Abstract
The majority of potent new biologics today are IgG-based molecules that have demonstrated tissue-targeting specificity with favorable clinical response. Several factors determine the efficacy of these products, including target specificity, serum half-life and effector functions via complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity or drug conjugates. In this review, we will focus on the interaction between therapeutic antibody and neonatal Fc receptor (FcRn), which is one of the critical factors in determining the circulating antibody half-life. Specifically, we will review the fundamental biology of FcRn, FcRn functions in various organs, Fc mutations designed to modulate binding to FcRn, IgG-based therapeutics that directly exploit FcRn functions and tools and strategies used to study FcRn-IgG interactions. Comprehensive understanding of FcRn-IgG interactions not only allows for development of effective therapeutics, but also avoidance of potential adverse effects.
Key words: FcRn, Brambell receptor, fcgrt, IgG, antibody, transcytosis, Fc-fusion, Fab, albumin
Introduction
One of the first experiments in immunotherapy was performed more than 100 years ago when Behring and Kitasato demonstrated immune protection in rabbits by blood transfusions from other rabbits immunized with tetanus toxin.1 This eventually led to similar use in humans by transfusion of serum for passive immunity protection from toxin and infection, such as rubeola. Isolation of IgG began in the 1930s, and the process was further refined by Edwin J. Cohn, who was also able to isolate the albumin fraction from human serum. However, use of the Cohn method still resulted in severe anaphylaxis due to IgG re-aggregation; this approach was improved in the 1960s before it was accepted for clinical use. It was then another three decades until the first recombinant IgG therapeutic, infliximab (Remicade®), was approved by the US Food and Drug Administration for the treatment of Crohn disease.
The history of FcRn research parallels that of antibody therapeutics. Studies in passive transfer of immunity from mother to neonate led Francis William Rogers Brambell (1901–1970) to speculate 50 years ago that this process is mediated by a receptor that actively transports IgG at the neonatal rodent intestine. It was not until 1989 when Neil Simister and Keith Mostov cloned this receptor from the epithelial cells of the small intestine of an 11 day old rat.2 This review will focus on the current knowledge regarding the interactions between FcRn and IgG-based therapeutics with summaries of the fundamental biology of FcRn functions, the roles of FcRn in various organs and tissues, mutations in the Fc domains that modulate binding to FcRn, IgG-based therapeutics that directly co-opt FcRn functions, and tools used to study FcRn-IgG interactions.
Fundamental Biology of FcRn
FcRn, encoded by fcgrt and also known as the Brambell receptor, is a MHC class I like molecule associated with beta-2-microglobulin (β2m). It functions to protect IgG and albumin from catabolism, which explains the prolonged half-life of these two proteins compared with other immunoglobulins and liver synthesized proteins. FcRn is also known to mediate bidirectional transcytosis of IgG across epithelial cells as well as membrane recycling.3,4 FcRn-IgG interaction also functions in antigen presentation and cross-presentation in macrophage and dendritic cells.5,6 Each of these functions has important implications in therapeutic antibody development. Detailed review of the biology of FcRn function has also been covered elsewhere.7–9
Soon after the cloning of FcRn, the crystal structure of FcRn-IgG interaction was resolved by Pam Bjorkman and colleagues at the California Institute of Technology who had previously resolved the crystal structure of the MHC class I molecule. The crystal structure studies revealed that two FcRn molecules bind to a single IgG with 2:1 stoichiometry, FcRn-Fc binding was pH dependent with minimal conformational change, and the critical contact sites identified on both molecules were later confirmed by surface plasmon resonance approach.10,11 Two histidine residues, H250 and H251, at the α3 domain of FcRn were found to be responsible for pH depending binding to the CH2-CH3 domain of Fc region at H310, H430, I253, and to a lesser extent at H433 and Y436.12–14
Although Schultze and Heremans had proposed a putative albumin receptor that protects it from catabolism decades ago, it was not until 2003 that Clark Anderson's group demonstrated that FcRn also binds to albumin.15,16 Similar to FcRn-IgG interaction, FcRn binds albumin at acid pH, but not basic pH, and protects it from degradation in the liver, which is the predominant site of protection from catabolism. The FcRn site of albumin contact is H166, while the albumin contact sites are H464, H510 and H535.17,18 Using asymmetric flow field flow fraction and liquid chromatography-mass spectrometry, IgG:FcRn:albumin binding was determined to be at a 1:2:1 molar ratio.19 Data regarding whether FcRn can mediate albumin transcytosis or simultaneously bind to both IgG and albumin in physiologic condition has yet to be published.
Cross-species binding between FcRn and its ligands, IgG and albumin, has revealed distinct interactions that have been exploited for antibody testing in animal models. Murine FcRn has been recognized as being “promiscuous” due to its ability to bind to various species of IgG (including human IgG), while human FcRn can only bind to a limited species of IgG (mainly human and rabbit but not murine).20 Although the major structural difference is the presence of four N-glycans on rodent FcRn compared with the single N-glycan on human FcRn, “rodentization” by conferring additional N-glycans to human FcRn did not increase binding to rodent IgG.3 However, “murinization” of residues 137 and 121–131 at α2 domain of human FcRn allowed binding to mouse IgG.21 The opposite cross-species binding pattern was observed in FcRn-albumin interaction. Mouse FcRn cannot bind to human albumin, while human FcRn can bind to mouse albumin.22
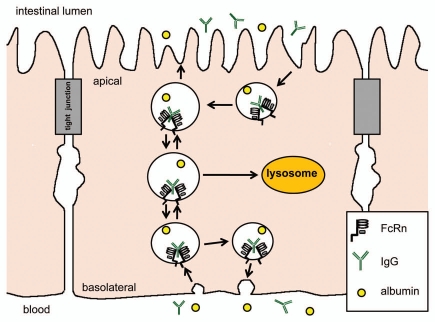
The intracellular pathway of FcRn mediated IgG transport begins with fluid phase pintocytosis of IgG (assuming neutral pH at the membrane surface), and binding to FcRn only occurs after endosome acidification. From there, IgG may be transcytosed to the opposite membrane surface, recycled back to the same membrane surface, or transported to the lysosome for degradation (Fig. 1). The regulation of each of these pathways is still not completely understood and is believed to differ between cell types. Studies using polarizable epithelial cells to dissect the mechanisms of transcytosis and recycling have revealed the importance of the FcRn cytoplasmic tail in determining basolateral membrane targeting. Amino acid residues at the cytoplasmic tail that mediate basolateral membrane targeting include the tryptophan and dileucine motifs, the phosphorylation site at serine-313, and the calmodulin binding site. Other regulators of transport included Rab 25 and myosin Vb in transcytosis, Rab11a in basolateral recycling and exocytosis, and Rab 7 in lysosome targeting.4,23 In dendritic cells, FcRn was found to mediate the transport of multimeric IgG-antigen complex (multiple IgGs attached to a single antigen) to the lysosome, but not that of monomeric IgG-antigen complex (antigen with a single IgG).5
Figure 1.
FcRn mediates bidirectional transport and membrane recycling of IgG in epithelial cells. IgG is believed to enter the cell by fluid phase pintocytosis (assuming neutral pH condition) and does not bind to FcRn until the endosome is acidified. However, in the duodenum, the acidic luminal environment may allow IgG to bind to FcRn at the apical membrane surface before endocytosis.79 Two FcRn bind to a single IgG molecule. IgG may be transcytosed to the opposite membrane surface or recycled back to the same membrane surface.11 The exact intracellular pathway is believed to differ between cells. IgG is dissociated from FcRn at the membrane surface at neutral pH.
FcRn Functions in Organs
FcRn is widely expressed in different organs where the functions can vary significantly. The known functions of FcRn in various organs are briefly reviewed here since they have important relevance to antibody catabolism, transport in drug delivery and potential tissue-specific side effects.
Intestine.
The original observations of passive immunity transfer from mother to neonate more than 50 years ago led Brambell to hypothesize the existence of this receptor in the neonate intestine and to the subsequent cloning from the same site. The biology of FcRn transport in polarized epithelium has been extensively studied and its function is known to extend beyond that of the neonatal period. Humanized rodent models have shown that FcRn in the intestine can transport IgG to the luminal surface to bind to the cognate antigen and transport the IgG-antigen complex back to the lamina propria for subsequent presentation to dendritic cells.24 This adaptive immune response was later demonstrated to be functional in a murine model of bacteria-induced colitis, in which FcRn in the intestine also transports bacteria-specific antibody into the intestinal lumen as a mucosal defense mechanism.25
Lung.
FcRn has been detected in the bronchial epithelial cells of human, non-human primate, rat, mouse and cow. It is not detected in alveoli of human but is detectable in those of rat. Bidirectional FcRn-mediated transport function in the pulmonary epithelial cells has been co-opted for the delivery of Fc-fusion proteins.26
Kidney.
Within the glomeruli, FcRn is expressed in the podocyte and functions to reabsorb IgG and prevent the deposition of immune complex.27 FcRn is not expressed in the distal tubule but is in the human proximal tubule where bidirectional transport has been demonstrated.28 Several in vitro polarized epithelial models that used renal tubule cells also confirmed similar findings of bidirectional transcytosis, and with the predominant vector being basal-to-apical direction. A recent report using a kidney transplant system demonstrated that FcRn in the kidney secretes IgG but salvages albumin from urinary loss.29 Therefore, FcRn in the urinary tract, like that of the intestine, probably also serves in immune surveillance and defense for mucosal protection.
Breast.
FcRn is expressed in the human mammary gland endothelial cells rather than the epithelial cells. In malignant tissues, FcRn has been detected in ductal, lobular and medullary carcinoma, as well as histiocytes within the breast cancer interstitium.30 FcRn is believed to salvage IgG to retain in the systemic circulation. A recent study showed the benefit of protective maternal antibody transport to the neonate in an asthma model and highlighted the role of FcRn in the transfer of passive immunity and tolerance induction.31
Placenta.
As opposed to rodents, in which passive immunity transfer is believed to be predominantly post-natal, human immunity occurs mostly in utero where IgG transfer to the fetus via syncytiotrophoblast and the fetal intestine increases from the second trimester until delivery. IgG1 is most efficiently transported to the fetus, while IgG2 is the least.32 This transport process has led to the design of Fc-fusion proteins for in utero fetus targeting the use of β-glucuronidase (GUS)-Fc fusion protein in a mouse model of GUS-deficiency.33 Despite the benefit of co-opting placental FcRn as a mechanism of drug delivery, it is also a portal for transplacental transport of pathogenic IgG and IgG-viron complex to the fetus.34 Although biologics used in Crohn disease do not appear to increase the risks for malformations, there are limited data on teratogenicity profile in most of therapeutic monoclonal antibodies (mAb) used today. It is possible that mAb toxicities are more associated with developments in the later trimesters than that of the early gestational period.
Hematopoietic cells.
FcRn is expressed in macrophages, monocytes, B cells and dendritic cells. FcRn in macrophages can mediate adaptive immunity by phagocytosis of bacteria that is complexed with bacteria-specific IgG.35 In dendritic cells, FcRn can mediate antigen presentation and cross-presentation, functions that are critical for adaptive immunity and potential therapeutic immunization process.5,6
Vascular endothelium.
By conditional deletion of FcRn in mice using Cre recombinase under the control of the Tie2 promoter, it was demonstrated that FcRn in the vascular endothelium and hematopoietic cells are the major sites for maintaining IgG homeostasis.36
Genital system.
Fcgrt rRNA has been detected in the testes of rats and IgG has been detected in rete testis fluid with a yet to be determined function in immunity.37 Recent studies showed that FcRn in the vaginal epithelium, similar to that of intestine, can mediate bidirectional transport of IgG and secretion of IgG for immune protection.38,39
Liver.
FcRn in the liver is the major site for the maintenance of albumin homeostasis.40 Although little has been published regarding its function in the liver, our preliminary data showed that FcRn in the liver prevents biliary loss of IgG and albumin.
Eye.
FcRn is not detected in the retinal pigmented epithelium or the choroid of the rodent eye, but it is expressed in the epithelium of the cornea, lens and non-pigmented ciliary body. It is also expressed in the blood vessels of the retina and ciliary body, as well as the conjunctiva lymphatic vessel and the optic nerve vessel. Bevacizumab (Avastin®) administered via intravitreal injection in mice was found to diffuse through the retina and transported by FcRn across the blood-retina barrier to the systemic circulation.41,42 Antibody transport was found to increase after laser-induced neovascularization, and this was believed to be caused by TNFα increase and subsequent FcRn upregulation. Because FcRn appeared to be the major conduit in intravitreal antibody elimination and choroidal neovascularization can develop after laser therapy, there has been suggestion that the dose of intraocular injection of anti-vascular endothelial growth factor may need to be adjusted to prevent extra-ocular side effects.
Brain.
FcRn expression has been found at the capillary endothelium and choroid plexus epithelium, and the expression is upregulated in astrocytomas and oligodendrogliomas. An early hypothesis that suggested FcRn in the blood-brain barrier functioned to remove IgG from the brain to systemic circulation via “reverse transcytosis” has not been proven in a subsequent study; thus, the function in the brain is still unclear.43
Skin.
FcRn has been detected in the hair follicle, sebaceous gland, epidermal keratinocyte and melanocytes.44 However, the exact role in each cell type is still unknown.
With the exception of intestines and dendritic cells, FcRn functions in various tissues and organs are still incompletely understood. Furthermore, FcRn-IgG interactions in multiple organs appear to be involved in coordinating immune responses as seen in mucosal epithelium and dendritic cells. Whether other tissues function similarly is still unknown.
Strategies in Modulating FcRn-IgG Binding Affinity and Antibody Half-life
Because FcRn is known to prolong the half-life of IgG, the obvious strategy has been to modulate FcRn-IgG interaction to either extend or shorten the antibody half-life. Half-life extension of therapeutic antibodies would help maintain drug therapeutic levels and reduce the frequency of administration, while half-life reduction would be ideal for diagnostic tests or toxicity control.
Attempts to prolong antibody half-life by mutations of the Fc region critical for FcRn binding have been relatively successful. However, increasing Fc to FcRn binding does not necessarily prolong serum half-life. In fact, IgG1 mutants created to significantly increase binding at pH 6.0 as well as pH 7.4 did not contribute to increase serum half-life, but instead offset the benefit of enhanced binding at pH 6.0 alone.45,46 It is believed that FcRn-IgG binding at pH 7.4 prevents IgG release into the circulation and instead diverts it to the degradation pathway. Furthermore, it has been suggested that the rate of dissociation at pH 7.4 is equally or perhaps more important in determining serum half-life.47
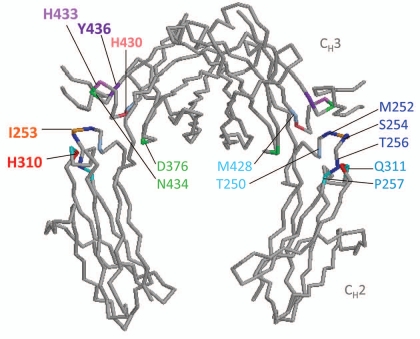
Five sets of published mutations in the Fc domain, most with relative success in both increasing binding to FcRn and extending serum half-life (Table 1), are reviewed here. Locations of amino acid residue mutations are illustrated in Figure 2.
Table 1.
A summary of IgG mutations developed to increase FcRn-IgG binding affinity and extend serum half-life
| Mutations | Mutation abbrev. | Antibody | FcRn binding at pH 6.0-fold increase | Serum half-life (T1/2β) fold increase | Ref. | Author affiliation |
| M252Y, S254T, T256E | YTE | IgG1 (MEDI-524) (α-RSV) | 10x (human), 10x (cynomolgus) | 4x (cynomolgus) | 48 | Medimmune |
| M252Y, S254T, T256E | YTE | α-VEGF | 2.5x (cynomolgus) | 58 | Xencor | |
| T250Q, M428L | QL | IgG2 (α-HBV OST577) | 28x (human), 27x (rhesus) | 1.9x (rhesus) | 50 | Protein Bioscience |
| T250Q, M428L | QL | IgG1 (α-HBV OST577) | 29x (human), 37x (rhesus) | 2.5x (rhesus) | 50, 51 | Protein Design Lab |
| T250Q, M428L | QL | IgG1 (α-TNFα) | 40x (cynomolgus), 500x (mouse) | No difference (Cynomolgus) | 52 | Lilly Research Lab |
| P257I, Q311I | II | IgG1, (α-TNFα) | 19x (human), 80x (cynomolgus), 25x (mouse) | No difference (cynomolgus), 0.1x (mouse) | 52 | Lilly Research Lab |
| P257I, N434H | IH | IgG1 (α-TNFα) | 16x (human), 52x (cynomolgus), 197x (mouse) | 0.7x (cynomolgus), 0.03x (mouse) | 53 | Lilly Research Lab |
| D376V, N434H | VH | IgG1 (α-TNFα) | 15x (human), 52x (cynomolgus), 17x (mouse) | 0.8x (cynomolgus), 0.1x (mouse) | 53 | Lilly Research Lab |
| N434A | NA | IgG1 (Hu4D5, αHER2, trastuzumab) | 4x (cynomolgus) | 1.6–2.3x (cynomolgus) | 54 | Genentech |
| N434W | NW | Humanized IgG1 (Hu4D5, αHER2, trastuzumab) | 80x (cynomolgus) | No difference (cynomolgus) | 54 | Genentech |
| N434A | A | IgG1 (Hu4D5, αHER2) | 3.4x (human) | 2.2x (hFcRn-Tg mouse) | 56, 57 | Genentech, Jackson Lab |
| T307A, E380A, N434A | AAA | IgG1 (Hu4D5, αHER2) | 11.8x (human) | 2.5x (hFcRn-Tg mouse) | 56, 57 | Genentech, Jackson Lab |
| M428L, N434S | LS | IgG1 (αVEGF, bevacizumab) | 11x (human) | 3.2x (cynomolgus), 4x (hFcRn-Tg mouse) | 58 | Xencor |
| M428L, N434S | LS | IgG1 (αVEGFR, cetuximab) | 3.1x (cynomolgus), 5x (hFcRn-Tg mouse) | 58 | Xencor |
FcRn, neonatal Fc receptor; HBV, hepatitis B virus; HER2, human epidermal growth factor receptor 2; Tg, transgenic; TNF, tumor necrosis factor; RSV, respiratory syncytial virus; VEGF, vascular endothelial growth factor.
Figure 2.
Mutations in amino acid residues in the Fc region to enhance FcRn affinity. Diagram of the Fc portion of human IgG1 (protein data bank (PDB) ID: 1DN2) was generated using RasMol (OpenRasMol).80 FcRn binds at the CH2–CH3 hinge region of Fc at I253, H310, H430, and to a lesser extent H433 and Y436.12–14 Amino acid residues near the Fc hinge region mutated to increase binding to FcRn are grouped in colors according to the various combination listed in Table 1.
YTE.
Human IgG1 with M252Y/S254T/T256E (YTE) mutations (motavizumab, anti-respiratory syncytial virus, MedImmune Inc.,) resulted in a 10-fold increase in binding to FcRn (human and cynomolgus monkey) at pH 6.0 and a 4-fold increase in serum half-life (cynomolgus monkey). A concomitant 4-fold increase was also detected in the bronchio-alveolar fluid.48 Crystal structure analysis demonstrated that the effect of increased binding was likely due to favorable surface contact involving hydrogen bonds.49 In a different human IgG1 with mutations to enhance ADCC activity by increased binding to FcγRIIIA (etaracizumab, anti-human αvβ3 integrin complex, MedImmune), introduction of YTE residues resulted in >100-fold reduction in the ADCC activity. This negative effect was reversed with additional mutations that consisted of S239D/A330L/I332E and reconstitution of the ADCC activity to 10-fold that of the original MEDI-522. The serum half-life of the combined ADCC and YTE mutations in MEDI-522 was not reported.48
QL.
Although not known to contact FcRn, amino acid residues 250 and 428 (T250Q, M428L; QL) had been targeted for mutation due to their proximity to the CH2-CH3 interface; these two residues are also conserved in all four human IgG subtypes. Initial mutations in an IgG2 (anti-hepatitis B virus OST577, Protein Design Labs) resulted in a 27-fold increase in binding to human FcRn and a 1.9-fold increase in serum half-life in rhesus monkeys.50 Later studies using IgG1 resulted in a 29-fold increase in binding to human FcRn and 2.5-fold increase in serum half-live in rhesus monkeys.51 No negative effects on CDC or ADCC activities were observed with the same mutations on another antibody, Hu1D10-IgG1.
Anti-tumor necrosis factor α IgG1 mutations.
When the QL mutations were substituted in human anti-tumor necrosis factor (TNF)α IgG1 antibody, affinities to cynomolgus monkey and murine FcRn were found to increase by 40- and 500-fold, respectively.52 In the same study, P257I/Q311I substitution on anti-TNFα IgG1 antibody also resulted in an 80- and 25-fold increase in binding to cynomolgus monkey FcRn and murine FcRn, respectively. However, neither of these two mutant antibodies was found to have an increased serum half-life in cynomolgus monkeys. It was suspected that binding to the therapeutic target may have resulted in rapid antigen-antibody complex degradation.
A separate study also used humanized anti-TNFα IgG1, with three separate sets of mutations including P257I/Q311I, P257I/N434H and D376V/N434H. Each resulted in a significant increase in affinity for human, cynomolgus and mouse FcRn.53 However, the serum half-life in the cynomolgus monkeys were significantly less than that of the unmutated antibodies. Because none of the mutants showed association with FcRn at pH 7.4, this is unlikely the cause of the decreased serum half-life as seen in previous mutants that bind at a wide range of pH. Rather, the authors speculate that the “off-rate kinetic” at endosomal pH (6.0) may be a contributor in determining antibody transport to the recycling or the degradation pathway.
N434 mutations.
The association between binding affinity and serum half-life was further demonstrated when a single mutation at amino acid residue 434 was mutated to increase affinity to FcRn. The humanized anti-human epidermal growth factor receptor 2 (HER2) IgG1 trastuzumab (Herceptin®) with either N434A or N434W substitution resulted in 4- and 80-fold increased binding to cynomolgus monkey FcRn at pH 6. Only the N434W mutant was found to also bind at pH 7.4. Serum half-life in cynomolgus monkey was 1.6- to 2.3-fold increased in the N434A mutant compared with the wild-type antibody, but no increase was observed in the N434W mutant.54 This further substantiates the previous studies showing dramatic increases in pH binding does not result in increased serum half-life. Similar results were also seen with regards to binding affinity and serum half-life when N434A and N434H substitutions in the humanized anti-TNFα IgG1 were tested in the severe combined immune deficiency (SCID) mice and cynomolgus monkeys.55
AAA.
From a previous comprehensive screen of Fc mutations in FcRn binding study, N434A and T307A/E380A/N434A (AAA), were shown to have 3.4-fold and 11.8-fold increases in binding to FcRn (human).56 Substitutions in trastuzumab resulted in 1.3- and 3.3-fold increases in binding to FcRn (human) using a cell based assay and 2.2- and 2.5-fold increases in the serum half-life in mice that expressed the human FcRn transgene and deficient in the endogenous FcRn (hFcRn-Tg).57 Despite the differences in FcRn affinity, the serum half-life extensions were similar. The authors speculated that additional mutations may have resulted in a slight increase in pH 7.4 binding or that the maximal benefit was already achieved with the single N434A mutation. Mouse anti-human antibody was not detected and thus was not believed to be a factor.
LS.
In a study aimed to demonstrate positive correlation between FcRn binding affinity and effector anti-tumor activity, anti-vascular endothelial growth factor (VEGF) IgG1 bevacizumab with M428L/N434S (LS) substitutions were generated. This resulted in 11-fold improved binding to FcRn (human and cynomolgus monkey).58 Serum half-life in cynomolgus monkey was extended from 9.7 to 31 days, representing a 3.2-fold improvement, which is equivalent to serum half-life of 50 days in human. The same amino acid substitutions in anti-EGFR IgG1 cetuximab (Erbitux®) also resulted in extended serum half-life in cynomolgus monkey from 1.5 to 4.7 days, representing a 3-fold improvement. When these two mutated antibodies were used in a murine tumor model (SKOV-3 tumor in hFcRn-tg/Rag-/- mice), tumor burden decreased at a faster rate than that of the unmutated antibodies and thus indicating a positive correlation between antibody half-life and antibody effector response.
Current therapeutic antibodies in clinical use have reported serum half-lives that vary from 0.75 to 27 days, and these include a range of different IgG platforms such as chimeric (human and mouse) antibodies, human antibodies and Fc-fusion proteins. A recent study by Suzuki and colleagues reported that, although most current therapeutic antibodies have the same Fc domain, affinity to FcRn differs, and the Fc affinity for FcRn at pH 6 correlates with the serum half-life.59 The differences in FcRn affinity were not due to FcγRI binding or amino acid differences at the Fc portion. Instead, the receptor binding domain can influence Fc binding to FcRn. Papain cleavage of the receptor domain from the Fc segment resulted in increased binding of the Fc fragment to FcRn, thus suggesting receptor domain may somehow negatively impact Fc:FcRn binding. This hypothesis was also supported by the additional finding that ligand-antibody complexes, such as infliximab-TNFα or adalimumab-TNFα complexes, have decreased FcRn binding compared with the respective antibody alone. Similarly, another recent study by Wang and colleagues also showed that different therapeutic antibodies with the same Fc portion can also have different affinity for FcRn.47 They also confirmed the previous hypothesis regarding the importance of dissociation rate at neutral pH, where slower off-rate at neutral pH is associated with decreased T1/2. However, the positive correlation between KD at pH 6 and T1/2 seen in the Suzuki et al. study was not observed. A closer analysis of the antibodies examined by Suzuki and colleagues revealed no significant differences in KD and T1/2 among the mAbs studied, but the Fc-fusion proteins had lower KD and lower T1/2 compared with the mAbs. These two studies emphasize the notion that Fc:FcRn binding affinity may not be the only factor in the determination of the antibody serum half-life, and other structural domains may also impact the antibody:FcRn affinity and serum half-life.
Other factors have also been suggested and demonstrated to explain the unexpected altered half-life of different therapeutic antibodies, such as antibody endocytosis, ligand:antibody ratio, antibody structural stability, antibody isoelectric point and methionine oxidation. The decreased therapeutic half-lives of anti-CD20 rituximab (Rituxan®) and anti-HER2 trastuzumab are believed to be caused by target antigen-mediated antibody endocytosis and subsequent degradation. Methionine oxidation at the Met252 and Met428 of IgG can also result in decreased binding to FcRn and subsequent loss of protection from catabolism.60,61
To improve the circulating half-life of Fc-fusion protein, Fc-fusion “monomers” were created where single rather than dimeric therapeutic protein was fused to the dimeric Fc domain.62 Fc-fusion monomer of erythropoietin, interferon (IFN)α, IFNβ and Factor IX have all been shown to have longer circulating half-lives in mouse or cynomolgus monkey than its dimeric counterpart and administration by pulmonary or oral route also resulted in enhanced bioavailability of the agents.63 Improved circulating half-life with concurrent improved therapeutic efficacy was demonstrated when monomeric factor IX Fc-fusion protein corrected the whole blood clotting time in factor IX deficient mice for 144 h compared with only 72 h for recombinant Factor IX.64
The new generation of IgG-based therapeutics also includes Fab heavy and light chain fragments in various combinations and variations. However, these Fab fragments have very short circulating half-life. For example, bispecific single chain diabodies (scDb), which are linked variable heavy and light chain fragments from two antibodies, have a half-life of <6 h. Various strategies have been attempted to prevent early scDb degradation, including conjugation to polyethylene glycol (PEG), addition of N-glycosylation, fusion with albumin and fusion with albumin binding domain (ABD) from streptococcal protein G. The latter two strategies still rely upon interaction with FcRn, which is known to bind to serum albumin. When fused to ABD, scDb was found to have extended half-life and improved tumor penetration compared to scDb conjugation to PEG.65 Similar strategies and results were also seen in Fab fragment fused with albumin or albumin targeting proteins, such as HER2 Fab fused with albumin, ABD or albumin binding peptide.66,67
Direct Target of FcRn for Therapy
Various therapeutic strategies have co-opted FcRn functions either by blocking FcRn-IgG binding to facilitate endogenous IgG degradation or by facilitating FcRn-IgG binding for extension of circulating half-life or drug delivery.
Intravenous immunoglobulin.
Purified human immunoglobulin was first used in the 1960s for primary immunodeficiencies and later for the treatment of autoimmune diseases.1 Approved treatment for various autoimmune diseases includes Guillain-Barre syndrome, Kawasaki disease, idiopathic thrombocytopenia purpura and chronic inflammatory demyelinating polyneuropathy. The list for “off-label” use is much longer. It is now recognized that one of the mechanisms of action is due to oversaturation of FcRn that results in accelerated degradation of pathogenic antibodies. Studies using murine models of autoimmune myasthenia gravis and skin blistering diseases confirmed the critical role of FcRn in mediating disease severity by maintaining the circulating half-life of pathogenic antibodies, and that inhibition of FcRn-IgG interaction leads to accelerated pathogenic antibody degradation and disease amelioration.38,68
Abdegs and peptide inhibitors.
Disruption of FcRn-IgG interaction can also be achieved through a compound that blocks the binding interface. Abdegs (antibodies that enhance IgG degradation) are recombinant antibodies that bind to FcRn with high affinity at both pH 6 and 7.2 and thus allow for accelerated degradation of the endogenous IgG.69 In a similar approach, a 26 amino acid dimeric peptide SYN1436 (Syntonix Pharmaceuticals, now Biogen Idec) was shown to bind FcRn similarly to the Fc domain at H310 and I253, as well as being able to bridge two FcRn molecules.70 When administered to hFcRn-Tg mice or cynomolgus monkeys, the endogenous IgG reduced at a faster rate without altering albumin levels.71
Fc-fusion protein.
Fc-fusion proteins have been developed to extend the circulating half-life or exploit endogenous FcRn transcytosis function at mucosal surfaces for trans-epithelial drug delivery. So far, recombinant Fc-fusion proteins in clinical use have yet to achieve the same serum persistence as that of the whole IgG molecules. Transepithelial transport in the lung using monomeric erythropoietin-Fc fusion protein (a single erythropoietin protein fused with Fc dimer) is effective and safe in human trials with 70% deposition of aerosolized doses.62,72 In a different configuration of Fc fusion with follicular stimulating hormone (FSH), which is composed of α and β subunits, both the single chain and the heterodimer FSH-Fc fusion proteins were successfully delivered to the circulation via oral and pulmonary administration in rats and cynomolgus monkeys.73
Immunization.
Lessons from studies in mucosal immune surveillance and protection in the intestine and antigen presentation function in dendritic cells have led to the logical conclusion that coordination of these two functions can be utilized for the development of vaccine mediated immunity. A recent study demonstrated this principle by immunization of mice with a Fc-fusion protein that consisted of herpes simplex virus type-2 glycoprotein gD, which led to the generation of protective antibodies that prevented subsequent intravaginal infection of a virulent HSV-2 strain.39 The multi-functioning roles of FcRn in different tissues appear to coordinate adaptive immunity, and this process appears to be a rational strategy for vaccine development in the prevention of mucosal infections.
Tools to Study FcRn-IgG Interaction
In vitro analysis of FcRn-IgG/ligand interactions still relies heavily on surface plasmon resonance data. Although cell-based platforms represent an alternative approach for larger scale screening, these lack the sensitivity in determining precise FcRn-IgG binding affinity. Perhaps a more appropriate role for the cell-based analysis of FcRn-IgG interaction would be examination of the physiologic properties of transcytosis and recycling using polarized epithelial cells. FcRn mediated transcellular transport of IgG has been demonstrated using MDCK cells, as well as several other commonly used polarizable epithelial cells, with stable transfection and expression of FcRn and β2m.3 This may be a potential “physiologic screen” where pH dependent kinetics for ligand “on” and “off” rates can be examined under physiologic conditions.
In vivo analysis using mice has taken advantage of cross-species differences in FcRn-IgG binding. However, this is also the Achilles heel of the murine model due to varied binding affinities between murine FcRn and other species of IgG. Higher than physiologic affinity between FcRn and IgG may lead to increased IgG degradation and thus provide experimental results that would lead to the wrong conclusion. Prior to the generation of FcRn-deficient mice, β2m-deficient mice were commonly used, but these mice also lack other MHC class I molecules, such as CD1 and transferrin receptors. The generation of mice that express human FcRn and β2m transgenes and without the endogenous mouse FcRn has allowed for a better model to interrogate human FcRn interactions in vivo.74 Although human FcRn does not bind to murine IgG, it can bind to murine albumin. Furthermore, in the absence of endogenous human IgG, the effect of competitive binding between normal circulating human IgG and recombinant Fc fragments cannot be determined. To date, nonhuman primates continue to be the more physiologically accurate model, but the cost has limited their use.
FcRn in Livestock
The study of FcRn in different species has included a wide range of various mammals, including novel studies of bovine FcRn.75 A mouse model has been introduced with bovine FcRn transgene expression, which coincidentally appears to be functional in binding to murine β2m as well as to both mouse and human IgG.76 This murine model has demonstrated usefulness in revealing that FcRn can induce robust antibody response, as well as expansion of B cells and plasma cells after immunization.77,78 The authors of these studies have also proposed the provocative idea of human antibody production in a large animal, such as the cow, and the extraction of antibodies from milk.
Conclusion
It is almost certain that most IgG-based therapeutics, today and in the future, will somehow interact with FcRn, and this interaction can be modulated for optimal pharmacologic effect while avoiding off-target complications. It is not inconceivable that future designs in Fab fragments can somehow achieve extended half-life without fusing to a ligand that binds FcRn, but such technology for clinical use may still be years away. In the meantime, it is critical to understand the functions of FcRn in various organs and tissues, so that we may not only understand the role of FcRn and IgG in health and disease, but also optimize our current arsenal of therapeutics.
Acknowledgments
The author is grateful to those in his group and collaborators in the study of FcRn, including R. Blumberg, E. de Munick, K. Baker, T. Kraemer, M. Yoshida, S. Claypool, T. Nagaishi, W. Lencer, D. Roopenian and J. Cusick. The author is also grateful for the support from the funding agencies over the years, including National Institute of Health, American Liver Foundation and Harvard Digestive Disease Center.
References
- 1.Good RA, Lorenz E. Historic aspects of intravenous immunoglobulin therapy. Cancer. 1991;68:1415–1421. doi: 10.1002/1097-0142(19910915)68:6+<1415::aid-cncr2820681402>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 3.Kuo TT, de Muinck EJ, Claypool SM, Yoshida M, Nagaishi T, Aveson VG, et al. N-glycan moieties in neonatal Fc receptor determine steady-state membrane distribution and directional transport of IgG. J Biol Chem. 2009;284:8292–8300. doi: 10.1074/jbc.M805877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzaban S, Massol RH, Yen E, Hamman W, Frank SR, Lapierre LA, et al. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185:673–684. doi: 10.1083/jcb.200809122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci USA. 2008;105:9337–9342. doi: 10.1073/pnas.0801717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker KQS, Kuo TT, Aveson VG, Platzer B, Andersen JT, Sandlie I, et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. PNAS. 2011;108:9927–9932. doi: 10.1073/pnas.1019037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, et al. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol. 2010;30:777–789. doi: 10.1007/s10875-010-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker K, Qiao SW, Kuo T, Kobayashi K, Yoshida M, Lencer WI, et al. Immune and non-immune functions of the (not so) neonatal Fc receptor, FcRn. Semin Immunopathol. 2009;31:223–236. doi: 10.1007/s00281-009-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 10.Raghavan M, Gastinel LN, Bjorkman PJ. The class I major histocompatibility complex related Fc receptor shows pH-dependent stability differences correlating with immunoglobulin binding and release. Biochemistry. 1993;32:8654–8660. doi: 10.1021/bi00084a037. [DOI] [PubMed] [Google Scholar]
- 11.Huber AH, Kelley RF, Gastinel LN, Bjorkman PJ. Crystallization and stoichiometry of binding of a complex between a rat intestinal Fc receptor and Fc. J Mol Biol. 1993;230:1077–1083. doi: 10.1006/jmbi.1993.1220. [DOI] [PubMed] [Google Scholar]
- 12.Raghavan M, Chen MY, Gastinel LN, Bjorkman PJ. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity. 1994;1:303–315. doi: 10.1016/1074-7613(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim JK, Firan M, Radu CG, Kim CH, Ghetie V, Ward ES. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur J Immunol. 1999;29:2819–2825. doi: 10.1002/(SICI)1521-4141(199909)29:09<2819::AID-IMMU2819>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Medesan C, Matesoi D, Radu C, Ghetie V, Ward ES. Delineation of the amino acid residues involved in transcytosis and catabolism of mouse IgG1. J Immunol. 1997;158:2211–2217. [PubMed] [Google Scholar]
- 15.Schultze HE, Heremans JF. Nature and Metabolism of Extracellular Proteins. New York: Elsevier; 1966. Molecular biology of human proteins: with special reference to plasma proteins. [Google Scholar]
- 16.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen JT, Dee Qian J, Sandlie I. The conserved histidine 166 residue of the human neonatal Fc receptor heavy chain is critical for the pH-dependent binding to albumin. Eur J Immunol. 2006;36:3044–3051. doi: 10.1002/eji.200636556. [DOI] [PubMed] [Google Scholar]
- 18.Kenanova VE, Olafsen T, Salazar FB, Williams LE, Knowles S, Wu AM. Tuning the serum persistence of human serum albumin domain III:diabody fusion proteins. Protein Eng Des Sel. 2010;23:789–798. doi: 10.1093/protein/gzq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollastrini J, Dillon TM, Bondarenko P, Chou RY. Field flow fractionation for assessing neonatal Fc receptor and Fcgamma receptor binding to monoclonal antibodies in solution. Anal Biochem. 414:88–98. doi: 10.1016/j.ab.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Johnson JE, Ghetie V, Ober RJ, Ward ES. Generation of mutated variants of the human form of the MHC class I-related receptor, FcRn, with increased affinity for mouse immunoglobulin G. J Mol Biol. 2003;332:901–913. doi: 10.1016/s0022-2836(03)00952-5. [DOI] [PubMed] [Google Scholar]
- 22.Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J Biol Chem. 2010;285:4826–4836. doi: 10.1074/jbc.M109.081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc Natl Acad Sci USA. 2004;101:11076–11081. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitonti AJ, Dumont JA. Pulmonary administration of therapeutic proteins using an immunoglobulin transport pathway. Adv Drug Deliv Rev. 2006;58:1106–1118. doi: 10.1016/j.addr.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci USA. 2008;105:967–972. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi N, Suzuki Y, Tsuge T, Okumura K, Ra C, Tomino Y. FcRn-mediated transcytosis of immunoglobulin G in human renal proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2002;282:358–365. doi: 10.1152/ajprenal.0164.2001. [DOI] [PubMed] [Google Scholar]
- 29.Sarav M, Wang Y, Hack BK, Chang A, Jensen M, Bao L, et al. Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol. 2009;20:1941–1952. doi: 10.1681/ASN.2008090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cianga P, Cianga C, Cozma L, Ward ES, Carasevici E. The MHC class I related Fc receptor, FcRn, is expressed in the epithelial cells of the human mammary gland. Hum Immunol. 2003;64:1152–1159. doi: 10.1016/j.humimm.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Nakata K, Kobayashi K, Ishikawa Y, Yamamoto M, Funada Y, Kotani Y, et al. The transfer of maternal antigen-specific IgG regulates the development of allergic airway inflammation early in life in an FcRn-dependent manner. Biochem Biophys Res Commun. 2010;395:238–243. doi: 10.1016/j.bbrc.2010.03.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 33.Grubb JH, Vogler C, Tan Y, Shah GN, MacRae AF, Sly WS. Infused Fc-tagged beta-glucuronidase crosses the placenta and produces clearance of storage in utero in mucopolysaccharidosis VII mice. Proc Natl Acad Sci USA. 2008;105:8375–8380. doi: 10.1073/pnas.0803715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol. 2006;168:1210–1226. doi: 10.2353/ajpath.2006.050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, et al. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood. 2006;108:3573–3579. doi: 10.1182/blood-2006-05-024539. [DOI] [PubMed] [Google Scholar]
- 36.Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci USA. 2009;106:2788–2793. doi: 10.1073/pnas.0810796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knee RA, Hickey DK, Beagley KW, Jones RC. Transport of IgG across the blood-luminal barrier of the male reproductive tract of the rat and the effect of estradiol administration on reabsorption of fluid and IgG by the epididymal ducts. Biol Reprod. 2005;73:688–694. doi: 10.1095/biolreprod.105.041079. [DOI] [PubMed] [Google Scholar]
- 38.Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115:3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol. 2011;29:158–163. doi: 10.1038/nbt.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Bronson CL, Hayton WL, Radmacher MD, Roopenian DC, Robinson JM, et al. Albumin turnover: FcRn-mediated recycling saves as much albumin from degradation as the liver produces. Am J Physiol Gastrointest Liver Physiol. 2006;290:G352–360. doi: 10.1152/ajpgi.00286.2005. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Robinson SB, Csaky KG. FcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eye. Mol Vis. 2009;15:2803–2812. [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H, Fariss RN, Zhang C, Robinson SB, Thill M, Csaky KG. Mapping of the neonatal Fc receptor in the rodent eye. Invest Ophthalmol Vis Sci. 2008;49:2025–2029. doi: 10.1167/iovs.07-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garg A, Balthasar JP. Investigation of the influence of FcRn on the distribution of IgG to the brain. Aaps J. 2009;11:553–557. doi: 10.1208/s12248-009-9129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cianga P, Cianga C, Plamadeala P, Branisteanu D, Carasevici E. The neonatal Fc receptor (FcRn) expression in the human skin. Virchows Arch. 2007;451:859–860. doi: 10.1007/s00428-007-0467-7. [DOI] [PubMed] [Google Scholar]
- 45.Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, et al. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol. 2002;169:5171–5180. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]
- 46.Gurbaxani B, Dela Cruz LL, Chintalacharuvu K, Morrison SL. Analysis of a family of antibodies with different half-lives in mice fails to find a correlation between affinity for FcRn and serum half-life. Mol Immunol. 2006;43:1462–1473. doi: 10.1016/j.molimm.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Lu P, Fang Y, Hamuro L, Pittman T, Carr B, et al. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab Dispos. 2011;39:1469–1477. doi: 10.1124/dmd.111.039453. [DOI] [PubMed] [Google Scholar]
- 48.Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 49.Oganesyan V, Damschroder MM, Woods RM, Cook KE, Wu H, Dall'acqua WF. Structural characterization of a human Fc fragment engineered for extended serum half-life. Mol Immunol. 2009;46:1750–1755. doi: 10.1016/j.molimm.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 50.Hinton PR, Johlfs MG, Xiong JM, Hanestad K, Ong KC, Bullock C, et al. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem. 2004;279:6213–6216. doi: 10.1074/jbc.C300470200. [DOI] [PubMed] [Google Scholar]
- 51.Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176:346–356. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- 52.Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Wroblewski VJ. Monoclonal antibody clearance. Impact of modulating the interaction of IgG with the neonatal Fc receptor. J Biol Chem. 2007;282:1709–1717. doi: 10.1074/jbc.M607161200. [DOI] [PubMed] [Google Scholar]
- 53.Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Jiang W, Wroblewski VJ. Humanized IgG1 variants with differential binding properties to the neonatal Fc receptor: relationship to pharmacokinetics in mice and primates. Drug Metab Dispos. 2007;35:86–94. doi: 10.1124/dmd.106.011734. [DOI] [PubMed] [Google Scholar]
- 54.Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol. 2009;182:7663–7671. doi: 10.4049/jimmunol.0804182. [DOI] [PubMed] [Google Scholar]
- 55.Deng R, Loyet KM, Lien S, Iyer S, DeForge LE, Theil FP, et al. Pharmacokinetics of humanized monoclonal anti-tumor necrosis factor-{alpha} antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys. Drug Metab Dispos. 38:600–605. doi: 10.1124/dmd.109.031310. [DOI] [PubMed] [Google Scholar]
- 56.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, et al. High resolution mapping of the binding site on human IgG1 for Fcgamma RI, Fcgamma RII, Fcgamma RIII and FcRn and design of IgG1 variants with improved binding to the Fcgamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 57.Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–1769. doi: 10.1093/intimm/dxl110. [DOI] [PubMed] [Google Scholar]
- 58.Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol. 184:1968–1976. doi: 10.4049/jimmunol.0903296. [DOI] [PubMed] [Google Scholar]
- 60.Pan H, Chen K, Chu L, Kinderman F, Apostol I, Huang G. Methionine oxidation in human IgG2 Fc decreases binding affinities to protein A and FcRn. Protein Sci. 2009;18:424–433. doi: 10.1002/pro.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang W, Vlasak J, Li Y, Pristatsky P, Fang Y, Pittman T, et al. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol Immunol. 48:860–866. doi: 10.1016/j.molimm.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Bitonti AJ, Dumont JA, Low SC, Peters RT, Kropp KE, Palombella VJ, et al. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc Natl Acad Sci USA. 2004;101:9763–9768. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dumont JA, Low SC, Peters RT, Bitonti AJ. Monomeric Fc fusions: impact on pharmacokinetic and biological activity of protein therapeutics. BioDrugs. 2006;20:151–160. doi: 10.2165/00063030-200620030-00002. [DOI] [PubMed] [Google Scholar]
- 64.Peters RT, Low SC, Kamphaus GD, Dumont JA, Amari JV, Lu Q, et al. Prolonged activity of factor IX as a monomeric Fc fusion protein. Blood. 2010;115:2057–2064. doi: 10.1182/blood-2009-08-239665. [DOI] [PubMed] [Google Scholar]
- 65.Stork R, Campigna E, Robert B, Muller D, Kontermann RE. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J Biol Chem. 2009;284:25612–25619. doi: 10.1074/jbc.M109.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dennis MS, Jin H, Dugger D, Yang R, McFarland L, Ogasawara A, et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 2007;67:254–261. doi: 10.1158/0008-5472.CAN-06-2531. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen A, Reyes AE, 2nd, Zhang M, McDonald P, Wong WL, Damico LA, et al. The pharmacokinetics of an albumin-binding Fab (AB.Fab) can be modulated as a function of affinity for albumin. Protein Eng Des Sel. 2006;19:291–297. doi: 10.1093/protein/gzl011. [DOI] [PubMed] [Google Scholar]
- 68.Liu L, Garcia AM, Santoro H, Zhang Y, McDonnell K, Dumont J, et al. Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol. 2007;178:5390–5398. doi: 10.4049/jimmunol.178.8.5390. [DOI] [PubMed] [Google Scholar]
- 69.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23:1283–1288. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 70.Mezo AR, Sridhar V, Badger J, Sakorafas P, Nienaber V. X-ray Crystal Structures of Monomeric and Dimeric Peptide Inhibitors in Complex with the Human Neonatal Fc Receptor, FcRn. J Biol Chem. 2010;285:27694–27701. doi: 10.1074/jbc.M110.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mezo AR, McDonnell KA, Hehir CA, Low SC, Palombella VJ, Stattel JM, et al. Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn. Proc Natl Acad Sci USA. 2008;105:2337–2342. doi: 10.1073/pnas.0708960105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dumont JA, Bitonti AJ, Clark D, Evans S, Pickford M, Newman SP. Delivery of an erythropoietin-Fc fusion protein by inhalation in humans through an immunoglobulin transport pathway. J Aerosol Med. 2005;18:294–303. doi: 10.1089/jam.2005.18.294. [DOI] [PubMed] [Google Scholar]
- 73.Low SC, Nunes SL, Bitonti AJ, Dumont JA. Oral and pulmonary delivery of FSH-Fc fusion proteins via neonatal Fc receptor-mediated transcytosis. Hum Reprod. 2005;20:1805–1813. doi: 10.1093/humrep/deh896. [DOI] [PubMed] [Google Scholar]
- 74.Roopenian DC, Christianson GJ, Sproule TJ. Human FcRn transgenic mice for pharmacokinetic evaluation of therapeutic antibodies. Methods Mol Biol. 2010;602:93–104. doi: 10.1007/978-1-60761-058-8_6. [DOI] [PubMed] [Google Scholar]
- 75.Cervenak J, Kacskovics I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Vet Immunol Immunopathol. 2009;128:171–177. doi: 10.1016/j.vetimm.2008.10.300. [DOI] [PubMed] [Google Scholar]
- 76.Lu W, Zhao Z, Zhao Y, Yu S, Zhao Y, Fan B, et al. Overexpression of the bovine FcRn in the mammary gland results in increased IgG levels in both milk and serum of transgenic mice. Immunology. 2007;122:401–408. doi: 10.1111/j.1365-2567.2007.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vegh A, Cervenak J, Jankovics I, Kacskovics I. FcRn overexpression in mice results in potent humoral response against weakly immunogenic antigen. mAbs. 3:173–180. doi: 10.4161/mabs.3.2.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cervenak J, Bender B, Schneider Z, Magna M, Carstea BV, Liliom K, et al. Neonatal FcR overexpression boosts humoral immune response in transgenic mice. J Immunol. 2011;186:959–968. doi: 10.4049/jimmunol.1000353. [DOI] [PubMed] [Google Scholar]
- 79.Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeLano WL, Ultsch MH, de Vos AM, Wells JA. Convergent solutions to binding at a protein-protein interface. Science. 2000;287:1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]