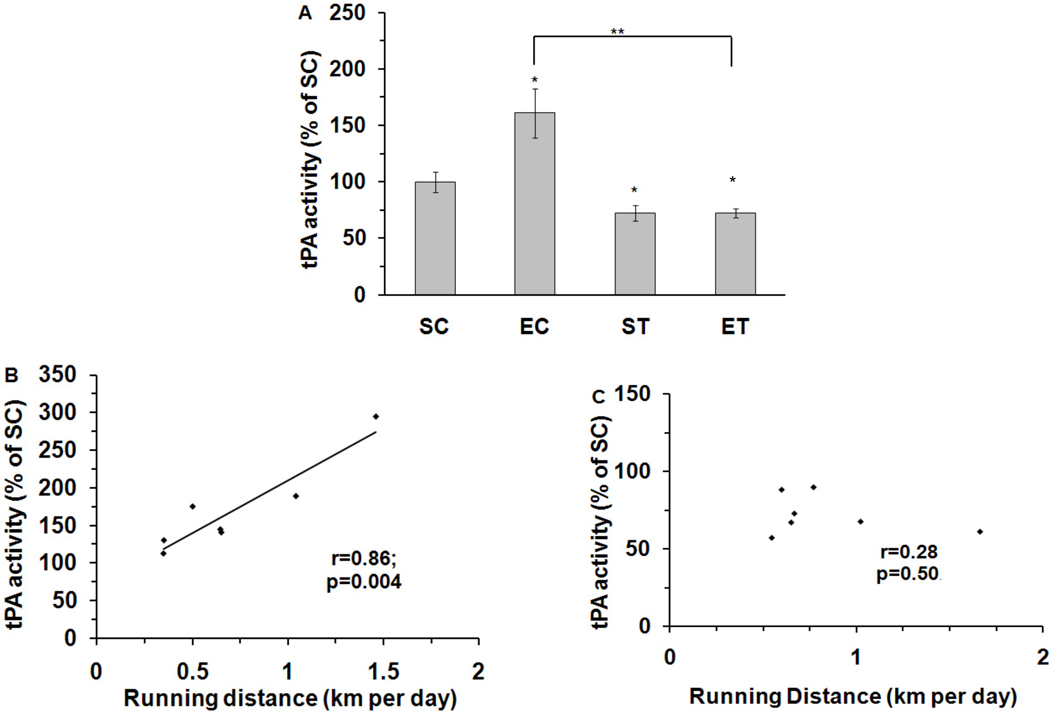
Figure 2.
Effects of exercise and tPA-STOP on tPA activity levels in the rat hippocampus. (A). tPA-STOP completely abrogated exercise-increased tPA activity (EC: 161%; ET: 72%). Its activity was also inhibited by tPA-STOP in the sedentary group (ST: 72%). (B). There was a positive correlation between running distance and tPA activity in EC animals (r=0.86, p=0.004). (C). This correction was disrupted after tPA-STOP administration (r=0.28, p=0.50, ET group). SC: Sedentary/Control; EC: Exercise/Control; ST: Sedentary/tPA-STOP; ET: Exercise/tPA-STOP. Results are expressed as means ± S.E.M. *p<0.05, **p<0.01, two-way ANOVA followed by Tukey’s range test, n=7/group.
Two-way ANOVA analysis for the tPA activity level showed a significant effect for both exercise (F(1, 24)=8.6, p=0.008) and tPA stop (F(1,24)=37.7, p=5.3E-06), and there was a significant interaction between exercise and drug (F(1,24)=8.4, p=0.009). A Tukey’s range test showed that exercise elevated tPA enzymatic activity to 160% of SC values (p=0.02). The injection of tPA-STOP to the hippocampus reduced the exercise-induced increase in tPA activity from 160% to 72% (p=0.001). The application of tPA-STOP also reduced tPA activity to 72% in ST animals (p=0.03,) compared to untreated SC animals (Fig. 2A). Furthermore, a significant positive correlation was observed between running distance and tPA activity (r=0.86; p=0.004; Fig. 2B), and this correlation was abolished by the application of tPA-STOP (r=0.28, p=0.50) (Fig. 2C).
tPA function blocking counteracts the effects of exercise on BDNF signaling (Figure 3)
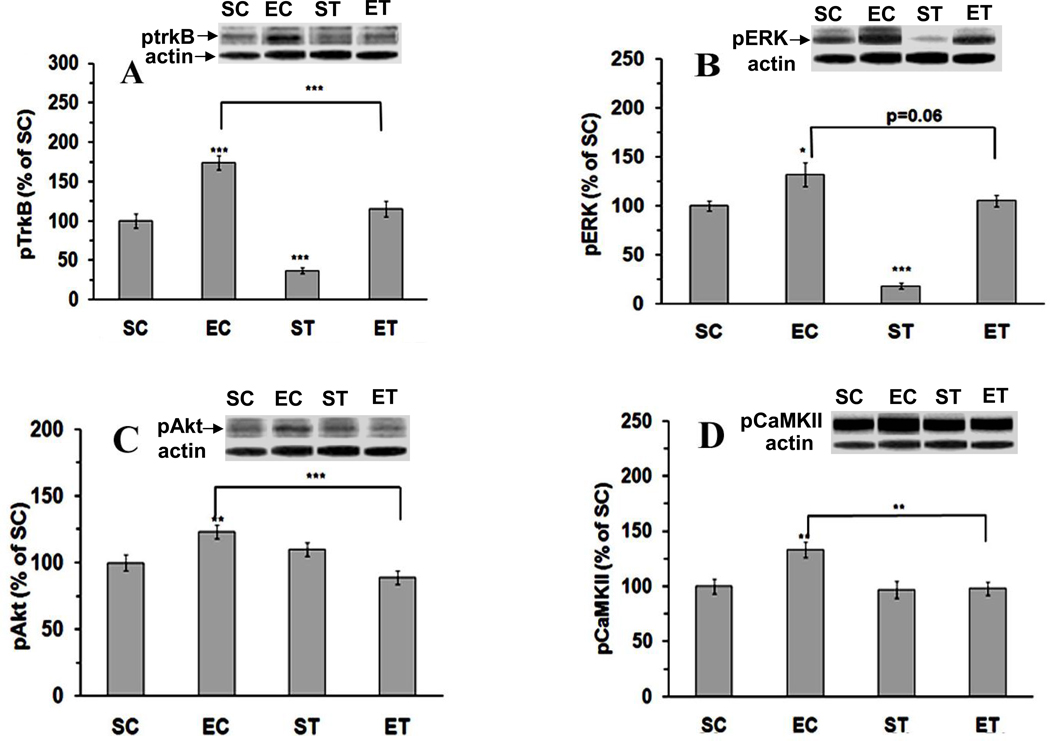
Figure 3.
Exercise-activated pTrkB (A), pERK (B), pAkt (C) and pCaMKII (D) were abrogated by tPA-STOP. SC: Sedentary/Control; EC: Exercise/Control; ST: Sedentary/tPA-STOP; ET: Exercise/tPA-STOP. Results are expressed as means ± S.E.M. *p<0.05, **p<0.01, ***p<0.001; two-way ANOVA followed by Tukey’s range test, n=6/group.
We next wanted to determine how disruptions in the BDNF processing would affect BDNF signaling events. BDNF binds TrkB receptor, activating ERK and Akt signaling systems (Frost, 2001, Yamada and Nabeshima, 2003). Two-way ANOVA showed that both exercise (F(1, 20)=61.7, p=1.5E-07) and tPA-STOP (F(1,20)=38.4, p=4.7E-06) affected TrkB phosphorylation. Likewise, pERK (exercise, F(1, 20)=49.4, p=8.1E-07; drug, F(1,20)=42.1, p=2.5E-06), pAkt (drug, F(1,20)=4.8, p=0.04), and pCaMKII (exercise, F(1,20)=4.2, p=0.05; drug, F(1,20)=5.2, p=0.03) also were affected by exercise and/or tPA-STOP. Tukey’s range test showed that exercise increased pTrkB to 174% (p=7.5E-05, EC vs SC), pERK to 132% (p=0.03), pAkt to 123% (p=0.009), and pCaMKII to133% (p=0.004) (Fig. 3A–D). In turn, tPA-STOP treatment attenuated the effects of exercise on pTrkB from 174% to 115% (p=9.6E-4, ET vs EC), pERK from 132% to 106% (p=0.06), pAkt from 123% to 89% (p=4.7E-4), and pCaMKII from 133% to 98% (p=0.002). In the sedentary/tPA-STOP animals (ST), no significant changes were observed in pCaMKII (97%) and pAkt (110%); however, pTrkB and pERK levels were decreased to 37% (p=2.0E-05) and 18% (p=5.2E-07) compared to SC group, respectively.
tPA function blocking counteracts the exercise-enhanced plasticity (Figure 4)
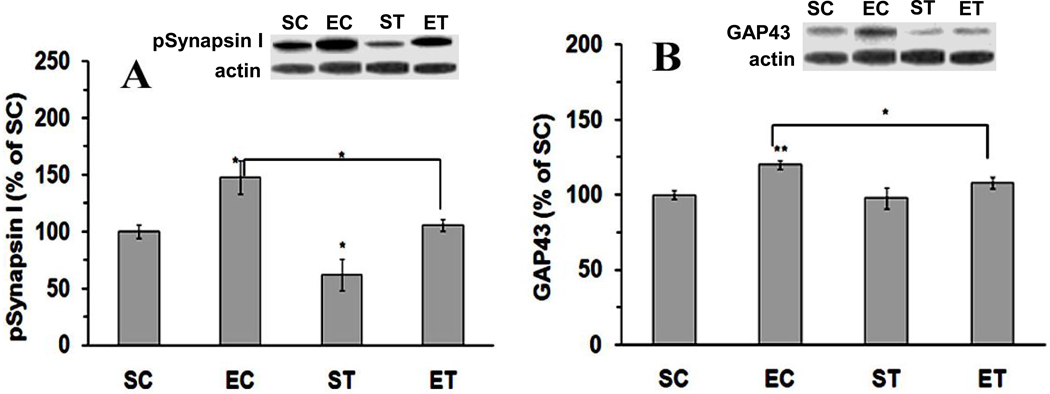
Figure 4.
Effects of tPA-STOP and exercise on neural plasticity-related molecules. The increased protein levels of both pSynapsin I (A) and GAP43 (B) were abolished with tPA-STOP administration. SC: Sedentary/Control; EC: Exercise/Control; ST: Sedentary/tPA-STOP; ET: Exercise/tPA-STOP. Results are expressed as means ± S.E.M. *p<0.05, ** p<0.01; two-factor ANOVA followed by Tukey’srange test, n=6/group.
Finally, we wanted to have an estimate of the effects of tPA function blocking on select markers of plasticity. We assessed phospho-synapsin I based on its role on synaptic function and neurotransmitter release. We assessed GAP-43 based on its role on dendrite spine formation and axonal growth. As shown in Fig. 4, both pSynapsin I (exercise, F(1,20)=13.3, p=0.001; tPA-STOP F(1,20)=10.9, p=0.004), and GAP-43 (exercise, F(1,20)=6.3, p=0.02) levels were influenced by exercise and/or tPA-STOP. We found that exercise increased levels of both pSynapsin I (148%, p=0.01; Fig. 4A) and GAP-43 (120%, p=0.002; Fig. 4B). The hippocampal injection of tPA-STOP reduced the effects of exercise on pSynapsin I from 148% in EC to 106% in ET (p=0.03), and GAP-43 levels from 120% to 108% (p=0.04). In sedentary rats, tPA-STOP reduced pSynapsin I levels (62%, p=0.03) but GAP43 levels (98%) remained unchanged.
Discussion
Based on information that BDNF is a critical intermediate for the effects of exercise on synaptic plasticity and cognition, we have investigated the possibility that the processing of BDNF into its mature form is crucial to accomplish these tasks. The processing of the mature form of BDNF (14kD) from its pro-BDNF precursor (32kD) involves the enzymatic action of tPA. Our results indicate that exercise exerts an influence on the levels of pro-BDNF, tPA, and mBDNF, and that tPA activation seems an important step for BDNF regulation during exercise. In particular, our results indicate that BDNF processing under the influence of tPA is critical for the action of exercise on synaptic plasticity. Indeed, our results showed that tPA blocking counteracted the effects of exercise on systems downstream to the action of BDNF on synaptic plasticity such as CaMKII and synapsin I. These results seem to portray a mechanism by which exercise can affect synaptic plasticity and cognition by interceding with the action of tPA.
Exercise and BDNF regulation
Our current results indicate that exercise has differential effects on the pro and mature forms of BDNF and that the function of tPA is critical for moderating levels of pro and mBDNF. According to these results, exercise elevated the tPA enzymatic activity in proportion to the distance run by individual animals. It is noteworthy that pro and mature BDNF play different and sometimes opposite roles in the brain, such that the function of BDNF has to be precisely regulated for proper neuronal function. For example, proBDNF over-accumulation may have severe consequences for neuronal plasticity and function, such as synapse withdrawal and neuronal death (Lu, 2003, Teng et al., 2005, Woo et al., 2005). In fact, our results showed that tPA activity blocking treatment elevated levels of proBDNF in sedentary animals, in conjunction with a reduction in the activated state of the synaptic marker synapsin I. Accumulation of proBDNF in the cell may occur by enhanced BDNF transcription and translation, in spite of low mature BDNF levels. According to our results, dysfunction in the tPA system may result in such case scenario with important consequences for BDNF signaling. Therefore, exercise appears as an important strategy to modulate levels of pro- and mBDNF in a homeostatic manner. Recent studies demonstrated that running wheel did not alter the level of pro-BDNF in hippocampus, but increases mature BDNF (Griesbach et al., 2009, Sartori et al., 2009, Sartori et al., 2011). The apparent discrepancy for proBDNF may be caused by different species (rat vs. mouse), timing and specifics of the exercise paradigm.
tPA modulates BDNF signaling
In the sedentary groups, it appears that tPA-STOP inhibited proBDNF conversion into mature BDNF, leading to an accumulation of proBDNF and a depletion of mBDNF. In turn, in the exercise groups, tPA activity blocking resulted in a decrease in both proBDNF and mBDNF. The reduction of both forms of BDNF in the exercise animals is suggestive that tPA blocking attenuated BDNF synthesis and processing. It is likely that tPA activity blocking also reduced BDNF signaling, as evidenced by a reduction in pTrkB levels in the sedentary and exercise rats. Given that the reduction in BDNF signaling occurred in conjunction with a decrease in pro- and mBDNF, it is possible that a reduction in BDNF signaling may have disrupted a positive feedback by which BDNF regulates its own synthesis through TrkB activation (Saarelainen et al., 2001, Yasuda et al., 2007). In support of this hypothesis, it has been previously shown that blocking of BDNF signaling by using a TrkB receptor blocker interrupted an exercise induction of BDNF mRNA in the hippocampus (Vaynman et al., 2004, Vaynman et al., 2006). Therefore, it appears that exercise by engaging the action of tPA enacts that newly-synthesized proBDNF can be converted into its mature product, resulting in TrkB signaling (Fig. 5). These results may have important implications for the regulation of the function of BDNF as our results showed that several molecular systems associated with BDNF signaling and plasticity were disrupted by the tPA blocking. Although the topic is still controversial, there are indications for the asymmetry in the normal contents of select proteins between right Vs left rat hippocampi (Samara et al., 2011). There is no reported evidence, however, about the potential of exercise to differentially influence right Vs left hippocampi. Accordingly, the fact that we measured tPA activity in a different hemisphere to the one used for protein analysis may not be critical for the final interpretation of the results.
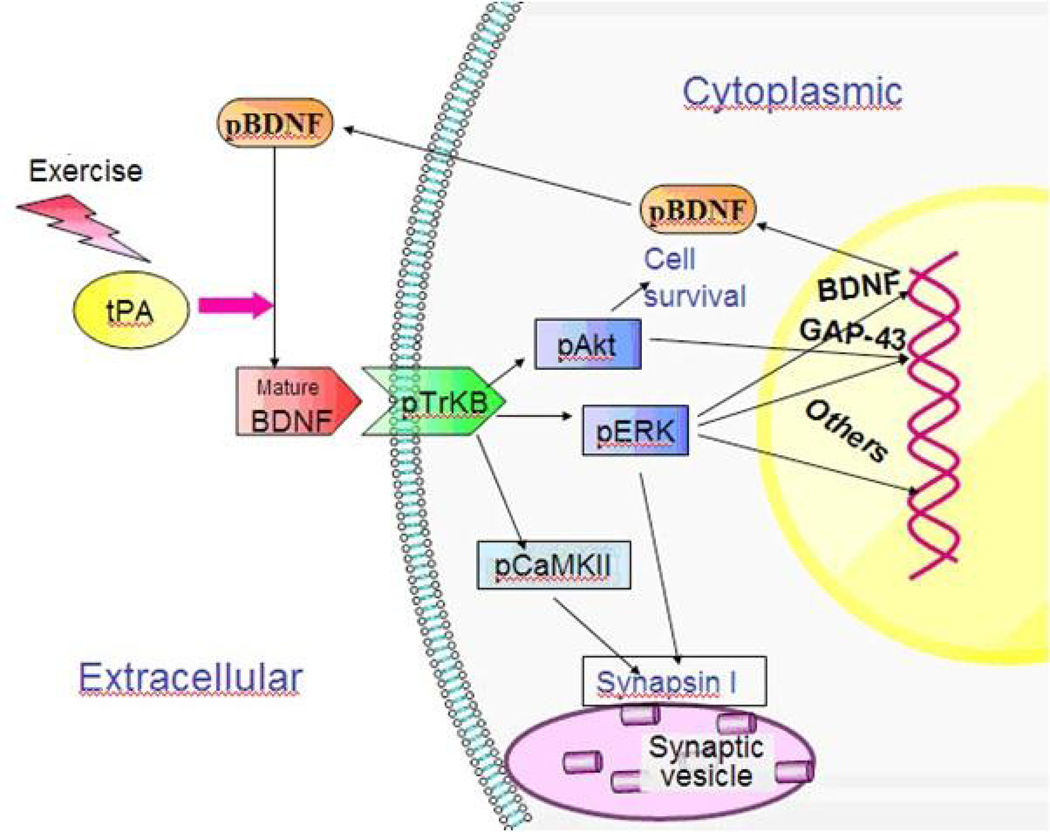
Figure 5.
A schematic diagram showing the involvement of tPA in BDNF maturation, TrkB signaling and neural plasticity in the hippocampus. Physical exercise enhances tPA activity and prompts BDNF maturation. Mature BDNF activates TrkB signaling in which several downstream effectors, including Akt, ERK and CaMKII, may be included. Phosphorlyated EKR and Akt regulate certain transcription factors and trigger gene expression and protein synthesis, such as GAP-43 and BDNF. Activated CaMKII phosphorylates synapsin I to promote neurotransmitter release.
Mature BDNF plays a critical role in exercise-enhanced neural plasticity
Our results showed that exercise elevated levels of various molecular systems involved with BDNF signaling such as pERK, pAkt, and pCaMKII. In addition, exercise also elevated levels of the synaptic marker synapsin I and the growth-associated protein GAP-43. It is significant that tPA function blocking counteracted the increases in all of these proteins, arguing in favor for an action of tPA and BDNF on the elevations of pERK, pAkt, and pCaMKII. Indeed, it has been previously shown that BDNF plays a central role in exercise-enhanced plasticity (Vaynman et al., 2003, 2004, Vaynman et al., 2006). The blocking of the function of the signaling transduction receptor for BDNF TrkB has been shown to abolish the effects of exercise on pCaMKII, with subsequent effects on learning and memory performance (Vaynman et al., 2007). CaMKII (Turner et al., 1999, Yamagata, 2003) can phosphorylate synaptic proteins such as synapin I to facilitate glutamate transmitter release. Long-term synaptic plasticity requires gene transcription and protein synthesis (such as GAP-43) in which MAPK/ERK and PI3K/Akt pathways are involved (Costa-Mattioli et al., 2009)(Fig. 5). Although tPA abolished the increase of GAP-43 in exercise rats, it did not change GAP-43 levels in the sedentary rats. This may be explained by taking into consideration the function of GAP-43, which seems more prevalent during challenging situations involving axonal growth.
These results are significant to define the importance of BDNF processing for controlling BDNF signaling and function. In particular, based on the demonstrated capacity of exercise to influence synaptic plasticity and cognition, it is highly significant that these events may be regulated by tPA action. Indeed, it has been described that tPA is important for regulation of long-term potentiation and memory formation (Pang and Lu, 2004, Pang et al., 2004), in which BDNF is also involved. In addition, it is significant that the regulatory action of tPA during exercise has also been associated with the antidepressive effects of exercise and BDNF (Sartori et al., 2011).
It should be noted that tPA has multiple functions in the central nervous system which are indirectly associated with the function of BDNF. For example, tPA has been shown to affect NMDA receptors cleavage and function (Nicole et al., 2001, Matys and Strickland, 2003), with resulting effects on enhancing glutamate transmission (Mali et al., 2005). It can be noted that regulation of glutamatergic neurotransmission is also under the spectrum of action of BDNF (Ferrero and Cereseto, 2004). The action of tPA has also been associated with the reduction of apoptotic cell death in models of cerebral ischemia (Flavin and Zhao, 2001, Liot et al., 2006). Indeed, tPA has gained recognition as a strong therapeutic agent for the treatment of brain ischemic injury such as stroke (Turley et al., 2005, Deb et al., 2010). Given the pro-apoptotic action of pro-BDNF and the protective action of mBDNF, it is plausible that the protective action of tPA on stroke can be related to an stimulation of BDNF processing. Furthermore, tPA is a strong promoter of the late phase of LTP, in which BDNF has been shown to be an important player; tPA-cleaved NMDA receptor and subsequently activated signaling may also mediate LTP (Centonze et al., 2002). Last but not least, tPA also binds to low density lipoprotein receptor-related protein-1 (LRP-1), which has been implicated in hippocampal LTP formation (Zhuo et al., 2000). These considerations imply that exercise may have an influence in several of the functions associated with tPA in the brain, and more studies are required in this fertile area of research.
Conclusions and Significance
In summary, we found that voluntary wheel running can enhance BDNF maturation by acting on tPA. These events seem important for regulation of TrkB signaling, with potential benefits for neural plasticity. Based on the multiple effects of BDNF in the brain, it seems crucial to maintain its levels within a homeostatic range. For example, given the apoptotic affects of proBDNF, its accumulation in the brain can be troublesome. Exercise appears to act as an excellent homeostatic regulator of BDNF processing and function with important implications for brain plasticity. Given the important actions of tPA and BDNF in the brain, our results are fundamental to understand how exercise plays its multiple beneficial actions on brain plasticity. In particular, our studies show that the action of exercise on BDNF processing is important for regulation of neuronal signaling and synaptic plasticity.
Acknowledgements
This work was supported by National Institutes of Health Grant NS50465 and NS56413.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- ERK
extracellular-signal related kinase
- GAP-43
Growth Associated Protein 43
- tPA
tissue-type plasminogen activator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Brock TO, O'Callaghan JP. Quantitative changes in the synaptic vesicle proteins synapsin I and p38 and the astrocyte-specific protein glial fibrillary acidic protein are associated wwith chemical-induced injury to the rat central nervous system. J Neurosci. 1987;7:931–942. doi: 10.1523/JNEUROSCI.07-04-00931.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Napolitano M, Saulle E, Gubellini P, Picconi B, Martorana A, Pisani A, Gulino A, Bernardi G, Calabresi P. Tissue plasminogen activator is required for corticostriatal long-term potentiation. Eur J Neurosci. 2002;16:713–721. doi: 10.1046/j.1460-9568.2002.02106.x. [DOI] [PubMed] [Google Scholar]
- Chytrova G, Ying Z, Gomez-Pinilla F. Exercise normalizes levels of MAG and Nogo-A growth inhibitors after brain trauma. Eur J Neurosci. 2008;27:1–11. doi: 10.1111/j.1460-9568.2007.05982.x. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17:197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Ferrero A, Cereseto M. Glutamatergic neurotransmission, depression and antidepressants. Vertex. 2004;15:91–98. [PubMed] [Google Scholar]
- Festoff BW, Reddy RB, VanBecelaere M, Smirnova I, Chao J. Activation of serpins and their cognate proteases in muscle after crush injury. J Cell Physiol. 1994;159:11–18. doi: 10.1002/jcp.1041590103. [DOI] [PubMed] [Google Scholar]
- Flavin MP, Zhao G. Tissue plasminogen activator protects hippocampal neurons from oxygen-glucose deprivation injury. J Neurosci Res. 2001;63:388–394. doi: 10.1002/1097-4547(20010301)63:5<388::AID-JNR1033>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Frost DO. BDNF/trkB signaling in the developmental sculpting of visual connections. Prog Brain Res. 2001;134:35–49. doi: 10.1016/s0079-6123(01)34004-9. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualandris A, Jones TE, Strickland S, Tsirka SE. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J Neurosci. 1996;16:2220–2225. doi: 10.1523/JNEUROSCI.16-07-02220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Keifer J, Sabirzhanov BE, Zheng Z, Li W, Clark TG. Cleavage of proBDNF to BDNF by a tolloid-like metalloproteinase is required for acquisition of in vitro eyeblink classical conditioning. J Neurosci. 2009;29:14956–14964. doi: 10.1523/JNEUROSCI.3649-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozai T, Yamanaka H, Dai Y, Obata K, Kobayashi K, Mashimo T, Noguchi K. Tissue type plasminogen activator induced in rat dorsal horn astrocytes contributes to mechanical hypersensitivity following dorsal root injury. Glia. 2007;55:595–603. doi: 10.1002/glia.20483. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Liot G, Benchenane K, Léveillé F, López-Atalaya JP, Fernández-Monreal M, Ruocco A, Mackenzie ET, Buisson A, Ali C, Vivien D. 2,7-Bis-(4-amidinobenzylidene)-cycloheptan-1-one dihydrochloride, tPA stop, prevents tPA-enhanced excitotoxicity both in vitro and in vivo. J Cereb Blood Flow Metab. 2004;24:1153–1159. doi: 10.1097/01.WCB.0000134476.93809.75. [DOI] [PubMed] [Google Scholar]
- Liot G, Roussel BD, Lebeurrier N, Benchenane K, López-Atalaya JP, Vivien D, Ali C. Tissue-type plasminogen activator rescues neurones from serum deprivation-induced apoptosis through a mechanism independent of its proteolytic activity. J Neurochem. 2006;98:1458–1464. doi: 10.1111/j.1471-4159.2006.03982.x. [DOI] [PubMed] [Google Scholar]
- Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- Mali RS, Cheng M, Chintala SK. Plasminogen activators promote excitotoxicity-induced retinal damage. FASEB J. 2005;19:1280–1289. doi: 10.1096/fj.04-3403com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matys T, Strickland S. Tissue plasminogen activator and NMDA receptor cleavage. Nat Med. 2003;9:371–372. doi: 10.1038/nm0403-371. author reply 372–373. [DOI] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Nagai N, Urano T, Napiorkowska-Pawlak D, Ihara H, Takada Y, Collen D, Takada A. Rapid, specific and active site-catalyzed effect of tissue-plasminogen activator on hippocampus-dependent learning in mice. Neuroscience. 2002;113:995–1001. doi: 10.1016/s0306-4522(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Rao BS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci U S A. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Cantallops I, Zaffuto S, Serrano P, Namgung U. Enhanced learning after genetic overexpression of a brain growth protein. Proc Natl Acad Sci U S A. 2000;97:7657–7662. doi: 10.1073/pnas.97.13.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen T, Vaittinen S, Castrén E. trkB-receptor activation contributes to the kainate-induced increase in BDNF mRNA synthesis. Cell Mol Neurobiol. 2001;21:429–435. doi: 10.1023/A:1012775808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles FJ, Strickland S. Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus. J Neurosci. 2002;22:2125–2134. doi: 10.1523/JNEUROSCI.22-06-02125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samara A, Vougas K, Papadopoulou A, Anastasiadou E, Baloyanni N, Paronis E, Chrousos GP, Tsangaris GT. Proteomics reveal rat hippocampal lateral asymmetry. Hippocampus. 2011;21:108–119. doi: 10.1002/hipo.20727. [DOI] [PubMed] [Google Scholar]
- Sartori CR, Pelágio FC, Teixeira SA, Valentinuzzi VS, Nascimento AL, Rogério F, Muscará MN, Ferrari EA, Langone F. Effects of voluntary running on spatial memory and mature brain-derived neurotrophic factor expression in mice hippocampus after status epilepticus. Behav Brain Res. 2009;203:165–172. doi: 10.1016/j.bbr.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Sartori CR, Vieira AS, Ferrari EM, Langone F, Tongiorgi E, Parada CA. The antidepressive effect of the physical exercise correlates with increased levels of mature brain-derived neurotrophic factor, and probrain-derived neurotrophic factor proteolytic cleavage-related genes, p11 and tissue plasminogen activator. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358:961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley KR, Toledo-Pereyra LH, Kothari RU. Molecular mechanisms in the pathogenesis and treatment of acute ischemic stroke. J Invest Surg. 2005;18:207–218. doi: 10.1080/08941930591004449. [DOI] [PubMed] [Google Scholar]
- Turner KM, Burgoyne RD, Morgan A. Protein phosphorylation and the regulation of synaptic membrane traffic. Trends Neurosci. 1999;22:459–464. doi: 10.1016/s0166-2236(99)01436-8. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between BDNF and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144:825–833. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Wang T, Xie K, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- Yamagata Y. New aspects of neurotransmitter release and exocytosis: dynamic and differential regulation of synapsin I phosphorylation by acute neuronal excitation in vivo. J Pharmacol Sci. 2003;93:22–29. doi: 10.1254/jphs.93.22. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Fukuchi M, Tabuchi A, Kawahara M, Tsuneki H, Azuma Y, Chiba Y, Tsuda M. Robust stimulation of TrkB induces delayed increases in BDNF and Arc mRNA expressions in cultured rat cortical neurons via distinct mechanisms. J Neurochem. 2007;103:626–636. doi: 10.1111/j.1471-4159.2007.04851.x. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, Jacquin M, Bu G. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]