Abstract
The relationship between alcohol consumption, sensitivity and tolerance is an important question that has been addressed in humans and rodent models. Studies have shown that alcohol consumption and risk of abuse may correlate with (1) increased sensitivity to the stimulant effects of alcohol, (2) decreased sensitivity to the depressant effects of alcohol and (3) increased alcohol tolerance. However, many conflicting results have been observed. To complement these studies, we utilized a different organism and approach to analyze the relationship between ethanol consumption and other ethanol responses. Using a set of 20 Drosophila melanogaster mutants that were isolated for altered ethanol sensitivity, we measured ethanol-induced hyperactivity, ethanol sedation, sedation tolerance and ethanol consumption preference. Ethanol preference showed a strong positive correlation with ethanol tolerance, consistent with some rodent and human studies, but not with ethanol hyperactivity or sedation. No pairwise correlations were observed between ethanol hyperactivity, sedation and tolerance. The evolutionary conservation of the relationship between tolerance and ethanol consumption in flies, rodents and humans indicates that there are fundamental biological mechanisms linking specific ethanol responses.
Key words: ethanol consumption, ethanol sensitivity, tolerance, correlation, Drosophila
Introduction
Ethanol elicits diverse behavioral responses in humans as well as animal models. Acute responses to ethanol include both stimulant and depressant effects, which in humans are typically perceived as positive and negative, respectively.1 Ethanol tolerance is defined as a decrease in any given ethanol response after previous exposure, and can develop both within and between intoxicating sessions.2 Despite the large body of work on ethanol sensitivity and tolerance, an important question remains unresolved: to what extent do ethanol sensitivity and tolerance influence ethanol consumption and abuse? Understanding the relationship between these behaviors is essential for both medical and scientific reasons. These relationships may aid in predicting which individuals are at risk for alcohol use disorders (AUDs), allowing for early intervention. In addition, determining which ethanol responses are most closely associated with addictive behavior may guide future studies using animal models and highlight potential neural and molecular mechanisms underlying addiction.
Landmark studies by Schuckit and colleagues demonstrated that individuals with decreased sensitivity to the acute effects of alcohol (such as postural instability and subjective “high”) are at greater risk for developing an AUD.3–5 However, other studies have come to the opposite conclusion, associating risk for AUDs with increased alcohol sensitivity.6,7 A potential explanation for these contradictory findings arises from the biphasic nature of alcohol intoxication. Newlin and Thomson proposed a “differentiator model”, in which individuals at risk for AUDs have increased sensitivity to the euphoric, stimulant effects of ethanol that occur earlier (as blood alcohol concentration [BAC] is rising) and decreased sensitivity to its negative, depressant effects that occur later (as BAC falls).1,6,8 This altered balance of positive and negative effects would cause these individuals to find alcohol particularly rewarding. Several studies support specific aspects of this model,9–11 but it remains to be tested rigorously (reviewed in ref. 12).
Animal studies have also addressed the relationship between ethanol sensitivity and addictive behaviors, focusing mainly on ethanol consumption preference.13 An association between low sensitivity to the sedative/hypnotic effects of ethanol and high ethanol preference has been observed in rodents selectively bred for high versus low ethanol preference14,15 as well as in many mouse mutants.16,17 However, this correlation is by no means universal, as several exceptions have been reported.18–20 In support of the differentiator model, some high-preferring lines show greater sensitivity to ethanol-induced locomotor stimulation than low-preferring lines.21,22
The influence of ethanol tolerance on ethanol consumption and addiction has not been extensively studied, despite the fact that tolerance is one of the DSM-IV criteria for alcohol dependence.23 Although tolerance often develops in non-alcoholics, it has been correlated with heavy drinking and alcohol abuse.24 Tolerance is thought to promote greater alcohol consumption by diminishing the aversive effects of alcohol as well as reducing its rewarding properties, which would require individuals to drink more alcohol to achieve the same positive effects.2 Similar to studies of ethanol sensitivity, some studies of selectively bred rodent lines14,25 and specific mutants26,27 point to a positive correlation between tolerance and alcohol preference, while other studies do not support this correlation.28,29
In summary, we are still in the process of gaining a clear understanding of how alcohol sensitivity and tolerance contribute to alcohol consumption and AUDs. While the differentiator model may explain some of the conflicting results from human studies, some of the discrepant findings are likely to be due to the confounding factors inherent to human studies. First, cognitive and emotional factors influence ethanol intoxication and consumption in humans. Second, the previous alcohol experience of subjects cannot be precisely controlled. Third, ethanol consumption in a laboratory study is dissimilar from real-life drinking. On the other hand, studies that ask subjects to recall real-life drinking experiences are susceptible to inaccurate or biased recollections. Rodent studies also have limitations. First, lines that have been selectively bred for high versus low alcohol preference differ in many other behavioral and neurobiological measures, including anxiety, novelty-seeking and startle responses,30 which may produce confounding biases during assays of ethanol sensitivity and tolerance. Second, correlation studies that examine different behavioral responses in the same animals (example in ref. 31) face the problem that previous exposure to alcohol alters subsequent alcohol responses (i.e., naive responses cannot be obtained for more than one behavior). Finally, it is difficult to make correlations from studies of mutant mice because different mutants have been generated and tested in different laboratories, often using different protocols and genetic backgrounds.32
To circumvent many of these limitations and complement the valuable studies that have been performed in humans and rodents, we have used a different organism and approach to examine the relationships between ethanol sensitivity, tolerance and consumption preference. The fruit fly Drosophila melanogaster is an established model for studying acute and chronic responses to ethanol. Flies exhibit acute ethanol responses similar to those of mammals: as ethanol concentration increases, flies exhibit locomotor stimulation,33 loss of postural control,34 and eventually sedation.35,36 With repeated exposure, flies develop tolerance to the motor-impairing and sedating effects of ethanol.37,38 Flies also have an innate preference for consuming ethanol-containing solutions.39 Furthermore, they exhibit several features of alcohol addiction, such as an increase in ethanol consumption over time, willingness to overcome an aversive stimulus in order to consume ethanol and relapse-like behavior.39
We set out to test whether ethanol sensitivity, tolerance and consumption preference are correlated in Drosophila. We utilized a set of mutants that had been isolated for altered ethanol responses. Our ability to test a relatively large number of mutants in the same genetic background under the same experimental conditions affords an ideal opportunity to observe meaningful correlations between ethanol-related behaviors. Based on the findings from human and rodent studies, we hypothesized that ethanol consumption preference would be positively correlated with sensitivity to the stimulant effects of ethanol and with resistance to the sedative effects of ethanol. We also predicted that ethanol tolerance and preference would be positively correlated. We found that ethanol preference was indeed positively correlated with tolerance but was uncorrelated with other ethanol responses, suggesting that there are shared genetic mechanisms underlying ethanol tolerance and preference that have been conserved from flies to humans.
Results
To examine the relationships between ethanol sensitivity, tolerance and consumption, we measured these responses in 20 Drosophila mutants that we isolated for altered ethanol sensitivity (Table 1), some of which have been published.35,36,40–42 All 20 mutants contain P element insertions, and the genes located nearest to the insertion sites are listed in Table 1. These are the genes that are most likely to be responsible for the behavioral phenotypes of the mutants, though functional studies have not been conducted to confirm their role (aside from the studies listed in Table 1). We chose to test a panel of ethanol sensitivity mutants rather than wild type strains or random mutants to ensure that a wide spectrum of ethanol responses would be represented. In the 20 mutants we assessed four different behavioral responses to ethanol: (1) consumption preference, (2) locomotor stimulation, (3) sedation and (4) sedation tolerance.
Table 1.
Mutants tested for ethanol responses
| Mutant | Nearest Gene(s) to Insertion Site | Original phenotype | Reference |
| 2–10 | PQBP1 | S inebriometer | 41 |
| 2–67 | path | R inebriometer | |
| 3–68 | CG31886 | S inebriometer | 41 |
| 4–12 | R sedation | ||
| 5–10 | Tsp42Ee | S hyperactivity | |
| 5–21 | sgg | S hyperactivity | 40 |
| 5–89 | inx7 | R sedation | |
| 6-6 | cv-2 | R sedation | |
| 8–128 | aru | S inebriometer | 42 |
| 8–169 | Sgs3 | R inebriometer | |
| 8–222 | Gs1 | R sedation | |
| 9–91 | CG13386 | S inebriometer | |
| 9–220 | l(2)01289, phtf | S inebriometer | 41 |
| 10–110 | elm | S inebriometer | 41 |
| 13–66 | R sedation | ||
| 14–45 | CG8498 | S inebriometer | |
| 1514 | RhoGAP18B | R sedation | 35 |
| 17-3 | Kdm2 | S hyperactivity | |
| 17–51 | hppy | R sedation | 36 |
| 20–29 | eyg, CG32102 | R sedation |
Mutants were identified in genetic screens for sensitivity to ethanol hyperactivity, sedation or loss of postural control in the inebriometer.34 The original phenotype identified is listed for each mutant (S, sensitive; R, resistant) as well as the reference for mutants that have been previously published. The gene(s) implicated by the p element insertion site are listed for all mutants except 4–12 and 13–66, which will be characterized in separate publications.
Ethanol consumption preference.
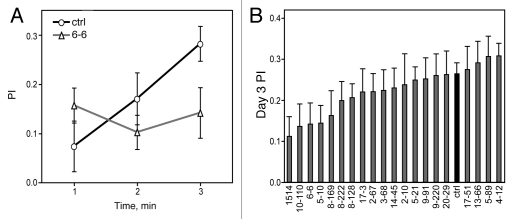
Flies preferentially consume ethanol-containing food over non-ethanol food in a continuous access two-choice feeding assay.39 Ethanol preference is relatively low and variable during the first one to two days, but subsequently increases and becomes more stable.39 We measured preference for 15% ethanol over three days. Because we expected that preference would be variable on days 1 and 2, we planned to primarily use the preference value on day 3 for correlation analyses. Preference was quantified by calculating a preference index (PI) defined as (volume of ethanol food consumption - volume of non-ethanol food consumption)/total consumption. PI can vary between −1 and +1, with positive values indicating preference. Control flies had a PI of 0.06 ± 0.03 on day 1 which increased to 0.26 ± 0.03 on day 3 (Fig. 1A). PI values on day 3 for the 20 mutants ranged from 0.11 to 0.31 (Fig. 1B). Three mutants (6–6, 10–110, 1514) showed PI values that were significantly different from the respective control tested in the same experiment (p < 0.05, t-test); one example is shown in Figure 1A. Two additional mutants (8–128, 8–222) showed a trend toward altered preference (0.05 < p < 0.06, t-test).
Figure 1.
Ethanol consumption preference of 20 Drosophila ethanol sensitivity mutants. (A) Ethanol preference of the control strain and mutant 6-6 in a 3-day assay. Line 6-6 showed decreased preference compared with the control on day 3 (p < 0.05, t-test, n = 18). (B) Ethanol PI on day 3 for each mutant tested (n = 18 for mutants, n = 36 for control).
Ethanol hyperactivity.
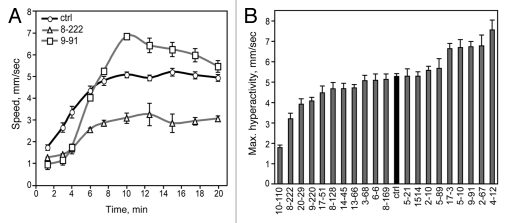
Locomotor hyperactivity and sedation represent different aspects of ethanol sensitivity in flies. Hyperactivity occurs at moderate ethanol concentrations33 and represents a stimulant effect of ethanol, whereas sedation occurs at high ethanol concentrations35 and represents a depressant effect of ethanol. We first measured ethanol hyperactivity in our set of mutants using a locomotor tracking system.33 When exposed to a moderate concentration of ethanol vapor (47% ethanol vapor in humidified air), flies exhibit a gradual increase in activity as their internal ethanol concentration increases (Fig. 2A).33 Maximum ethanol hyperactivity was quantified as the average of the 3 consecutive time points showing the highest locomotor speed during the 20 min assay, a measure that has been previously used.35 Control flies had a maximum hyperactivity of 5.27 ± 0.14 mm/sec and hyperactivity values for the 20 mutants ranged from 1.78 to 7.55 mm/sec (Fig. 2B). Tracking profiles of a mutant exhibiting increased hyperactivity (9–91) and one exhibiting decreased hyperactivity (8–222) are shown in Figure 2A (p < 0.001, t-tests).
Figure 2.
Sensitivity to ethanol-induced hyperactivity. (A) Exposure to a moderate concentration of 47% ethanol vapor causes locomotor hyperactivity in flies. Line 8–222 exhibited decreased maximum ethanol hyperactivity and line 9–91 exhibited increased maximum hyperactivity compared with the control (p < 0.001, t-tests, n = 10). (B) Maximum ethanol-induced hyperactivity of each mutant (n = 10 for mutants, n = 20 for control).
Ethanol hyperactivity shows a U-shaped relationship with ethanol concentration: hyperactivity initially increases as ethanol concentration increases, but decreases at high ethanol concentrations as flies become sedated.33 We wondered whether mutants that showed altered hyperactivity at 47% ethanol would also exhibit the same phenotypes at a lower concentration, given that some of those mutants may also have altered sedation sensitivity (Fig. 3). Using a lower concentration of 33% ethanol, we retested the 11 mutants that showed significantly different maximum hyperactivity from the respective control tested on the same day (p < 0.05, t-test). Six of the 11 mutants exhibited the same phenotype at both ethanol concentrations (data not shown; p < 0.05, t-test), including lines 8–222 and 9–91 shown in Figure 2A. Of the other five mutants, three mutants showed no significant phenotype at 33% ethanol (2–67, 9–220, 17-3; though 17-3 showed a trend in the same direction), and two mutants showed the opposite phenotype (4–12, 14–45). The majority of hyperactivity mutants therefore showed the same phenotype at multiple concentrations, although there were exceptions.
Figure 3.
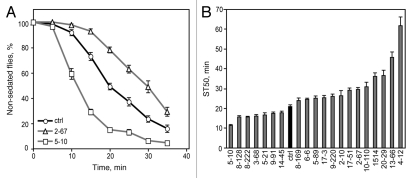
Sensitivity to ethanol-induced sedation in naive flies. (A) Flies exhibit sedation during exposure to a high concentration of ethanol vapor (67%). Line 2–67 exhibited decreased sedation sensitivity (p < 0.001) and line 5–10 exhibited increased sedation sensitivity (p < 0.001) compared with the control (t-tests comparing ST50 values, n = 8–9). (B) ST50 of each mutant (n = 8–13 for mutants, n = 16 for control).
Ethanol sedation.
We next measured sensitivity to ethanol sedation in the 20 mutants by testing naive flies in a loss of righting reflex assay.35,36 We counted the number of non-sedated flies at 5-min intervals during exposure to a high concentration of ethanol vapor (67% ethanol; Fig. 3A) and calculated the time required for 50% of flies to sedate (ST50). Control flies had an ST50 of 20.8 ± 0.9 min. ST50 values for all mutants are shown in Figure 3B and ranged from 11.3 to 61.7 min. Sedation curves for a mutant showing increased sedation sensitivity (5–10) and one showing decreased sensitivity (2–67) as compared with the control are shown in Figure 3A (p < 0.001 for each mutant vs. control ST50, t-tests).
Ethanol tolerance.
Tolerance to the sedating effects of ethanol has been previously characterized in flies.37,38 We measured sedation tolerance in the mutants by exposing naive flies to a sedating dose of ethanol (similar to the assays conducted above) and then re-exposing the same flies four hours later. The decrease in edation sensitivity during the second exposure as compared to the first exposure reflects the development of tolerance. The duration of the first exposure was set at 30 min in order to avoid lethality that can occur with longer exposures. Even though we used a higher ethanol concentration (73% ethanol vapor) than had been used for the sedation assays described above, several vials of many genotypes failed to reach 50% sedation during the first and/or second exposure. We therefore quantified sedation in this assay as the time required for 25% of flies to sedate (ST25), and tolerance was calculated as the increase in ST25 from the first to the second exposure. Tolerance values calculated from ST25 values were very similar to those calculated from ST50 values (data not shown). Two mutants (4–12 and 20–29) consistently failed to reach even 25% sedation during the first and/or second exposure. This prevented us from calculating their ST25 and tolerance values, so they were excluded from the analysis.
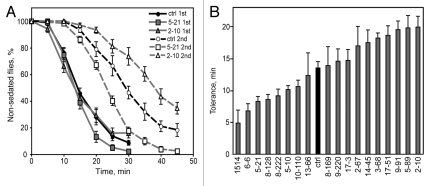
Control flies had an average sedation tolerance of 13.5 ± 1.0 min, indicating that flies took an average of 13.5 minutes longer to sedate during the second exposure as compared to the first (Fig. 4A and B). Tolerance values for all mutants tested are shown in Figure 4B and ranged from 4.9 to 19.9 min. Sedation curves for a mutant showing decreased tolerance (5–21) and one showing increased tolerance (2–10) relative to the control are shown in Figure 4A. Both 5–21 and 2–10 exhibited initial sedation sensitivity similar to the control (p > 0.05 for each mutant vs. control ST25, t-tests). However, during the second exposure 5–21 showed increased sensitivity relative to the control, indicating decreased tolerance, while 2–10 showed decreased sensitivity relative to the control, indicating increased tolerance (p < 0.01 for each mutant vs. control ST25, t-tests).
Figure 4.
Tolerance to ethanol-induced sedation. (A) Flies given two exposures to 73% ethanol vapor become less sensitive to sedation during the second exposure (dotted lines, open symbols) as compared to the first exposure (solid lines, filled symbols), reflecting tolerance. Two tolerance mutants are shown: both 5–21 and 2–10 exhibited sedation sensitivity similar to the control during the first exposure (p > 0.05 for each mutant vs. control ST25, t-tests), but during the second exposure respectively showed increased sensitivity (=decreased tolerance) or decreased sensitivity (=increased tolerance) relative to the control (p < 0.01 for each mutant vs. control ST25, t-tests). (B) Ethanol sedation tolerance for each mutant (n = 5–7 for mutants, n = 18 for control).
Ethanol pharmacokinetics.
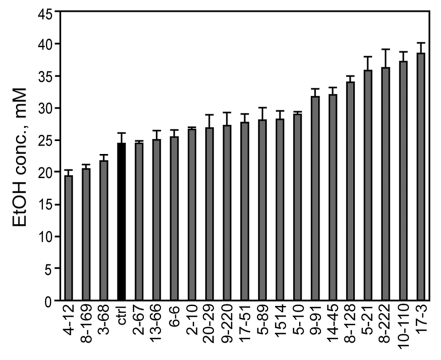
Since our panel of mutants displayed a wide range of ethanol responses in all four behavioral assays, we tested whether the mutants might also vary in their ethanol pharmacokinetics. We measured the internal ethanol concentration of flies that had been exposed for 15 min to a moderate concentration of 47% ethanol vapor. Control flies contained 24.4 ± 1.6 mM ethanol (Fig. 5). The ethanol concentration in the mutants ranged from 19.3 to 38.4 mM ethanol (Fig. 5), indicating that altered ethanol absorption or metabolism may underlie the behavioral phenotypes in some of these lines.
Figure 5.
Ethanol pharmacokinetics of the mutants. Flies were exposed to 47% ethanol vapor for 15 min and their internal ethanol concentration was measured (n = 3).
Correlations between ethanol sensitivity, tolerance and consumption preference.
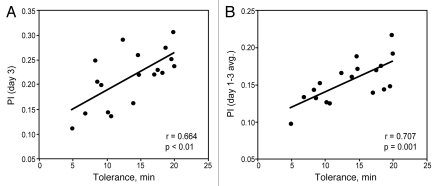
After measuring ethanol preference, hyperactivity, sedation and tolerance in the 20 mutants, we asked whether behavioral responses in different assays were correlated. We were most interested in determining which behaviors might correlate with ethanol preference. We found that ethanol preference on day 3 of the assay correlated positively with ethanol tolerance (r = 0.664, p < 0.01, Pearson's correlation; Fig. 6A). In addition, tolerance correlated slightly more strongly with the average ethanol preference across all 3 days (r = 0.707, p = 0.001, Pearson's correlation; Fig. 6B).
Figure 6.
Ethanol consumption preference correlates positively with ethanol tolerance. (A) Ethanol preference on day 3 correlated positively with ethanol tolerance (r = 0.664, p < 0.01, Pearson's correlation, n = 18). (B) Average ethanol preference across all three days of the preference assay correlated positively with ethanol tolerance (r = 0.707, p = 0.001, Pearson's correlation, n = 18).
We found no significant correlations between ethanol preference and either ethanol hyperactivity, sedation or internal ethanol concentration (Table 2). Furthermore, there were no significant correlations for any pairwise comparisons of ethanol hyperactivity, sedation or internal ethanol concentration (Table 2). However, the correlation between ethanol concentration and both maximum hyperactivity (r = -0.437, p = 0.054) and sedation ST50 (r = -0.429, p = 0.059) were close to significance (Pearson's correlation). These correlations would suggest that flies that achieve higher internal ethanol levels tend to exhibit decreased hyperactivity and increased sedation.
Table 2.
Results of correlation analyses comparing different behavioral responses to ethanol
| Pref. vs. Hyp. | Pref. vs. Sed. | Pref. vs. Tol. | Pref. vs. EtOH | |
| day 1 | −0.006 (0.979) |
−0.211 (0.372) |
0.211 (0.399) |
−0.104 (0.662) |
| day 2 | −0.051 (0.829) |
0.039 (0.871) |
0.288 (0.247) |
−0.184 (0.438) |
| day 3 | 0.238 (0.312) |
0.348 (0.133) |
0.664 (0.003*) |
−0.206 (0.384) |
| day 1–3 avg. | 0.136 (0.567) |
0.136 (0.568) |
0.707 (0.001*) |
−0.285 (0.229) |
| Hyp. vs. Sed. | Hyp. vs. Tol. | Hyp. vs. EtOH | Sed. vs. Tol. | Sed. vs. EtOH | Tol. vs. EtOH |
| 0.195 (0.410) |
0.289 (0.245) |
−0.437 (0.054) |
0.026 (0.920) |
−0.429 (0.059) |
−0.192 (0.446) |
We calculated all pairwise correlations between five responses to ethanol: ethanol preference on day 3 (pref.), maximum ethanol hyperactivity (hyp.), sedation ST50 (sed.), sedation tolerance (tol.) and internal ethanol concentration (EtOH). r values for Pearson's correlation are shown with p values in parentheses (*p < 0.05, in bold). For analyses involving preference, correlations were calculated using PI values on each day of the assay as well as the average across all 3 days. n = 18 mutant lines for analyses involving tolerance and n = 20 mutant lines for all other analyses.
GAL4 expression patterns in the mutant lines.
The 20 mutant lines tested in this study are enhancer traps, in which the transcriptional activator GAL4 is expressed in cells likely to express the endogenous gene affected by each P element. We wondered whether these expression patterns might be related to the diverse behavioral phenotypes exhibited by the mutants. We visualized the GAL4 expression pattern in each mutant line by crossing the lines to a UAS-GFP reporter (Table 3), with the exception of lines 4–12 and 13–66, which will be described in separate publications.
Table 3.
GAL4 expression patterns in the adult brain for each line
| OL | AL | CC | MB | PI | SEG | Other | |
| 2–10 | + | + | + | + | |||
| 2–67 | |||||||
| 3–68 | + | + | + | ||||
| 5–10 | + | + | + | + | + | ||
| 5–21 | + | + | + | + | + | + | |
| 5–89 | + | + | |||||
| 6–6 | + | + | + | ||||
| 8–128 | + | + | + | ||||
| 8–169 | |||||||
| 8–222 | + | + | + | + | + | + | |
| 9–91 | + | + | + | + | |||
| 9–220 | |||||||
| 10–110 | + | + | + | + | |||
| 14–45 | + | + | + | + | |||
| 1514 | + | + | + | + | + | + | + |
| 17-3 | + | + | + | + | + | + | |
| 17–51 | + | + | + | + | + | + | + |
| 20–29 | + |
Abbreviations: OL, optic lobe; AL, antennal lobe; CC, central complex; MB, mushroom body; PI, pars intercerebralis; SEG, subesophageal ganglion.
Of the 18 lines analyzed, 15 showed GAL4 expression in the adult brain; the remaining lines may show expression in the adult ventral nerve cord or in the developing nervous system, which were not analyzed. Most lines showed GAL4 expression in multiple brain areas. The most common site of GAL4 expression in the adult brain was the subesophageal ganglion (SEG; 13/18 lines), mainly due to projections from cells in the pars intercerebralis (PI; 12/18 lines). To determine whether this high proportion of GAL4 expression in the PI and SEG was meaningful, we analyzed the number of random enhancer trap lines from the same collection expressing GAL4 in these regions. Of 50 random lines analyzed, 38% expressed GAL4 in the PI and 60% expressed GAL4 in the SEG. In comparison to the random lines, our ethanol sensitivity mutants showed a significant over-representation of GAL4 expression in the PI but not the SEG (p < 0.05, chi-square test), suggesting that this region may be important in regulating ethanol responses. Subsets of mutant lines also expressed GAL4 in the antennal lobe (nine lines), optic lobe (seven lines), central complex (five lines) and mushroom body (five lines). There was no clear relationship between the expression pattern and behavioral phenotypes of the mutant lines, as different lines expressing in the same brain region did not appear more likely to share the same behavioral phenotypes.
Discussion
Correlations between ethanol consumption and both sensitivity and tolerance to ethanol have been observed in human and rodent studies, though much of the data is conflicting. Because we have recently characterized ethanol consumption preference in Drosophila and shown that this paradigm models features of addiction-like behavior,39 we now had the opportunity to test whether the same relationships between ethanol consumption and other ethanol responses also exist in flies. In contrast to most rodent and human studies, we were able to test many Drosophila strains under the same conditions and in the same genetic background, representing a highly systematic and controlled approach.
We measured ethanol preference, hyperactivity, sedation and sedation tolerance in 20 mutants that had been identified as ethanol-sensitive or -resistant in various behavioral assays. Ethanol preference was the most variable behavior; this variability has been previously observed and likely reflects the complexity of ethanol preference as a choice assay in which flies must integrate chemosensory and experience-dependent information.39 For ethanol hyperactivity, sedation and tolerance, we identified some lines that showed an increased response and some with decreased response compared to the control. In contrast, we found mutants that had decreased ethanol consumption preference but none with increased preference.
While it was not our primary goal to find new genes modulating ethanol-induced behaviors, our use of P element mutants allowed us to easily identify the gene(s) likely to be affected in each mutant line. This lays the groundwork for future studies to confirm the role of these genes in regulating ethanol responses and uncover the molecular pathways in which they function. We have also characterized the GAL4 expression pattern in each mutant line, which represents the likely expression pattern of the affected gene, allowing future studies to more easily identify the neurons in which each gene functions. Interestingly, in our set of mutants there was an over-representation of lines expressing GAL4 in the PI, a region that contains neuropeptidergic cells, including insulin-producing cells.43 The PI has also been implicated in previous studies of ethanol sensitivity36,43,44 and may therefore represent an important locus for regulating ethanol responses.
We observed a strong positive correlation between ethanol tolerance and ethanol preference. This correlation suggests that tolerance might be one reason why flies increase their ethanol consumption over time. The correlation between ethanol consumption and tolerance is consistent with human and rodent studies that have addressed this question (see Introduction). However, in humans there is also evidence that risk of AUD correlates with sensitivity to the stimulant effects of alcohol and resistance to its depressant effects (see Introduction). We did not find a correlation between ethanol consumption preference and either locomotor hyperactivity, the major stimulant effect of ethanol in flies, or sedation, the major depressant effect. These data indicate that ethanol preference is more strongly linked to tolerance, a form of ethanol-induced plasticity that develops over time, than to naive ethanol responses. In humans the relative importance of ethanol tolerance versus naive sensitivity in influencing ethanol consumption has not been determined within a single study; it will be interesting to see whether the same relationship holds.
We did not observe any pairwise correlations between ethanol sedation, hyperactivity and tolerance. Berger et al. also did not observe a correlation between sedation and sedation tolerance in a set of 52 long-term memory mutants with widely varying ethanol sensitivities.45 Kong et al. did, however, observe correlations between sedation sensitivity and both sedation tolerance and hyperactivity, although the latter was fairly weak.46 These differences may be attributable to the different sets of mutants that were analyzed as well as the varying methods of quantifying behavior. In particular, Kong et al. conducted ethanol hyperactivity and sedation assays at the same ethanol concentration, which could account for the correlation they observed between these two behaviors, whereas we designed our experiments such that flies would not sedate during the hyperactivity assay. Kong et al. also conducted sedation assays at a lower concentration than we did and quantified both sedation and tolerance by the fraction of flies sedated at 26 minutes rather than the ST50. Finally, the strains analyzed by Kong et al. contained mutations in genes known to be transcriptionally regulated by ethanol, which represents a unique subset of strains different from our mutants which were chosen based on phenotype.
The lack of a strong correlation between internal ethanol concentration and any of the ethanol responses tested suggests that the primary effect of the mutations in this study is to alter the way that the nervous system reacts to ethanol rather than simply disrupting ethanol absorption or metabolism. In addition, the lack of correlation between ethanol hyperactivity, sedation and tolerance supports the view that these assays measure distinct ways by which ethanol affects the nervous system. However, it is interesting that many mutants isolated for a phenotype in one of the assays also exhibited phenotypes in a different assay. For example, several mutants isolated for altered ethanol-induced sedation or loss of postural control (e.g., 2–10, 4–12, 10–110, 1514) also exhibited altered ethanol hyperactivity (4–12, 10–110) or tolerance (1514, 2–10). These results suggest that shared genetic pathways mediate different ethanol responses, even if the responses themselves are not correlated, which may be informative in identifying the molecular mechanisms underlying these behaviors.
In this study we measured only one of two types of tolerance characterized in flies: rapid tolerance, which is induced by a relatively brief ethanol exposure that causes intoxication.47 A second form of tolerance, chronic tolerance, is induced by prolonged (∼24 hr) exposure to a low ethanol concentration that does not produce overt intoxication, and is mechanistically distinct from rapid tolerance.47 It remains to be seen whether chronic tolerance also correlates with ethanol preference in flies. Both rapid and chronic tolerance develop between discrete ethanol exposures; a third form of tolerance present in mammals, acute functional tolerance, develops within a single intoxicating session.48 However, acute functional tolerance has not yet been characterized in flies as it is difficult to distinguish from naive ethanol sensitivity using our current assays.
Our results indicate that shared genetic mechanisms underlie ethanol tolerance and preference, but an open question is whether this relationship occurs at the mechanistic or behavioral level. Specifically, one possibility is that the neural or molecular pathways that are shared between ethanol tolerance and preference directly promote both of these behaviors in parallel. However, an alternative model (commonly applied to humans and rodents) posits that animals that develop greater tolerance choose to consume more ethanol because they require a greater internal ethanol concentration in order to achieve a certain desired level of behavioral intoxication. This model suggests that ethanol tolerance and preference share the same genetic mechanisms only because ethanol preference is directly modulated by tolerance. Regardless of the mechanism, the positive correlation between ethanol preference and tolerance appears to be shared by flies, rodents and humans. Thus, there are fundamental, evolutionarily conserved biological mechanisms linking these ethanol-induced behaviors.
Materials and Methods
Fly stocks and maintenance.
Flies were reared at 25°C and 70% relative humidity on standard cornmeal/molasses food. Flies tested for ethanol consumption preference were kept in a 12/12 hr light/dark cycle since feeding behavior is strongly modulated by circadian rhythm. Flies tested in all other behavioral assays, which only measure short term responses, were kept in constant light in order to minimize the effect of circadian variation. All assays were performed on 3- to 4-day-old males that were collected by anesthetization with CO2. Nineteen of 20 mutants were obtained from the Heberlein Lab P[GAL4] collection; mutant 1514 was obtained from the Japanese NP consortium (NP1514). Mutants were outcrossed for at least five generations to the w Berlin control strain to remove unlinked modifiers and homogenize the genetic background.
Mutant characterization.
The genomic DNA flanking the P elements was isolated by inverse PCR, allowing us to identify the insertion sites. GAL4 expression was imaged in dissected brains of GAL4/UAS-GFP adult males under a fluorescence microscope; any observable expression within a specified region (regardless of intensity) was noted in Table 3.
Behavioral assays.
Ethanol consumption preference was measured as described using eight flies per vial.39 Briefly, flies choose between liquid food containing 0% or 15% ethanol presented in 5 µL capillary tubes placed vertically through the top of their vials. The volumes consumed are determined daily by measuring the descent of each liquid column. Ethanol hyperactivity, sedation and sedation tolerance were measured in the booz-o-mat, an 8-chambered apparatus in which flies are exposed to a specific concentration of ethanol vapor by mixing pure ethanol vapor with humidified air at a fixed ratio.33 Ethanol hyperactivity was measured by video tracking of flies as described,33 using an ethanol vapor concentration of 47% or 33% as specified. Maximum hyperactivity was calculated for each vial as the average of the three consecutive time points with the highest speed; these points varied from vial to vial. Ethanol sedation was assayed manually as described36 using an ethanol concentration of 67% for general sedation assays and 73% for sedation tolerance assays. Twenty flies per vial were used for ethanol hyperactivity, sedation and tolerance assays.
General sedation assays were initially conducted using a 35-min ethanol exposure. Sedation sensitivity was quantified as the time required for 50% of flies to sedate (ST50). The ST50 was calculated by linear interpolation (or linear extrapolation if the last time point was close to 50%). Four mutants (20–29, 1514, 13–66, 4–12) did not reach 50% sedation by 35 min and were therefore retested in a 60 min (20–29, 1514, 13–66) or 90 min (4–12) assay using different flies.
Tolerance assays were conducted using a 30-min ethanol exposure followed by a 45-min ethanol exposure 4 hrs later. Because many vials did not reach ST50 during either the first or second exposure, we quantified sedation in this assay as the time required for 25% of flies to sedate (ST25), and tolerance was calculated as ST252nd exposure - ST251st exposure. For two mutants (4–12 and 20–29), most vials did not come close to reaching 25% sedation; these lines were excluded from analysis because their tolerance could not be calculated.
Every fly strain was tested in each behavioral assay on at least two separate days (n = 18 for ethanol consumption preference, n = 10 for ethanol hyperactivity, n = 8–13 for sedation and n = 5–7 for tolerance). Unless otherwise specified, n refers to the number of vials. The control line used was w Berlin, the genetic background for all strains tested. Mean values for the control were obtained by averaging all experiments, but when comparing specific mutants to the control (Figs. 1A–4A) we only compared samples that were tested simultaneously.
Measurement of ethanol concentration.
Flies were frozen in liquid nitrogen and homogenized in 50 mM Tris-HCl (pH 7.5, 200 µL for 20 flies). Ethanol concentrations were measured in fly homogenates using the Ethanol Assay kit from Diagnostic Chemicals Limited (catalog no. 229-29). To calculate the ethanol concentration in flies, the volume of one fly was estimated to be 2 µL as previously reported.34
Statistical analyses.
Statistical analyses were performed using GraphPad Prism, Version 4. Statistical significance of mutant phenotypes was established using Student's t-test. Pearson's correlation was used for correlation analyses. All graphs represent mean ± SEM.
Acknowledgments
We thank Liqun Luo for some GAL4 expression images, Chris Kliethermes for advice on statistical analyses and members of the Heberlein laboratory for comments on the manuscript. This work was supported by an NSF predoctoral fellowship (Anita V. Devineni) and grants from NIH/NIAAA (Ulrike Heberlein).
References
- 1.Babor TF, Berglas S, Mendelson JH, Ellingboe J, Miller K. Alcohol, affect, and the disinhibition of verbal behavior. Psychopharmacology (Berl) 1983;80:53–60. doi: 10.1007/BF00427496. [DOI] [PubMed] [Google Scholar]
- 2.Kalant H. Current state of knowledge about the mechanisms of alcohol tolerance. Addict Biol. 1996;1:133–141. doi: 10.1080/1355621961000124756. [DOI] [PubMed] [Google Scholar]
- 3.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- 4.Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- 5.Trim RS, Schuckit MA, Smith TL. The relationships of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcohol Clin Exp Res. 2009;33:1562–1570. doi: 10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- 7.Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcohol Clin Exp Res. 2010;34:199–202. doi: 10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 8.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 9.Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–794. [PubMed] [Google Scholar]
- 10.King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- 11.Kaplan RF, Hesselbrock VM, O'Connor S, DePalma N. Behavioral and EEG responses to alcohol in nonalcoholic men with a family history of alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:873–885. doi: 10.1016/0278-5846(88)90083-8. [DOI] [PubMed] [Google Scholar]
- 12.Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- 13.Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: Is better consilience possible? Addict Biol. 2010;15:125–144. doi: 10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtz DL, Stewart RB, Zweifel M, Li TK, Froehlich JC. Genetic differences in tolerance and sensitization to the sedative/hypnotic effects of alcohol. Pharmacol Biochem Behav. 1996;53:585–591. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- 15.Froehlich JC, Wand GS. Adenylyl cyclase signal transduction and alcohol-induced sedation. Pharmacol Biochem Behav. 1997;58:1021–1030. doi: 10.1016/s0091-3057(97)00305-5. [DOI] [PubMed] [Google Scholar]
- 16.Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- 17.Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, et al. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- 18.Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, et al. Different sensitivity to ethanol in alcohol-preferring sP and -nonpreferring sNP rats. Alcohol Clin Exp Res. 2000;24:1603–1608. [PubMed] [Google Scholar]
- 19.Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, et al. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- 20.Boehm SL, Peden L, Jennings AW, Kojima N, Harris RA, Blednov YA. Overexpression of the fyn-kinase gene reduces hypnotic sensitivity to ethanol in mice. Neurosci Lett. 2004;372:6–11. doi: 10.1016/j.neulet.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Waller MB, Murphy JM, McBride WJ, Lumeng L, Li TK. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1986;24:617–623. doi: 10.1016/0091-3057(86)90567-8. [DOI] [PubMed] [Google Scholar]
- 22.Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, et al. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol. 2001;23:123–126. doi: 10.1016/s0741-8329(00)00144-0. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association, author. Diagnostic and Statistical Manual of Mental Disorders. Vol. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 24.Schuckit MA, Smith TL, Hesselbrock V, Bucholz KK, Bierut L, Edenberg H, et al. Clinical implications of tolerance to alcohol in nondependent young drinkers. Am J Drug Alcohol Abuse. 2008;34:133–149. doi: 10.1080/00952990701877003. [DOI] [PubMed] [Google Scholar]
- 25.Le AD, Kiianmaa K. Characteristics of ethanol tolerance in alcohol drinking (AA) and alcohol avoiding (ANA) rats. Psychopharmacology (Berl) 1988;94:479–483. doi: 10.1007/BF00212841. [DOI] [PubMed] [Google Scholar]
- 26.Bowers BJ, Owen EH, Collins AC, Abeliovich A, Tonegawa S, Wehner JM. Decreased ethanol sensitivity and tolerance development in gamma-protein kinase C null mutant mice is dependent on genetic background. Alcohol Clin Exp Res. 1999;23:387–397. [PubMed] [Google Scholar]
- 27.Bowers BJ, Wehner JM. Ethanol consumption and behavioral impulsivity are increased in protein kinase Cgamma null mutant mice. J Neurosci. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-21-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grahame NJ, Rodd-Henricks K, Li TK, Lumeng L. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology (Berl) 2000;151:252–260. doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- 29.Wallace MJ, Newton PM, Oyasu M, McMahon T, Chou WH, Connolly J, et al. Acute functional tolerance to ethanol mediated by protein kinase Cepsilon. Neuropsychopharmacology. 2007;32:127–136. doi: 10.1038/sj.npp.1301059. [DOI] [PubMed] [Google Scholar]
- 30.Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- 31.Chappell AM, Weiner JL. Relationship between ethanol's acute locomotor effects and ethanol self-administration in male Long-Evans rats. Alcohol Clin Exp Res. 2008;32:2088–2099. doi: 10.1111/j.1530-0277.2008.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- 33.Wolf FW, Rodan AR, Tsai LTY, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- 35.Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LTY, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett S, et al. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137:949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 38.Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila Homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27:4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf FW, Eddison M, Lee S, Cho W, Heberlein U. GSK-3/Shaggy regulates olfactory habituation in Drosophila. Proc Natl Acad Sci USA. 2007;104:4653–4657. doi: 10.1073/pnas.0700493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaFerriere H, Guarnieri DJ, Sitaraman D, Diegelmann S, Heberlein U, Zars T. Genetic dissociation of ethanol sensitivity and memory formation in Drosophila melanogaster. Genetics. 2008;178:1895–1902. doi: 10.1534/genetics.107.084582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eddison M, Guarnieri DJ, Cheng L, Liu CH, Moffat KG, Davis G, et al. arouser reveals a role for synapse number in the regulation of ethanol sensitivity. Neuron. 2011;70:979–990. doi: 10.1016/j.neuron.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 43.Corl AB, Rodan AR, Heberlein U. Insulin signaling in the nervous system regulates ethanol intoxication in Drosophila melanogaster. Nat Neurosci. 2005;8:18–19. doi: 10.1038/nn1363. [DOI] [PubMed] [Google Scholar]
- 44.Rodan AR, Jr, Heberlein U. Functional dissection of neuroanatomical loci regulating ethanol sensitivity in Drosophila. J Neurosci. 2002;22:9490–9501. doi: 10.1523/JNEUROSCI.22-21-09490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res. 2008;32:895–908. doi: 10.1111/j.1530-0277.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, et al. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol Clin Exp Res. 2010;34:302–316. doi: 10.1111/j.1530-0277.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger KH, Heberlein U, Moore MS. Rapid and chronic: two distinct forms of ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2004;28:1469–1480. doi: 10.1097/01.alc.0000141817.15993.98. [DOI] [PubMed] [Google Scholar]
- 48.Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev. 1971;23:135–191. [PubMed] [Google Scholar]