Abstract
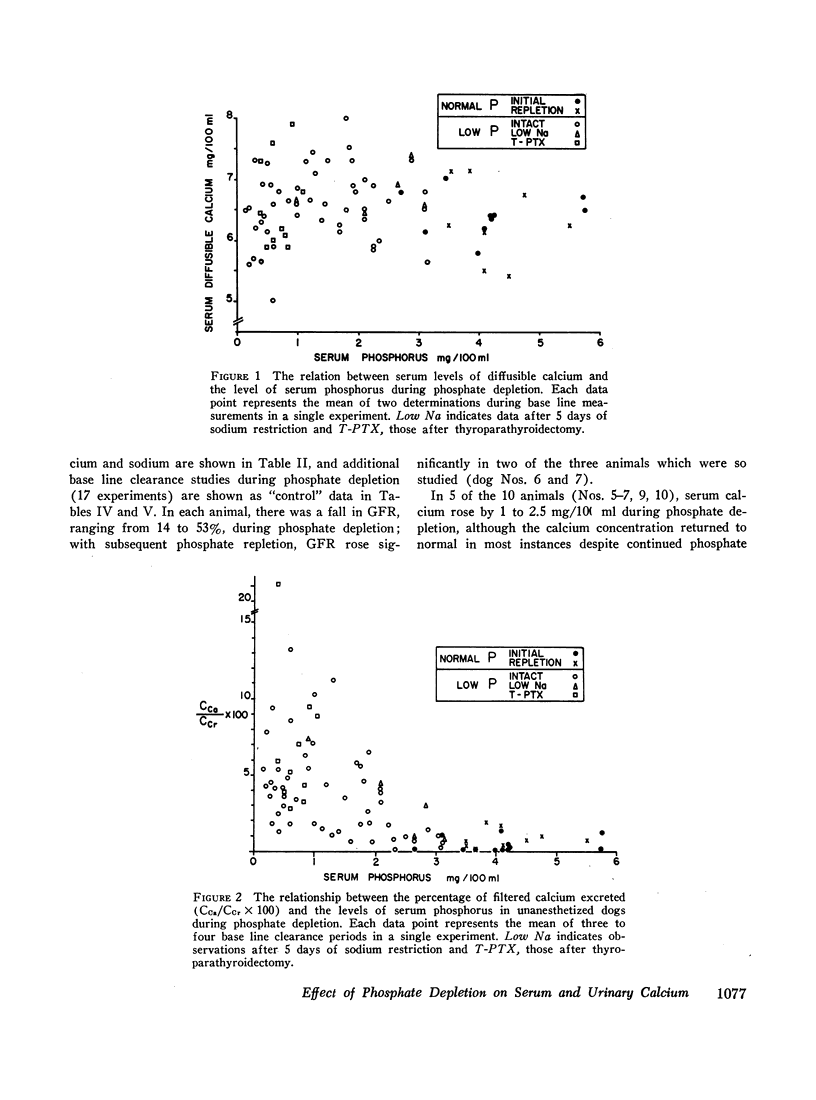
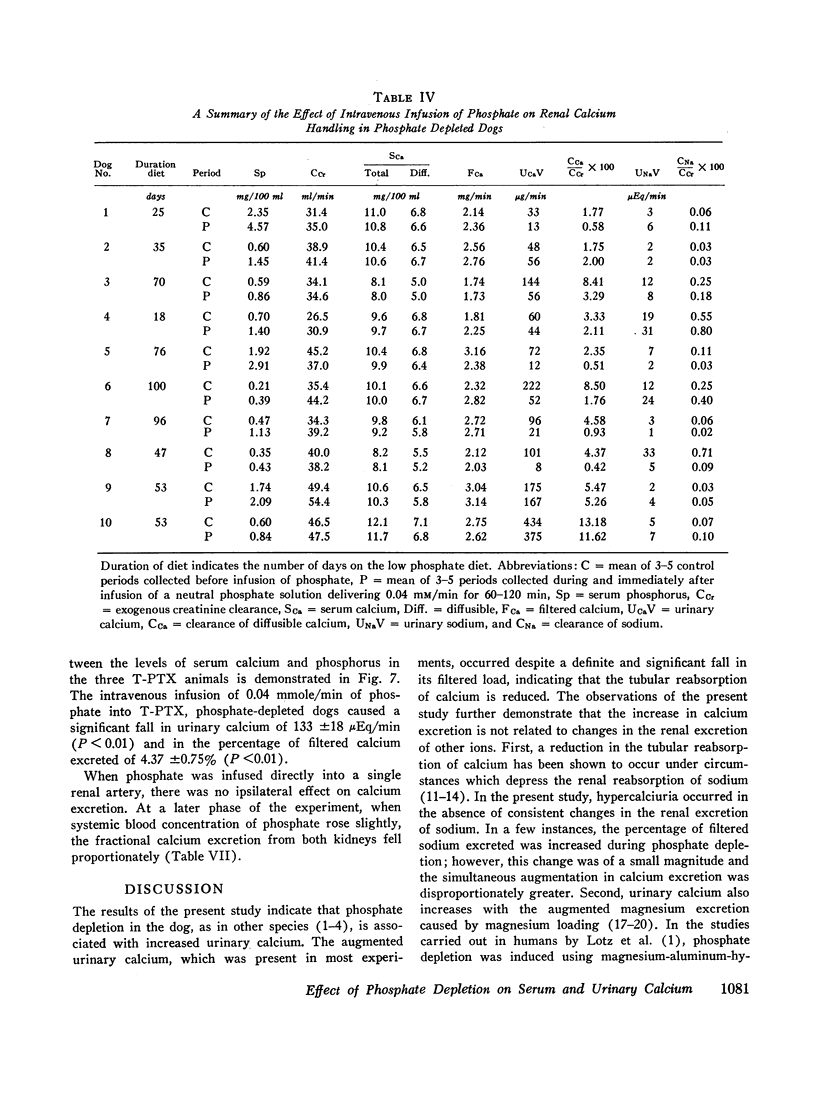
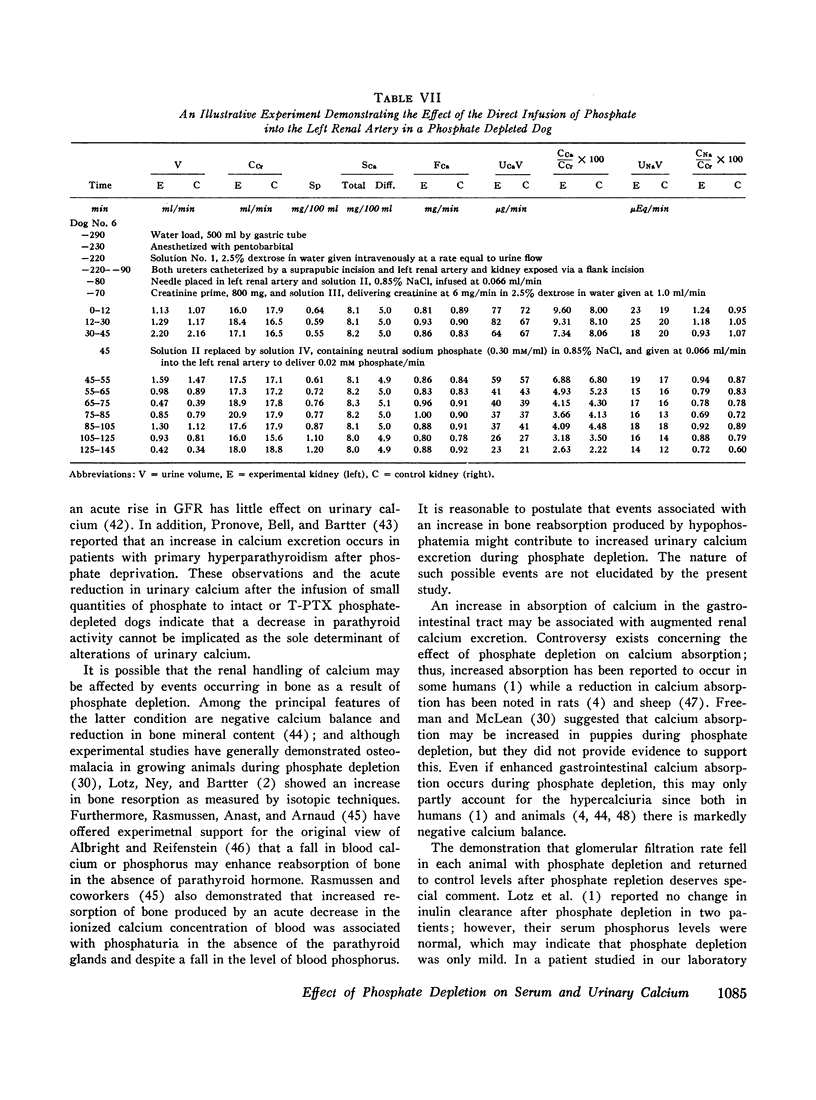
The changes in serum calcium and the renal handling of this ion were evaluated during phosphate depletion. 96 renal clearance studies were carried out in 10 dogs before and after prolonged phosphate depletion (30-160 days) and after repletion. Depletion was produced by reducing phosphate intake and administering aluminum hydroxide gel while intakes of sodium, calcium, and magnesium were constant. With phosphate depletion, serum phosphorus fell to less than 1.0 mg/100 ml and diffusible serum calcium either remained unchanged or rose transiently. Glomerular filtration rate (GFR) fell by 15 to 53%. Despite the reduced filtered load of calcium, its fractional excretion increased in most experiments. This hypercalciuria was not dependent upon changes in sodium or magnesium excretion, or the urinary concentration of complexing anions, and persisted after sodium restriction. Phosphate repletion reversed the effects on GFR and calcium excretion. The intravenous infusion of small quantities of phosphate (0.04 mmole/min) into either intact or thyroparathyroidectomized (T-PTX), phosphate-depleted animals caused a significant reduction in fractional excretion of calcium, but the intrarenal infusion of 0.02 mmole/min of phosphate into one kidney failed to produce an ipsilateral effect. The administration of parathyroid extract reduced fractional calcium excretion, but the latter remained significantly elevated. After T-PTX, fractional calcium excretion did not increase in the phosphate-depleted animals. Furthermore, serum calcium was normal after T-PTX until serum phosphorus increased slightly, and only then did hypocalcemia develop. These observations indicate that (a) phosphate depletion produces hypercalciuria through a reduction in tubular reabsorption of calcium which is not due to changes in the tubular reabsorption of other ions; this effect is not reversed by the direct intrarenal infusion of phosphate; (b) a state of functional hypoparathyroidsm occurs during phosphate depletion which may, in part, cause reduced tubular reabsorption of calcium; (c) other extra renal mechanism(s), possibly related to events occurring in bone as a result of phosphate depletion, may have an effect on urinary calcium excretion; and (d) in the phosphatedepleted state, parathyroid hormone is not required for the maintenance of a normal level of serum calcium.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUGUST J. T., NELSON D. H., THORN G. W. Response of normal subjects to large amounts of aldosterone. J Clin Invest. 1958 Nov;37(11):1549–1555. doi: 10.1172/JCI103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright F., Bauer W., Claflin D., Cockrill J. R. STUDIES IN PARATHYROID PHYSIOLOGY: III. The Effect of Phosphate Ingestion in Clinical Hyperparathyroidism. J Clin Invest. 1932 Mar;11(2):411–435. doi: 10.1172/JCI100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER E. S., ELKINTON J. R., CLARK J. K. Studies of the renal excretion of magnesium in man. J Clin Invest. 1959 Oct;38:1733–1745. doi: 10.1172/JCI103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNSTEIN D., KLEEMAN C. R., CUTLER R. E., DOWLING J. T., MAXWELL M. H. Comparison of renal clearance of calcium during infusion of calcium chloride and calcium gluconate. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:671–673. doi: 10.3181/00379727-110-27612. [DOI] [PubMed] [Google Scholar]
- BEUTLER E., YEH M. K. A simplified method for the determination of citric acid. J Lab Clin Med. 1959 Jul;54(1):125–131. [PubMed] [Google Scholar]
- Bell N. H. Observations concerning the effects of fasting on collagen metabolism in man. J Clin Endocrinol Metab. 1969 Mar;29(3):338–345. doi: 10.1210/jcem-29-3-338. [DOI] [PubMed] [Google Scholar]
- Bernstein D. S., Newton R. The effect of oral sodium phosphate on the formation of renal calculi and on idiopathic hypercalcuria. Lancet. 1966 Nov 19;2(7473):1105–1107. doi: 10.1016/s0140-6736(66)92195-7. [DOI] [PubMed] [Google Scholar]
- Bethune J. E., Turpin R. A., Inoue H. Effect of parathyroid hormone extract on divalent ion excretion in man. J Clin Endocrinol Metab. 1968 May;28(5):673–678. doi: 10.1210/jcem-28-5-673. [DOI] [PubMed] [Google Scholar]
- Blythe W. B., Gitelman H. J., Welt L. G. Effect of expansion of the extracellular space on the rate of urinary excretion of calcium. Am J Physiol. 1968 Jan;214(1):52–57. doi: 10.1152/ajplegacy.1968.214.1.52. [DOI] [PubMed] [Google Scholar]
- Briscoe A. M., Ragan C. Effect of magnesium on calcium metabolism in man. Am J Clin Nutr. 1966 Nov;19(5):296–306. doi: 10.1093/ajcn/19.5.296. [DOI] [PubMed] [Google Scholar]
- CARONE F. A., EPSTEIN F. H., BECK D., LEVITIN The effects upon the kidney of transienthypercalcemia induced by parathyroid extract. Am J Pathol. 1960 Jan;36:77–103. [PMC free article] [PubMed] [Google Scholar]
- CHEN P. S., Jr, NEUMAN W. F. Renal excretion of calcium by the dog. Am J Physiol. 1955 Mar;180(3):623–631. doi: 10.1152/ajplegacy.1955.180.3.623. [DOI] [PubMed] [Google Scholar]
- Hebert L. A., Lemann J., Jr, Petersen J. R., Lennon E. J. Studies of the mechanism by which phosphate infusion lowers serum calcium concentration. J Clin Invest. 1966 Dec;45(12):1886–1894. doi: 10.1172/JCI105493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEEMAN C. R., BERNSTEIN D., ROCKNEY R., DOWLING J. T., MAXWELL M. H. Studies on the renal clearance of diffusible calcium and the role of the parathyroid glands in its regulation. Yale J Biol Med. 1961 Aug;34:1–30. [PMC free article] [PubMed] [Google Scholar]
- LOTZ M., NEY R., BARTTER F. C. OSTEOMALACIA AND DEBILITY RESULTING FROM PHOSPHORUS DEPLETION. Trans Assoc Am Physicians. 1964;77:281–295. [PubMed] [Google Scholar]
- Lemann J., Jr, Piering W. F., Lennon E. J. Possible role of carbohydrate-induced calciuria in calcium oxalate kidney-stone formation. N Engl J Med. 1969 Jan 30;280(5):232–237. doi: 10.1056/NEJM196901302800502. [DOI] [PubMed] [Google Scholar]
- Lemann J., Litzow J. R., Lennon E. J. Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest. 1967 Aug;46(8):1318–1328. doi: 10.1172/JCI105624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Zisman E., Bartter F. C. Evidence for a phosphorus-depletion syndrome in man. N Engl J Med. 1968 Feb 22;278(8):409–415. doi: 10.1056/NEJM196802222780802. [DOI] [PubMed] [Google Scholar]
- MANITIUS A., LEVITIN H., BECK D., EPSTEIN F. H. On the mechanism of impairment of renal concentrating ability in potassium deficiency. J Clin Invest. 1960 Apr;39:684–692. doi: 10.1172/JCI104084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Chapman L. W., Kleeman C. R. Effect of NaCl infusion on urinary Ca++ and Mg++ during reduction in their filtered loads. Am J Physiol. 1967 Nov;213(5):1218–1224. doi: 10.1152/ajplegacy.1967.213.5.1218. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Chapman L. W., Kleeman C. R. Role of serum Ca, parathyroid hormone, and NaCl infusion on renal Ca and Na clearances. Am J Physiol. 1968 Jun;214(6):1403–1409. doi: 10.1152/ajplegacy.1968.214.6.1403. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Kleeman C. R. Renal handling of magnesium in the dog. Am J Physiol. 1969 Jun;216(6):1460–1467. doi: 10.1152/ajplegacy.1969.216.6.1460. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Mueller E., Silverman A. G., Kleeman C. R. Inorganic phosphate treatment of hypercalcemia. Arch Intern Med. 1968 Apr;121(4):307–312. [PubMed] [Google Scholar]
- PRONOVE P., BELL N. H., BARTTER F. C. Production of hypercalciuria by phosphorus deprivation on a low calcium intake: a new clinical test for hyperparathyroidism. Metabolism. 1961 May;10:364–371. [PubMed] [Google Scholar]
- Parfitt A. M. The acute effects of mersalyl, chlorothiazide and mannitol on the renal excretion of calcium and other ions in man. Clin Sci. 1969 Apr;36(2):267–282. [PubMed] [Google Scholar]
- Rasmussen H., Anast C., Arnaud C. Thyrocalcitonin, EGTA, and urinary electrolyte excretion. J Clin Invest. 1967 May;46(5):746–752. doi: 10.1172/JCI105575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIKITA M., TSURUFUJI S., ITO Y. Adaptation in renal phosphorus excretion under the influence of parathyroids; a study in ureterally catheterized rats. Endocrinol Jpn. 1962 Sep;9:171–180. doi: 10.1507/endocrj1954.9.171. [DOI] [PubMed] [Google Scholar]
- Suki W. N., Schwettmann R. S., Rector F. C., Jr, Seldin D. W. Effect of chronic mineralocorticoid administration on calcium excretion in the rat. Am J Physiol. 1968 Jul;215(1):71–74. doi: 10.1152/ajplegacy.1968.215.1.71. [DOI] [PubMed] [Google Scholar]
- WALSER M., BROWDER A. A. Ion association. III. The effect of sulfate infusion on calcium excretion. J Clin Invest. 1959 Aug;38(8):1404–1411. doi: 10.1172/JCI103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M. Calcium clearance as a function of sodium clearance in the dog. Am J Physiol. 1961 May;200:1099–1104. doi: 10.1152/ajplegacy.1961.200.5.1099. [DOI] [PubMed] [Google Scholar]
- WESSON L. G., Jr Magnesium, calcium, and phosphate excretion during osmotic diuresis in the dog. J Lab Clin Med. 1962 Sep;60:422–432. [PubMed] [Google Scholar]
- WIDROW S. H., LEVINSKY N. G. The effect of parathyroid extract on renal tubular calcium reabsorption in the dog. J Clin Invest. 1962 Dec;41:2151–2159. doi: 10.1172/JCI104673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. R., Lofgreen G. P., Luick J. R. The effects of phosphorus depletion, and of calcium and phosphorus intake, on the endogenous excretion of these elements by sheep. Br J Nutr. 1966;20(4):795–805. doi: 10.1079/bjn19660081. [DOI] [PubMed] [Google Scholar]
- Young V. R., Luick J. R., Lofgreen G. P. The influence of dietary phosphorus intake on the rate of bone metabolism in sheep. Br J Nutr. 1966;20(4):727–732. doi: 10.1079/bjn19660074. [DOI] [PubMed] [Google Scholar]
- Young V. R., Richards W. P., Lofgreen G. P., Luick J. R. Phosphorus depletion in sheep and the ratio of calcium to phosphorus in the diet with reference to calcium and phosphorus absorption. Br J Nutr. 1966;20(4):783–794. doi: 10.1079/bjn19660080. [DOI] [PubMed] [Google Scholar]
- ZETTNER A., SELIGSON D. APPLICATION OF ATOMIC ABSORPTION SPECTROPHOTOMETRY IN THE DETERMINATION OF CALCIUM IN SERUM. Clin Chem. 1964 Oct;10:869–890. [PubMed] [Google Scholar]


