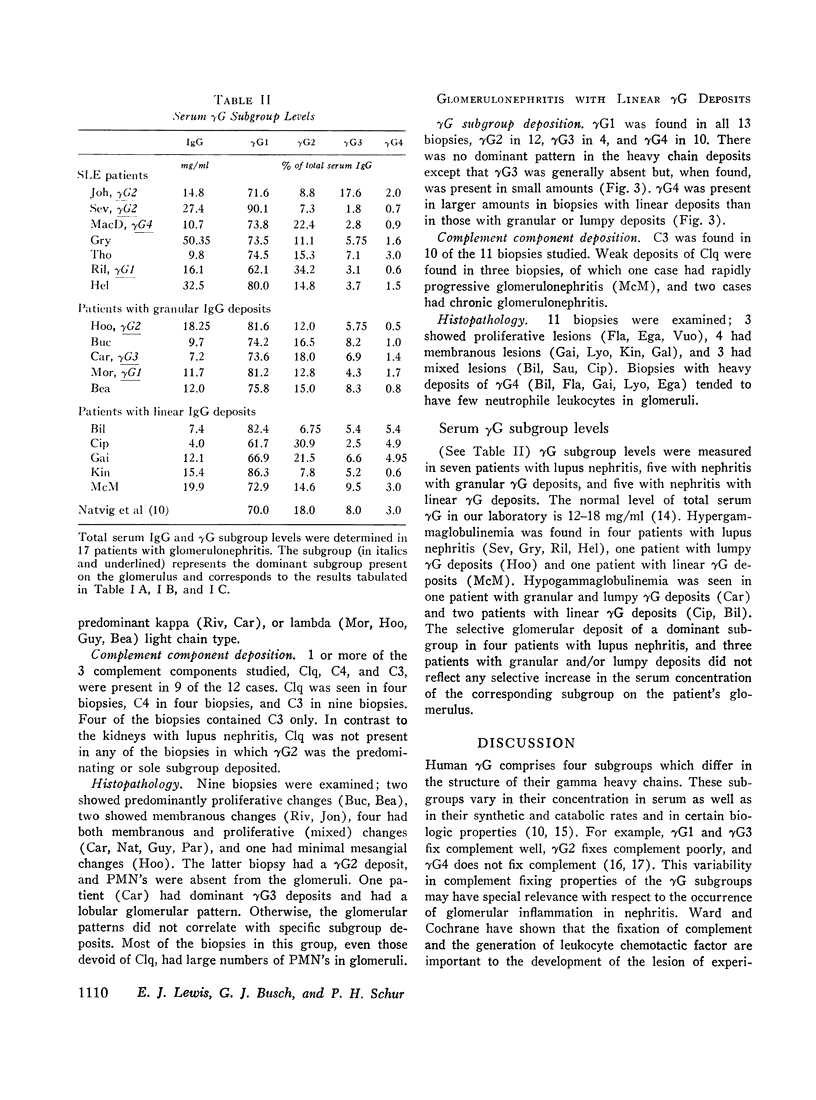
Abstract
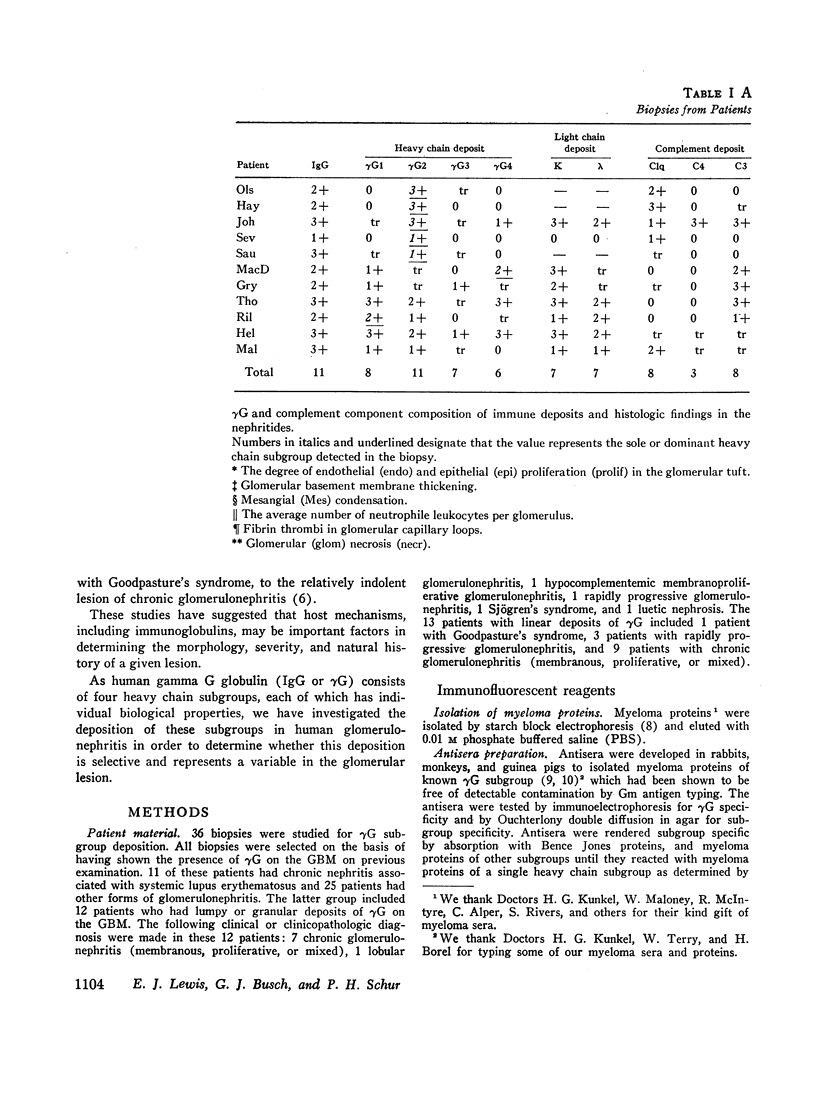
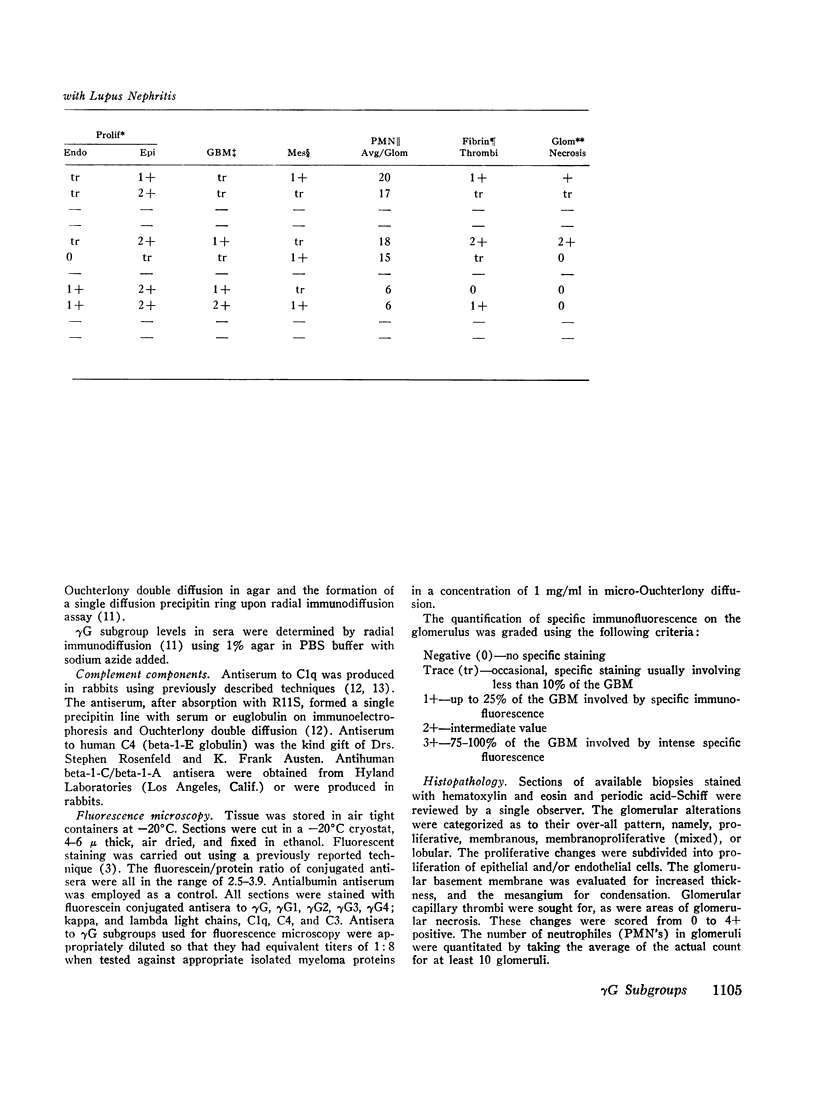
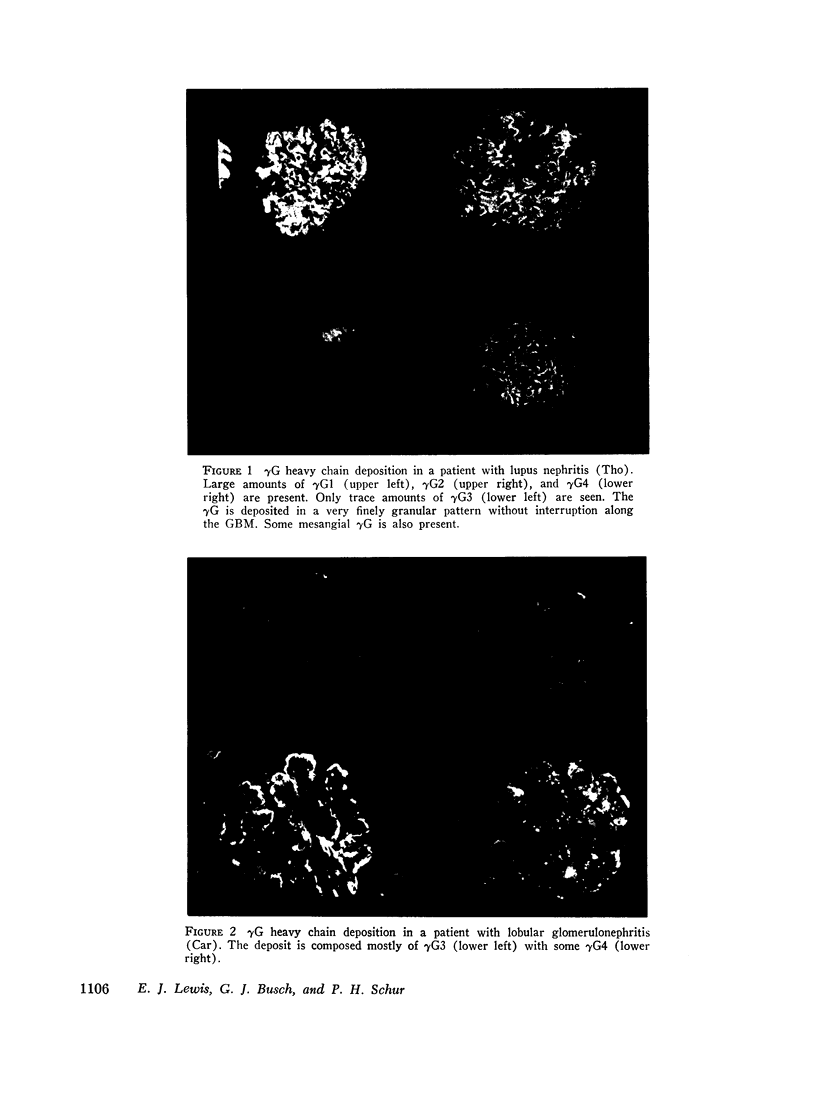
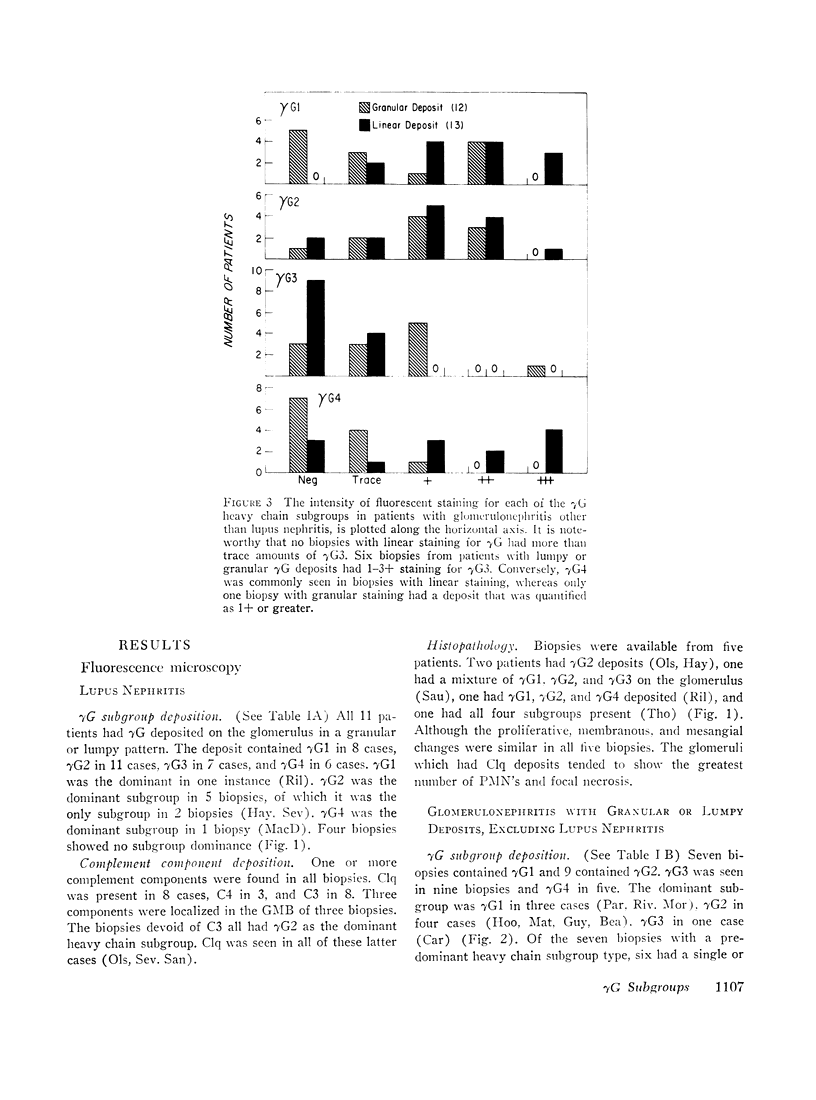
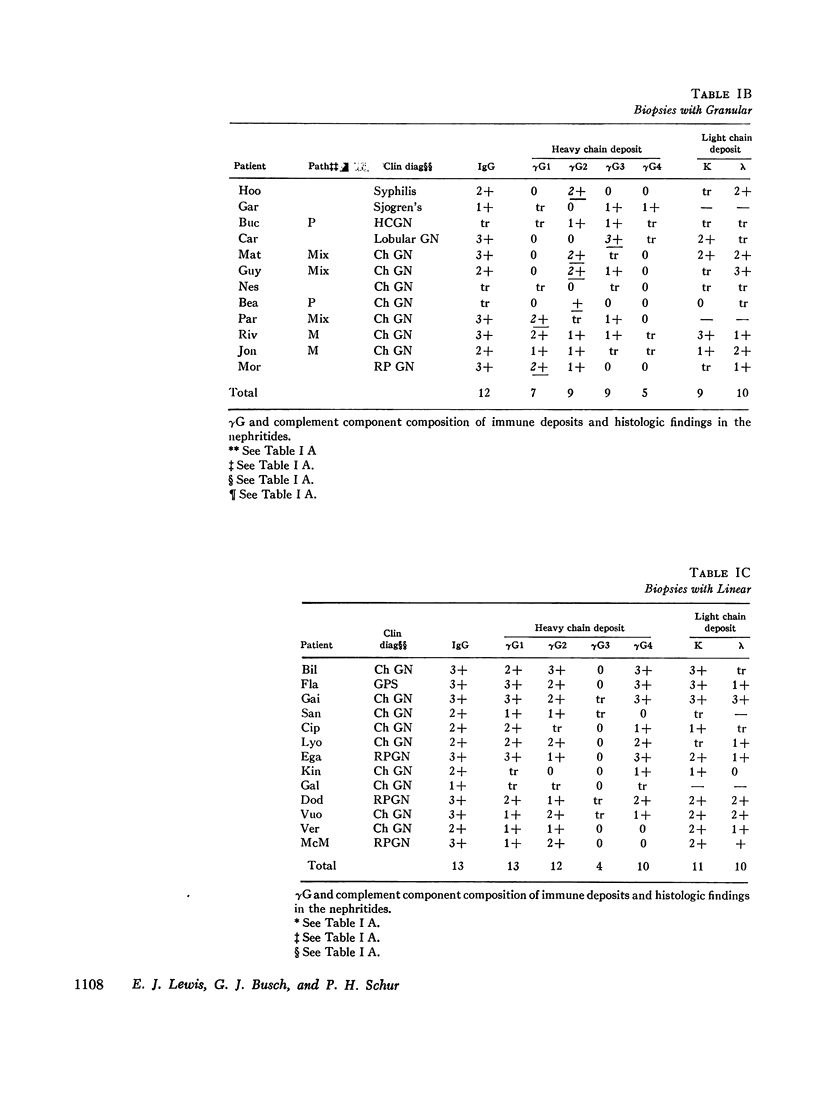
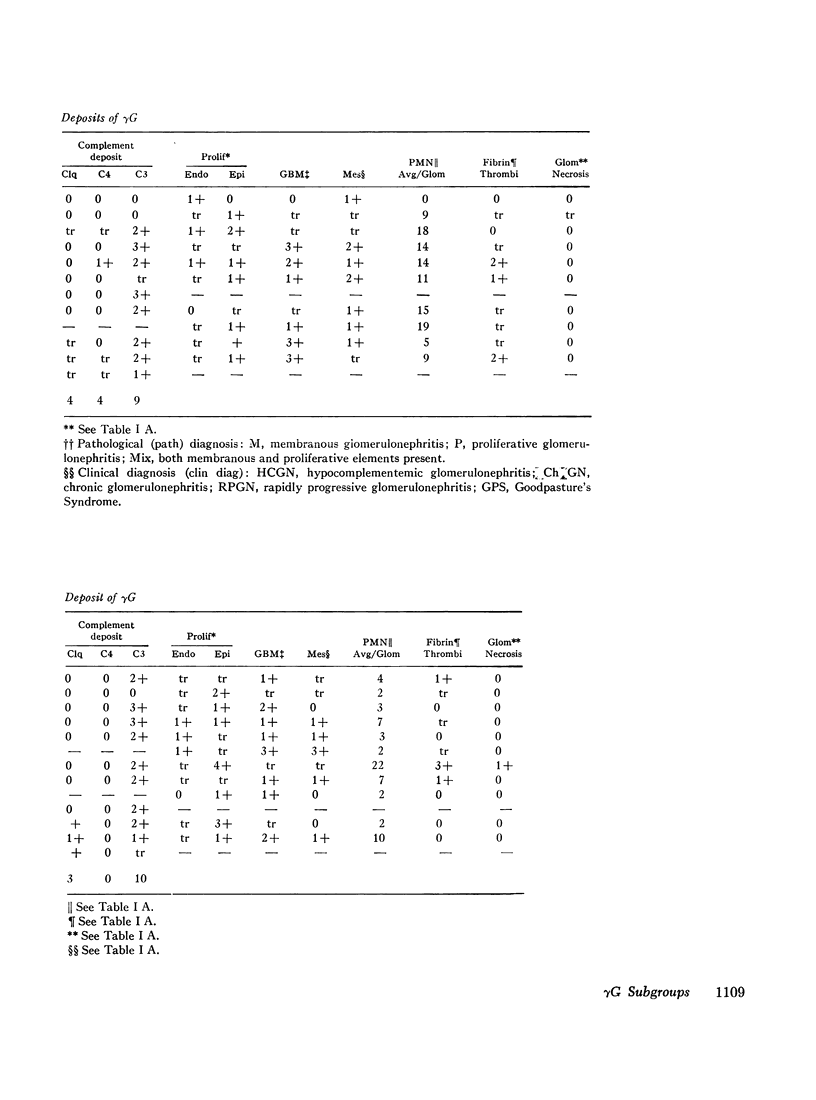
36 renal biopsies from patients with nephritis were studied for glomerular localization of the heavy chain subgroups of immunoglobulin G (IgG or γG). The deposition pattern of these subgroups was selective and did not reflect the normal serum concentration of these proteins. γG2, which comprises 18% of normal serum γG, was the predominant or unique subgroup deposited in five cases of lupus nephritis and four biopsies with other forms of nephritis associated with granular γG deposits. γG3, which normally makes up only 8% of the serum γG, was the dominant subgroup seen in one biopsy of lobular glomerulonephritis. Patients with linear γG deposits generally had a selective absence of γG3 and often had large amounts of γG4 (normally 3% of the serum γG) deposited. The deposition of complement components C1q, C4, and C3 was variable. One biopsy had only γG2 and no complement components in the deposits and had no neutrophile leukocyte infiltration. This latter observation correlates well with the poor ability of γG2 to fix complement in vitro. Similarly, deposits containing large amounts of γG4, which does not fix complement, also tended to have less inflammatory infiltrate than deposits devoid of this subgroup. The selective deposition of monotypic or restricted γG subgroups on the glomerulus supports the likelihood that the γG represents antibody. The nature of the subgroup involved in the deposit may represent one variable in the determination of the inflammatory and morphological picture that evolves in human glomerulonephritis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger J. IgA glomerular deposits in renal disease. Transplant Proc. 1969 Dec;1(4):939–944. [PubMed] [Google Scholar]
- COCHRANE C. G., UNANUE E. R., DIXON F. J. A ROLE OF POLYMORPHONUCLEAR LEUKOCYTES AND COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1965 Jul 1;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., FELDMAN J. D., VAZQUEZ J. J. Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J Exp Med. 1961 May 1;113:899–920. doi: 10.1084/jem.113.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- GREY H. M., KUNKEL H. G. H CHAIN SUBGROUPS OF MYELOMA PROTEINS AND NORMAL 7S GAMMA-GLOBULIN. J Exp Med. 1964 Aug 1;120:253–266. doi: 10.1084/jem.120.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germuth F. G., Jr, Senterfit L. B., Pollack A. D. Immune complex disease. I. Experimental acute and chronic glomerulonephritis. Johns Hopkins Med J. 1967 Apr;120(4):225–251. [PubMed] [Google Scholar]
- HAMMER D. K., DIXON F. J. Experimental glomerulonephritis. II. Immunologic events in the pathogenesis of nephrotoxic serum nephritis in the rat. J Exp Med. 1963 Jun 1;117:1019–1034. doi: 10.1084/jem.117.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- KUNKEL H. G., SLATER R. J. Zone electrophoresis in a starch supporting medium. Proc Soc Exp Biol Med. 1952 May;80(1):42–44. doi: 10.3181/00379727-80-19516. [DOI] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Lerner R. A., Glassock R. J., Dixon F. J. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967 Dec 1;126(6):989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORSE J. H., CHRISTIAN C. L. IMMUNOLOGICAL STUDIES OF THE 11S PROTEIN COMPONENT OF THE HUMAN COMPLEMENT SYSTEM. J Exp Med. 1964 Feb 1;119:195–209. doi: 10.1084/jem.119.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A. F., Jr, Drummond K. N., Good R. A., Vernier R. L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Invest. 1966 Feb;45(2):237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Kunkel H. G. Characteristics of a soluble nuclear antigen precipitating with sera of patients with systemic lupus erythematosus. J Immunol. 1966 Mar;96(3):464–471. [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G. BOUND COMPLEMENT AND IMMUNOLOGIC INJURY OF BLOOD VESSELS. J Exp Med. 1965 Feb 1;121:215–234. doi: 10.1084/jem.121.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]