Abstract
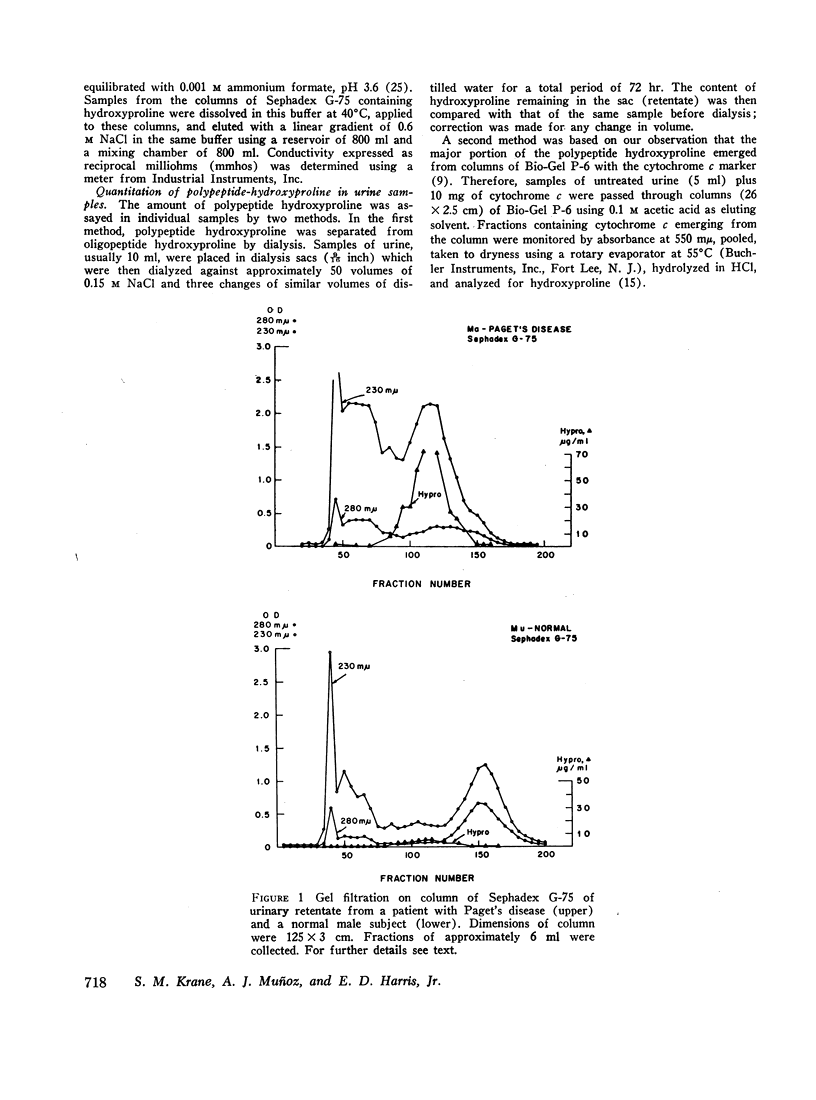
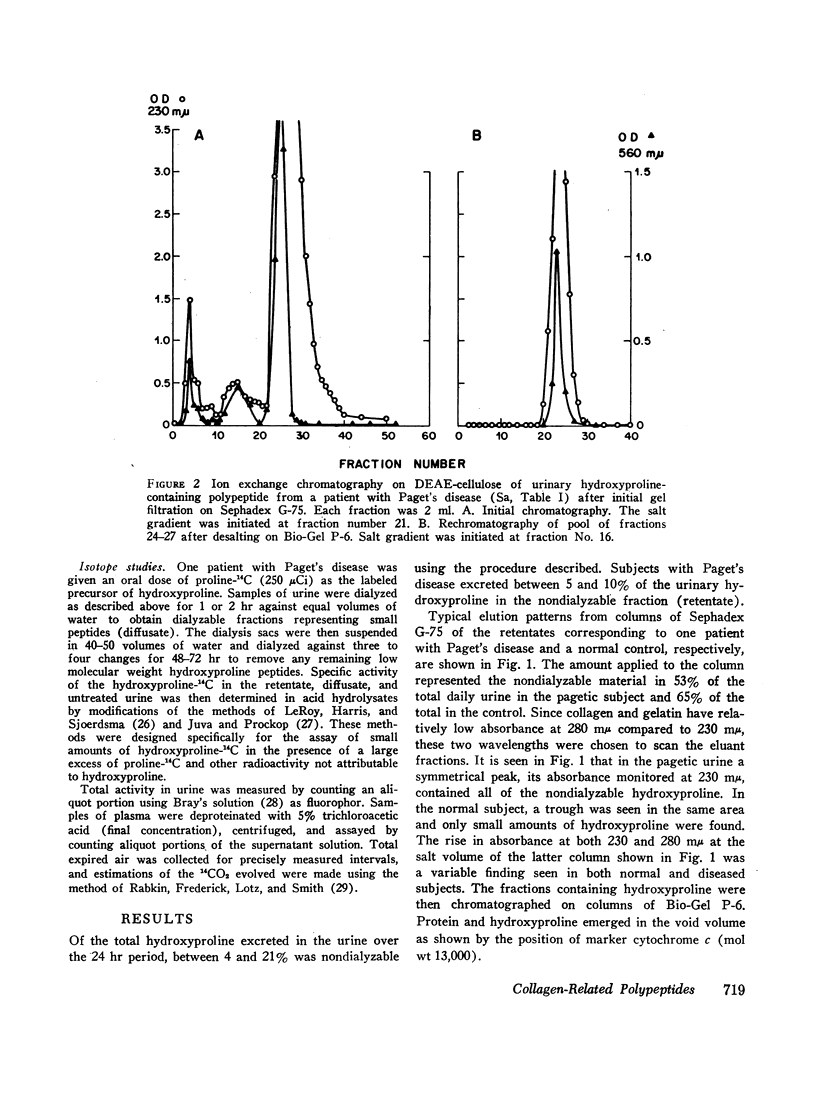
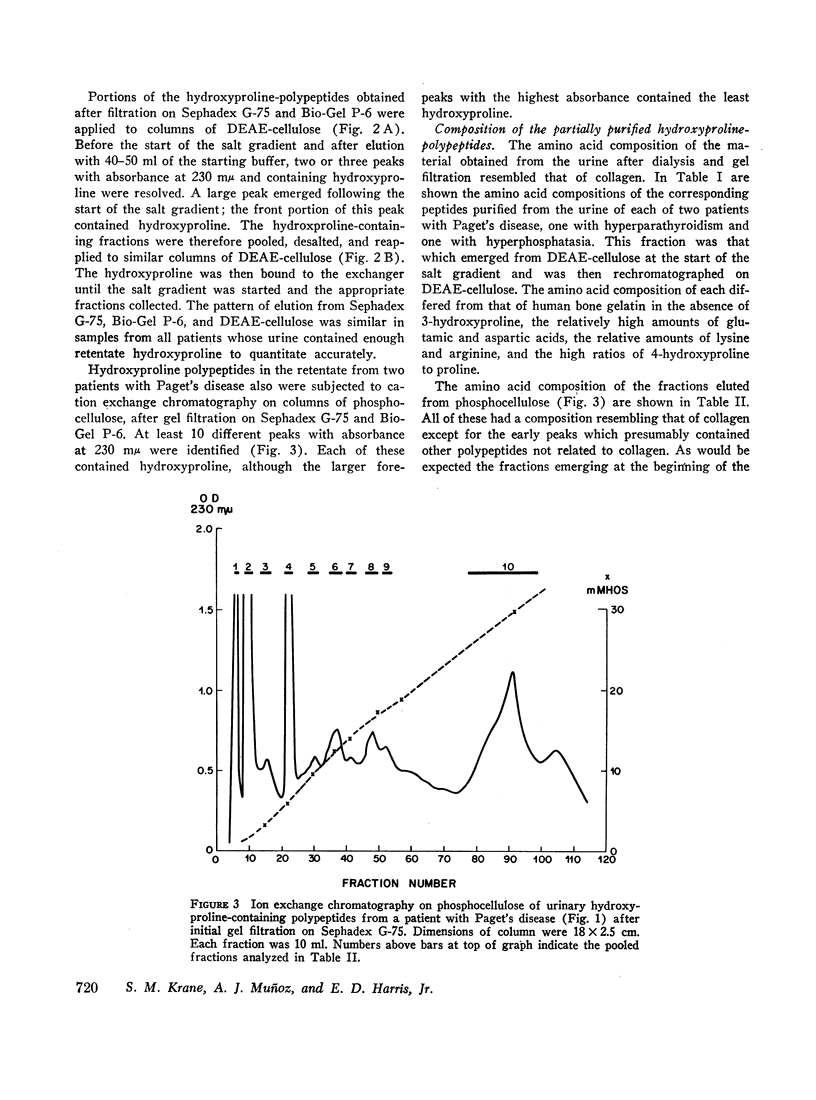
Of the total urinary hydroxyproline in normal subjects and those with skeletal disorders, between 4 and 20% was nondialyzable. In some patients with Paget's disease of bone, hyperparathyroidism with osteitis fibrosa, hyperphosphatasia, and extensive fibrous dysplasia the total urinary hydroxyproline was sufficiently high to permit purification of this polypeptide hydroxyproline by gel filtration and ion exchange chromatography. The partially purified polypeptides had molecular weights between 4500 and 10,000 and amino acid compositions and physical properties resembling those of gelatin. The polypeptide fractions also contained neutral sugar and glucosamine. These fragments had been shown to be susceptible to cleavage by purified bacterial collagenase suggesting the presence of the sequence-Pro-X-Gly-Pro-Y-.
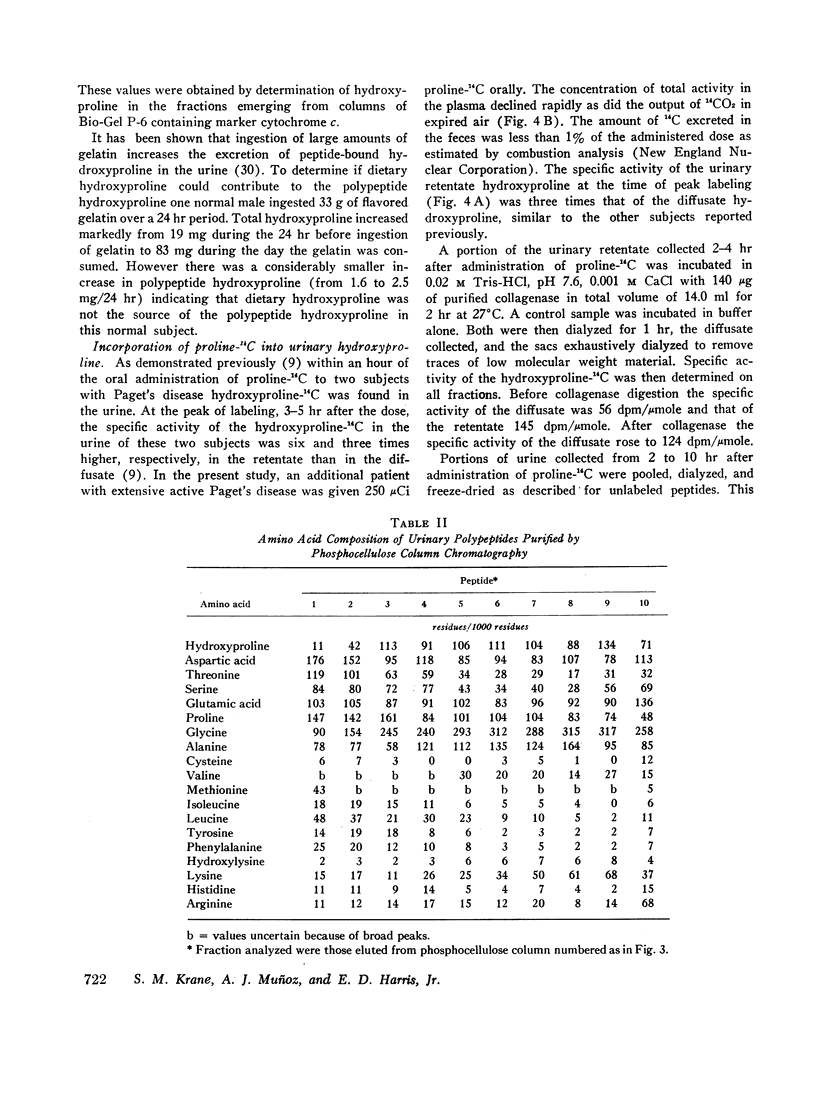
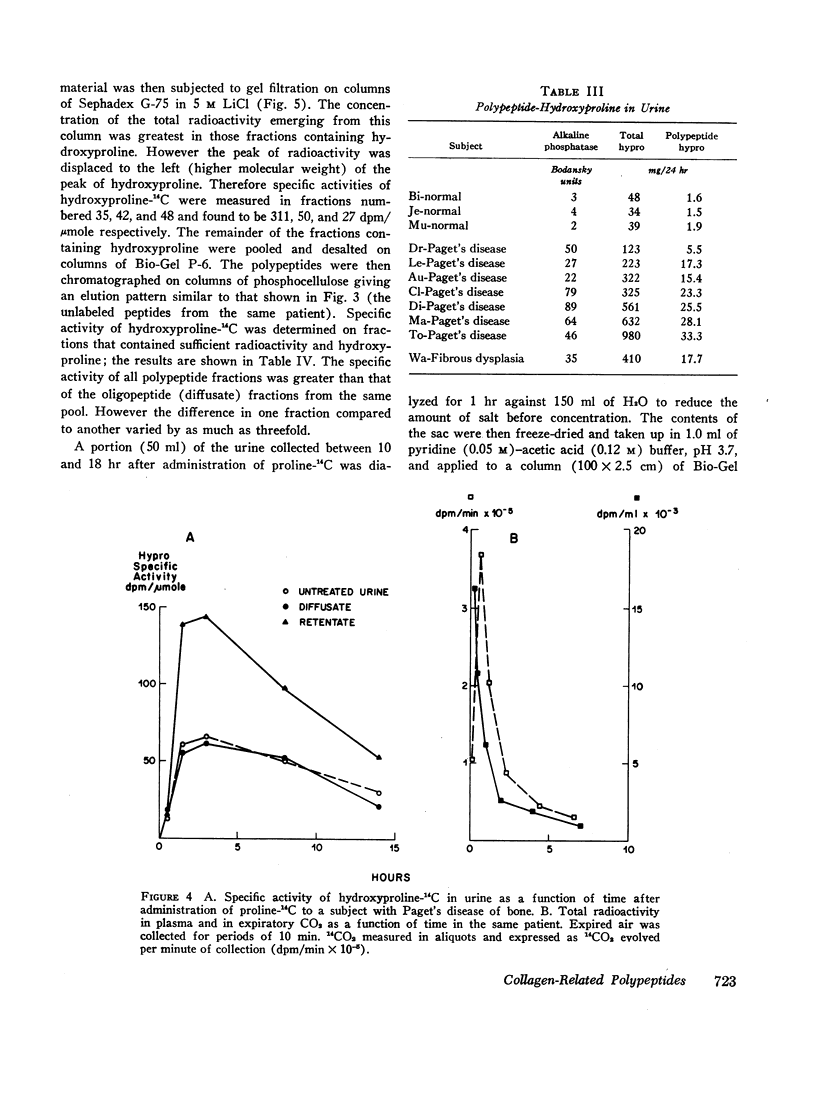
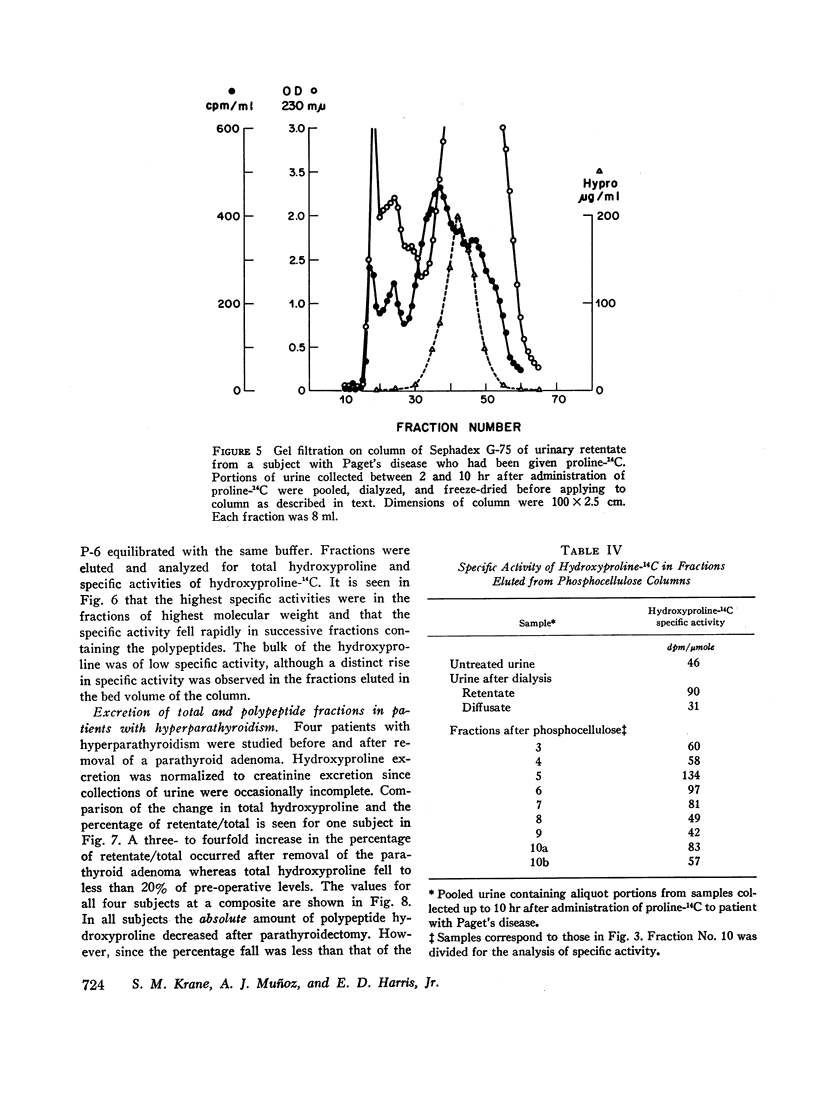
After administration of proline-14C to patients with Paget's disease hydroxyproline-14C was excreted in the urine. The hydroxyproline-14C specific activity reached a peak in 2-4 hr and declined rapidly. The specific activity of the polypeptide (retentate) portion was severalfold greater than that of the raw urine and diffusate. When the labeled urines were subjected to gel filtration the hydroxyproline-14C fractions of highest molecular weight which were eluted first from the columns had the highest specific activities. Exposure of the hydroxyproline-14C-containing polypeptides to bacterial collagenase rendered them dialyzable.
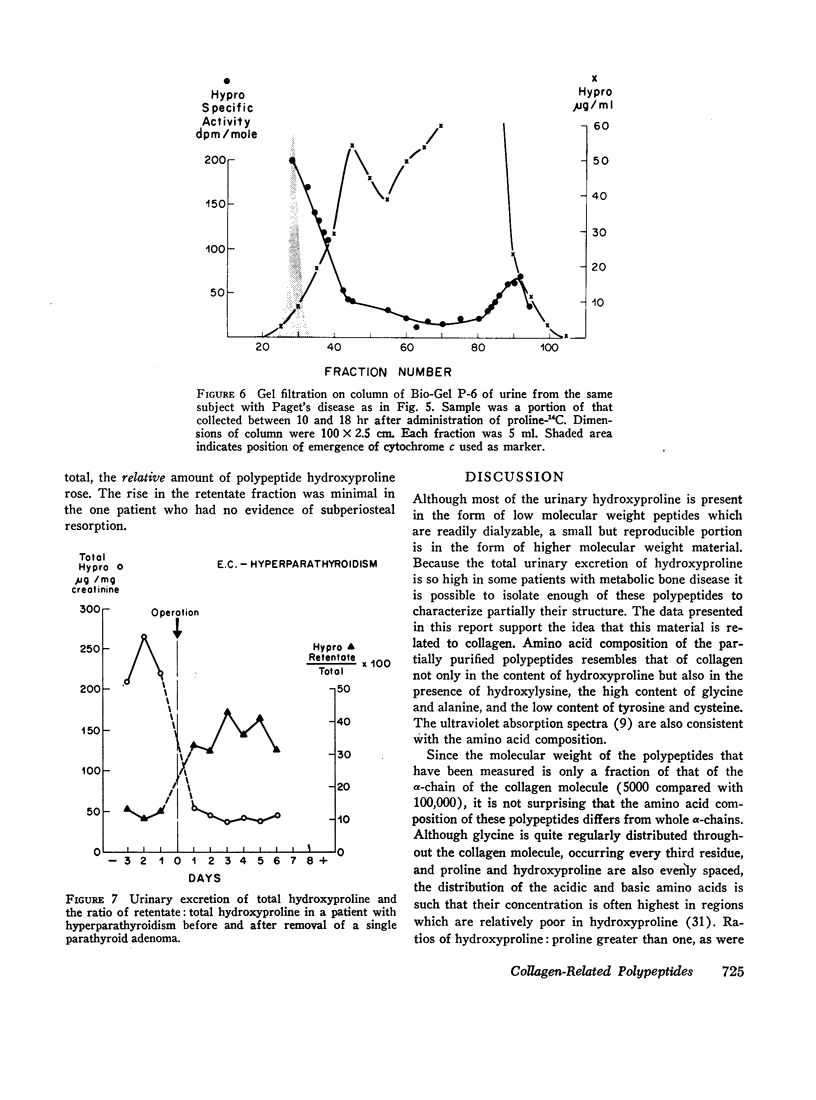
Four patients with hyperparathyroidism and osteitis fibrosa were studied before and after removal of a parathyroid adenoma, a period of transition from a predominance of bone collagen resorption to one of relatively increased bone collagen synthesis. The total urinary hydroxyproline fell rapidly after operation whereas the ratio of the polypeptide fraction to the total rose three- to fourfold. The results of these studies suggest that the urinary polypeptides represent fragments of collagen related to collagen synthesis. Changes in the ratio of these peptides to total hydroxyproline in the urine may serve as an index of new bone formation in patients with skeletal disorders.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourrillon R., Vernay J. L. Deux glycopeptides à hydroxyproline dans l'urine humaine normale. Biochim Biophys Acta. 1966 Apr 25;117(2):319–330. doi: 10.1016/0304-4165(66)90083-3. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Cunningham L. W. Evidence for the linkage of a disaccharide to hydroxylysine in tropocollagen. J Biol Chem. 1966 Sep 10;241(17):3882–3888. [PubMed] [Google Scholar]
- Cherian M. G., Radhakrishnan A. N. Isolation & characterization of a new glycopeptide containing hydroxyproline from human urine. Indian J Biochem. 1966 Jun;3(2):101–105. [PubMed] [Google Scholar]
- DE DEUXCHAISNES C. N., KRANE S. M. PAGET'S DISEASE OF BONE: CLINICAL AND METABOLIC OBSERVATIONS. Medicine (Baltimore) 1964 May;43:233–266. [PubMed] [Google Scholar]
- DUBOVSKY J., FORMANKOVA J., DUBOVSKA E. HYDROXYLZ'IN V MO CNI U OSTEOPATI'I S VYSOK'YM OBRATEM KOSTN'I MATRIX. Cas Lek Cesk. 1964 Feb 14;103:187–187. [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the cyanogen bromide peptides from the alpha 1 chain of chick skin collagen. Biochemistry. 1969 Apr;8(4):1506–1514. doi: 10.1021/bi00832a029. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Muñoz A. J., Harris E. D., Jr Collagen-like fragments: excretion in urine of patients with Paget's disease of bone. Science. 1967 Aug 11;157(3789):713–716. doi: 10.1126/science.157.3789.713. [DOI] [PubMed] [Google Scholar]
- LINDSTEDT S., PROCKOP D. J. Isotopic studies on urinary hydroxyproline as evidence for rapidly catabolized forms of collagen in the young rat. J Biol Chem. 1961 May;236:1399–1403. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laitinen O., Nikkilä E. A., Kivirikko K. I. Hydroxyproline in the serum and urine. Normal values and clinical significance. Acta Med Scand. 1966 Mar;179(3):275–284. doi: 10.1111/j.0954-6820.1966.tb05459.x. [DOI] [PubMed] [Google Scholar]
- LeRoy E. C., Harris E. D., Jr, Sjoerdsma A. A modified procedure for radioactive hydroxyproline assay in urine and tissues after labeled proline administration. Anal Biochem. 1966 Dec;17(3):377–382. doi: 10.1016/0003-2697(66)90173-4. [DOI] [PubMed] [Google Scholar]
- MATOLSTY A. G. A study on the structural protein of the vitreous body (vitrosin). J Gen Physiol. 1952 May;36(1):29–37. doi: 10.1085/jgp.36.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEILMAN E., URIVETZKY M. M., RAPOPORT C. M. Urinary hydroxyproline peptides. J Clin Invest. 1963 Jan;42:40–50. doi: 10.1172/JCI104694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagant de Deuxchaisnes C., Krane S. M. The treatment of adult phosphate diabetes and Fanconi syndrome with neutral sodium phosphate. Am J Med. 1967 Oct;43(4):508–543. doi: 10.1016/0002-9343(67)90177-5. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- PIEZ K. A., MORRIS L. A modified procedure for the automatic analysis of amino acids. Anal Biochem. 1960 Nov;1:187–201. doi: 10.1016/0003-2697(60)90045-2. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J. ISOTOPIC STUDIES ON COLLAGEN DEGRADATION AND THE URINE EXCRETION OF HYDROXYPROLINE. J Clin Invest. 1964 Mar;43:453–460. doi: 10.1172/JCI104930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCKOP D. J., SJOERDSMA A. Significance of urinary hydroxyproline in man. J Clin Invest. 1961 May;40:843–849. doi: 10.1172/JCI104318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- RABKIN M. T., FREDERICK E. W., LOTZ M., SMITH L. H., Jr Pyrimidine metabolism in man. V. The measurement in vivo of the biochemical effect of antineoplastic agents in animal and human subjects. J Clin Invest. 1962 Apr;41:871–883. doi: 10.1172/JCI104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D., Del Rio A., Akgun S., Frumin E. Novel proteins and peptides in the urine of patients with advanced neoplastic disease. Am J Med. 1969 Feb;46(2):174–187. doi: 10.1016/0002-9343(69)90002-3. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., GALLOP P. M., KLEIN L., MEILMAN E. Studies on collagen. II. Properties of purified collagenase and its inhibition. J Biol Chem. 1959 Feb;234(2):285–293. [PubMed] [Google Scholar]
- Spiro R. G. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J Biol Chem. 1960 Oct;235(10):2860–2869. [PubMed] [Google Scholar]
- Spiro R. G. Studies on the renal glomerular basement membrane. Nature of the carbohydrate units and their attachment to the peptide portion. J Biol Chem. 1967 Apr 25;242(8):1923–1932. [PubMed] [Google Scholar]
- Thompson R. C., Jr, Gaull G. E., Horwitz S. J., Schenk R. K. Hereditary hyperphosphatasia. Studies of three siblings. Am J Med. 1969 Aug;47(2):209–219. doi: 10.1016/0002-9343(69)90147-8. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]