Summary
Objective
Obesity is associated with reduced testosterone and growth hormone (GH). However, the interrelationship between these axes and their independent contributions to cardiovascular risk is unknown. The objectives of this study were to determine (1) the association between testosterone and GH in obesity, (2) whether excess adiposity mediates this association and (3) the relative contribution of reduced testosterone and GH to increased carotid intima-media thickness (cIMT) in obesity.
Design
Fifty obese men were studied with GH-releasing hormone–arginine testing, and morning free testosterone (FT) was measured by equilibrium dialysis. Metabolic, anthropometric and cardiovascular risk indices, including cIMT were measured. Twenty-six normal weight men served as controls.
Results
Obese subjects demonstrated lower mean (±SEM) peak stimulated GH (5·9 ± 0·6 vs 36·4 ± 3·9 µg/l; P < 0·0001) and FT (0·41 ± 0·03 vs 0·56 ± 0·03 nmol/l; P = 0·0005) compared to controls. GH was significantly associated with FT (r = +0·44; P < 0·0001) and both were inversely related to visceral adipose tissue (VAT) (GH: r = −0·65; P < 0·0001; FT: r = −0·51; P < 0·0001). In multivariate regression analysis controlling for VAT, FT was no longer related to GH. Both GH and FT were associated with cIMT in univariate analysis. However, in multivariate modelling including traditional cardiovascular risk markers, GH (β = 0·003; P = 0·04) but not FT (P = 0·35) was associated with cIMT.
Conclusions
These results demonstrate a strong relationship between FT and GH in obesity and suggest that this relationship is more a function of excess adiposity rather than a direct relationship. While reduced FT and GH are both related to increased cIMT, the relationship with reduced GH remains significant controlling for reduced FT and traditional cardiovascular disease risk markers.
Introduction
Obesity is associated with reduced pulsatile1–3 and stimulated growth hormone (GH) secretion.4,5 In addition, obesity is associated with reduced testosterone levels in men.6,7 However, relatively little is known regarding the interrelationship of these axes in obesity.
Prior studies suggest a relationship between reduced testosterone and GH. For example, paediatric studies have demonstrated an important association between the low testosterone levels in pre- to early puberty and reduced peak stimulated GH,8 and treatment of prepubertal to early pubertal boys with testosterone increases the peak stimulated GH on GH stimulation tests9,10 demonstrating potential interactions between the two hypothalamic-pituitary axes. An association between testosterone and the GH axis has also been demonstrated in adult hypogonadal men,11 elderly men12 and in healthy non-obese men.13 However, to our knowledge, the relationship between free testosterone (FT), as measured by equilibrium dialysis, and peak GH using a standardized stimulation testing algorithm in otherwise healthy obese men is not known. Moreover, no study has examined whether excess visceral adiposity or other body composition changes mediates the reduction in testosterone and GH in obesity and whether the relationship between testosterone and GH is independent of or a function of excess adiposity.
Cardiovascular disease risk is increased in obesity. Recent studies demonstrate reduced GH secretion is associated with increased cardiovascular disease risk, including dyslipidaemia and increased carotid intima-media thickness (cIMT) in obesity,14 while treatment of obese men with exogenous GH decreases visceral adiposity and improves unfavourable cholesterol profile.15,16 Similarly, reduced testosterone levels are associated with increased risk of diabetes, 17,18 unfavourable lipid profile19 and increased cardiovascular disease in men,20 and treatment of obese men with exogenous testosterone improves unfavourable lipid profile.19 Taken together, these data suggest that both reduced GH and testosterone may contribute to increased cardiovascular disease risk in obesity. However, prior studies have not simultaneously investigated the relationship of peak GH and FT to cIMT in obesity, controlling for other cardiovascular risk factors.
We hypothesized that reduced testosterone and GH would contribute independently to increased cardiovascular disease risk in obesity. In this study, we assessed the interrelationship between peak stimulated GH on GH-releasing hormone (GHRH)–arginine stimulation testing and FT by equilibrium dialysis in obesity, and the respective contributions of reduced GH and FT to increased cIMT in this population.
Methods
Study subjects
Fifty obese (BMI ≥ 30 kg/m2) men between the ages of 18 and 55 years from the Boston community were recruited between November 2007 and March 2009. Subjects were otherwise healthy without known pituitary dysfunction including dysfunction of the adrenal, GH, thyroid or gonadal axes. Subjects receiving GH, anabolic steroids, glucocorticoids, testosterone, hormone replacement, hormonal contraception or any medication known to affect GH or FT were excluded. Subjects with known diabetes mellitus, haemoglobin <11 g/dl, creatinine >1·5 mg/dl, aspartate aminotransferase >2·5-fold upper limit of normal and chronic illness such as HIV were also excluded. Subjects with a history and physical examination suggestive of pituitary dysfunction were also excluded. In addition, 26 normal weight subjects (BMI < 25 kg/m2) were recruited as a control group, using otherwise similar exclusion criteria. Written informed consent was obtained from each subject before testing, in accordance with the Committee on the use of Humans as Experimental Subjects of the Massachusetts Institute of Technology and the Subcommittee on Human Studies at the Massachusetts General Hospital. A subset of data from this sample (n = 65) were previously published in a study evaluating the association of peak stimulated GH secretion to carotid IMT in normal weight and obese men and women,14 but this prior study did not contain any data on testosterone in these subjects.
Biochemical assessment
All hormonal testing, including GHRH–arginine testing and measurement of FT, were performed in the morning after an overnight fast. GH stimulation testing was performed using standard GHRH–arginine stimulation test as previously reported.5,14 Briefly, after an overnight fast, sermorelin acetate (GHRH 1–29) (Geref; Serono Laboratories, Inc., Rockland, MA, USA) was administered intravenously at a dose of 1 µg/kg. Subsequently, arginine hydrochloride (30 g/300 ml) was administered at a dose of 0·5 g/kg (maximum 30 g) via intravenous pump at 600 ml/h over 30 min. GH levels were assessed at 0, 30, 45, 60, 90 and 120 min after sermorelin administration. Serum GH was measured using the Beckman Access Ultrasensitive human GH assay, a paramagnetic particle, chemiluminescent immunoassay (Beckman Coulter, Chaska, MN, USA). The analytical sensitivity of the assay is 0·002 µg/l. The intra-assay variation ranges from 1·90% to 2·78% and the inter-assay variation ranges from 1·77% to 2·65%. Relative GH deficiency of obesity was defined as peak stimulated GH ≤4·2 µg/l. In obese men and women with hypothalamic-pituitary disease, the cut-off value of 4·2 µg/l was previously validated as having the most discriminative power to diagnose GH deficiency.21 In a study comparing six different GH stimulation tests, Biller et al. similarly demonstrated a peak stimulated GH of ≤4·1 µg/l with the GHRH–arginine stimulation test conferred the best discriminatory power to diagnose true GH deficiency of pituitary disease in her study population which consisted of mostly obese subjects.22 We have previously utilized this cut-off value to demonstrate an increase in cardiovascular disease risk among obese subjects with reduced GH secretion.14 IGF-I was measured using a commercially available EIA from ALPCO Diagnostics (Salem, NH, USA) with an intra-assay coefficient of variation (CV) of 6·6–9·7% and inter-assay CV of 11·3–13·7%.
Fasting morning levels of total testosterone, SHBG and estradiol were measured using a Beckman Access chemiluminescent immunoassay (Beckman Coulter). The analytical sensitivity of the assay for testosterone is 0·35 nmol/l, SHBG is 0·33 nmol/l, and for estradiol is 73·4 pmol/l. Total testosterone was also assayed at the Mayo Clinic Medical Laboratories using a high throughput liquid chromatography and tandem mass spectrometry (LC-MS/MS) (API 5000; Applied Biosystems-MDS Sciex, Foster City, CA, USA) with an inter-assay variation of 2·2–11·6%. Inter-assay correlation between the chemiluminescent immunoassay and the LC-MS/MS was excellent (P < 0·0001) without systematic differences in the results. FT was measured by isotope dilution equilibrium dialysis at the Mayo Clinic Medical Laboratories. Briefly, samples were prepared in a pH 7·4 phosphate buffer using 30 ng/dl [3H]-testosterone per sample (Amersham, San Francisco, CA, USA) as the tracer. Radioactivity was measured in tubing and dialysate on a Taurus liquid scintillation counter following a 17 h dialysis at 37 °C (MicroMedic, Huntsville, AL, USA). The resulting FT percentage was then multiplied by the total testosterone concentration measured by LC-MS/MS (see above) to obtain the FT concentration. Inter-assay CV ranged from 10% to 19%. Relative hypogonadism was defined as fasting FT level of <0·31 nmol/l (9 ng/dl) using the reference ranges provided by the Mayo Clinical Medical Laboratories.
Fasting glucose and measurement of fasting cholesterol profile were determined using standard methodology. Insulin was measured using the Beckman Access paramagnetic particle chemiluminescence immunoassay (Beckman Coulter).
Anthropometric assessment
Height and body weight were obtained after an overnight fast. Measurement of waist circumference was performed in triplicate at the iliac crest in a standardized fashion with the subject in an upright position.5 One-centimetre cross-sectional abdominal computed tomography (CT) scans were performed at the level of L4 to assess the distribution of total abdominal adiposity, abdominal subcutaneous adipose tissue (SAT) and abdominal visceral adipose tissue (VAT) as previously described.5,23
Carotid intima-media thickness (cIMT)
Measurement of cIMT was performed as previous described.14,24 Briefly, ultrasound images were obtained using a high resolution 5–12 MHz linear array transducer (HDI 5000 SONOS CT; ATL Ultrasound, Bothell, WA, USA). Digital images were captured using a high-quality video frame capture card. Edge detection and mean cIMT calculation were accomplished with an in-house computer program. The published reproducibility of the technique is excellent with a SD of 0·007 mm.25 In further studies, we have demonstrated the reproducibility of the test to be 0·004.24 The average cIMT over the length of the measured segments on the left is reported.
Statistical analysis
Continuous variables were tested for normality of distribution with the use of the Shapiro–Wilk test and examination of the histogram distribution. Variables that were normally distributed were compared using the Student’s t-test, and variables that were not normally distributed were compared using the nonparametric Wilcoxon rank sum test. Nominal variables were compared using the χ2-test. Results are presented as mean ± SEM. Univariate regression analysis was performed comparing peak stimulated GH and IGF-I levels to hormonal and adiposity measures as well as cIMT using the Pearson correlation coefficient. Multivariate regression analysis with standard least squares modelling was performed for peak stimulated GH with FT, age, race and various measures of adiposity as the independent variables. The purpose of this modelling was to determine whether the relationship between FT and peak GH remained controlling for VAT, which we show in univariate modelling to be related to both FT and peak GH, and also simultaneously for variables such as age and race which might confound the relationship between FT and peak GH. Further multivariate regression analysis with standard least squares modelling was also performed for cIMT with peak stimulated GH, FT and known cardiovascular disease risk factors as the independent variables. Similar multivariate modelling was also performed for IGF-I in lieu of peak stimulated GH. Variables for entry into the multivariate modelling were chosen based on known physiological relationship with the dependent variable, cIMT. Obese subjects were stratified into GH sufficient (GHS) or relative GH deficient (GHD) based on peak stimulated GH of ≤4·2 µg/l and also stratified into eugonadal or hypogonadal based on fasting morning FT of <0·31 nmol/l (<9 ng/dl). Demographic, anthropometric, hormonal and metabolic parameters and cIMT were compared amongst normal weight and obese subjects stratified into the above groups using anova. Post hoc testing was performed for significant anova (P < 0·05) using the Tukey–Kramer post hoc test. Statistical analysis was performed using JMP Statistical Database Software (SAS Institute, Inc., Cary, NC, USA). Statistical significance was determined as P < 0·05.
Results
Clinical characteristics of study subjects
The characteristics of the study subjects are described in Table 1. Obese subjects demonstrated significantly lower mean (±SEM) peak stimulated GH (5·9 ± 0·6 vs 36·4 ± 3·9 µg/l; P < 0·0001), total testosterone (13·2 ± 0·9 vs 18·6 ± 1·2 nmol/l; P = 0·0006), FT (0·41 ± 0·03 vs 0·56 ± 0·03 nmol/l; P = 0·0005) and SHBG (29·6 ± 3·1 vs 45·3 ± 3·6 nmol/l; P = 0·003) compared to normal weight subjects (obese vs normal weight, respectively, in each comparison). In contrast, estradiol, LH and FSH levels did not differ between the groups (Table 1).
Table 1.
Characteristics of study subjects (n = 76). Data are presented as mean ± SEM
| Normal weight | Obese | P | |
|---|---|---|---|
| N | 26 | 50 | |
| Age | 43·4 ± 1·9 | 42·7 ± 1·5 | 0·77 |
| Race | |||
| Caucasian | 18 (69%) | 33 (66%) | 0·78 |
| Non-Caucasian | 8 (31%) | 17 (34%) | |
| Ethnicity | |||
| Hispanic | 1 (4%) | 7 (14%) | 0·14 |
| Non-Hispanic | 25 (96%) | 43 (86%) | |
| Tobacco use (pack years) | 5·9 ± 2·2 | 6·7 ± 2·0 | 0·80 |
| Blood pressure | |||
| Systolic BP (mmHg) | 121 ± 2 | 127 ± 2 | 0·06 |
| Diastolic BP (mmHg) | 76 ± 2 | 80 ± 1 | 0·11 |
| Body composition | |||
| BMI (kg/m2) | 22·5 ± 0·3 | 36·1 ± 0·7 | <0·0001 |
| Waist circumference (cm) | 81 ± 1 | 118 ± 2 | <0·0001 |
| VAT (cm2) | 57 ± 9 | 212 ± 12 | <0·0001 |
| SAT (cm2) | 104 ± 11 | 427 ± 21 | <0·0001 |
| Hormonal assessment | |||
| Basal GH (µg/l) | 0·7 ± 0·2 | 0·2 ± 0·1 | 0·03 |
| Peak stimulated GH (µg/l) | 36·4 ± 3·9 | 5·9 ± 0·6 | <0·0001 |
| IGF-1 (nmol/l) | 12·0 ± 0·6 | 10·6 ± 0·6 | 0·14 |
| Total testosterone (nmol/l) | 18·6 ± 1·2 | 13·2 ± 0·9 | 0·0006 |
| Free testosterone (nmol/l) | 0·56 ± 0·03 | 0·41 ± 0·03 | 0·0005 |
| SHBG (nmol/l) | 45·3 ± 3·6 | 29·6 ± 3·1 | 0·003 |
| Estradiol (pmol/l) | 103 ± 5 | 117 ± 7 | 0·15 |
| LH (IU/l) | 4·5 ± 0·4 | 4·0 ± 0·5 | 0·48 |
| FSH (IU/l) | 6·0 ± 0·7 | 5·7 ± 0·8 | 0·82 |
VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; GH, growth hormone.
Relationship between peak stimulated GH, reproductive hormones and body composition parameters
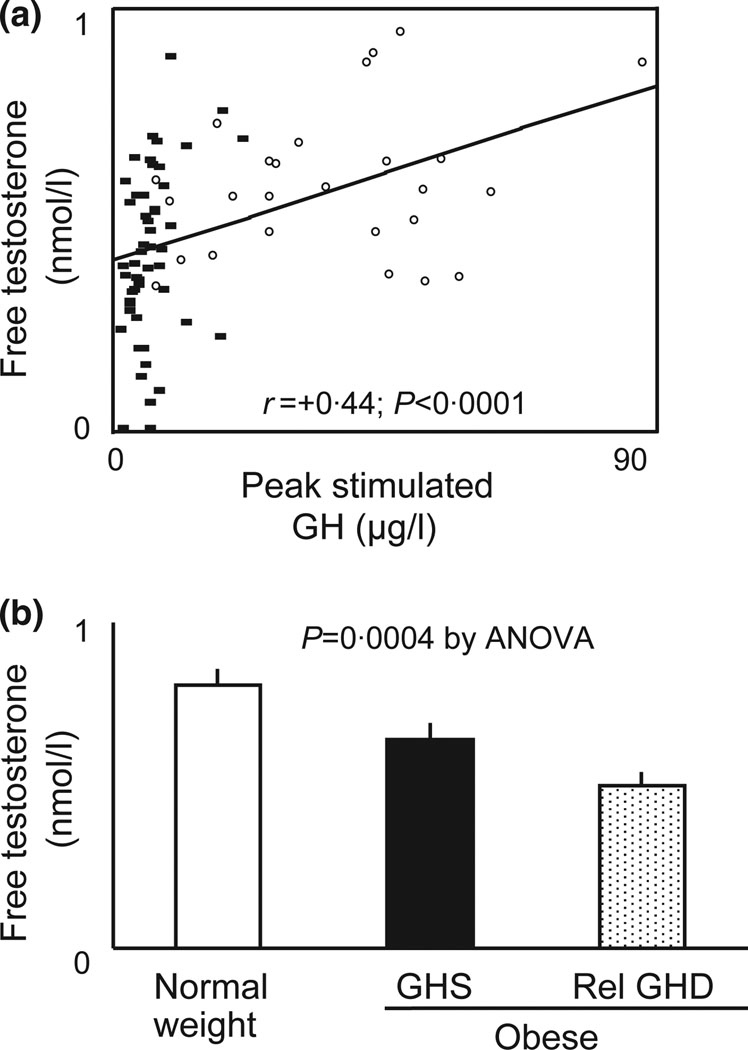
We first assessed the relationship between peak stimulated GH and reproductive hormones including FT by univariate analysis among all subjects (n = 76). Peak stimulated GH was significantly and positively associated with total testosterone (r = +0·26; P = 0·02), FT (r = +0·44; P < 0·0001) (Fig. 1a), and SHBG (r = +0·24; P = 0·04) but was not associated with estradiol (r = −0·19; P = 0·10), LH (r = 0·09; P = 0·45) or FSH (r = +0·03; P = 0·80). In analyses limited to obese subjects only, peak stimulated GH remained significantly associated with FT (r = +0·35; P = 0·01) but was not associated with total testosterone, SHBG, estradiol, LH or FSH.
Fig. 1.
(a) Univariate regression analysis of peak growth hormone (GH) on GH-releasing hormone (GHRH)–arginine stimulation test and free testosterone (FT) by equilibrium dialysis. Normal weight subjects are depicted by open circles while obese subjects are depicted by closed squares. (b) FT levels stratified by GH status. GH sufficiency or relative GH deficiency was determined by peak stimulated GH ≤4·2 µg/l on standard GHRH–arginine stimulation test. Statistical significance was determined by anova.
Both peak GH and FT were significantly related to measures of adiposity including VAT, SAT and BMI among all subjects (all P < 0·001) (Table 2A,B). In analyses limited to obese subjects, the relationship between peak GH and VAT, SAT and BMI remained significant, and the relationship was strongest for VAT in multivariate modelling including all three body composition variables. In the analyses limited to obese subjects, FT was significantly associated with VAT but was not significantly associated with BMI and SAT (Table 2C,D).
Table 2.
(A) Relationship of peak stimulated growth hormone (GH) on GH-releasing hormone (GHRH)–arginine stimulation test to measures of body composition in all subjects (n = 76) using univariate and multivariate regression analyses; (B) Relationship of FT by equilibrium dialysis to measures of body composition in all subjects (n = 76) using univariate and multivariate regression analyses; (C) Relationship of peak stimulated GH on GHRH–arginine stimulation test to measures of body composition in obese subjects only (n = 50) using univariate and multivariate regression analyses; (D) Relationship of FT by equilibrium dialysis to measures of body composition in obese subjects only (n = 50) using univariate and multivariate regression analyses
| Univariate regression | Multivariate regression | ||||
|---|---|---|---|---|---|
| r | P | β | SE | P | |
| (A) Peak GH (all subjects) | |||||
| BMI | −0·71 | <0·0001 | −1·42 | 0·58 | 0·02 |
| VAT | −0·65 | <0·0001 | −0·04 | 0·02 | 0·07 |
| SAT | −0·65 | <0·0001 | 0·003 | 0·02 | 0·86 |
| (B) FT (all subjects) | |||||
| BMI | −0·44 | <0·0001 | −0·005 | 0·007 | 0·48 |
| VAT | −0·51 | <0·0001 | −0·0007 | 0·0003 | 0·02 |
| SAT | −0·41 | 0·0002 | 0·00005 | 0·0003 | 0·84 |
| (C) Peak GH (obese only) | |||||
| BMI | −0·37 | 0·009 | −0·19 | 0·22 | 0·39 |
| VAT | −0·43 | 0·002 | −0·02 | 0·007 | 0·04 |
| SAT | −0·32 | 0·03 | −0·001 | 0·006 | 0·83 |
| (D) FT (obese only) | |||||
| BMI | −0·28 | 0·05 | −0·01 | 0·01 | 0·34 |
| VAT | −0·39 | 0·006 | −0·0007 | 0·0003 | 0·05 |
| SAT | −0·19 | 0·19 | 0·0001 | 0·0003 | 0·66 |
VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; FT, free testosterone.
Subsequently, multivariate regression analysis was performed, using standard least squares modelling, to determine the relationship between peak stimulated GH and FT while controlling for age, race and body composition measures. In a model adjusting for age, race and VAT, FT was no longer associated with peak stimulated GH (P = 0·10) while VAT was significantly associated with peak GH (β = −0·11; P < 0·0001) (Table 3). Substituting BMI, waist circumference, SAT, percent fat, or percent trunk fat by DEXA, in lieu of VAT did not significantly alter the results (data not shown).
Table 3.
Multivariate regression analysis for peak stimulated growth hormone controlling for age, race, VAT and free testosterone (FT) in men
| β | Standard error | P | |
|---|---|---|---|
| Age | 0·21 | 0·18 | 0·24 |
| Race | −0·29 | 1·77 | 0·87 |
| VAT | −0·11 | 0·02 | <0·0001 |
| FT | 17·2 | 10·4 | 0·10 |
VAT, visceral adipose tissue.
Overall R2 for the model is 0·45 (P < 0·0001).
Relationship between IGF-I, reproductive hormones and body composition parameters
IGF-I was not associated with total testosterone (r = −0·03; P = 0·81) but trended to a significant positive relationship with FT (r = +0·22; P = 0·06). IGF-I was significantly and negatively associated with SHBG (r = −0·25; P = 0·03) and estradiol (r = −0·27; P = 0·02) but not LH (r = +0·08; P = 0·52) or FSH (r = +0·01; P = 0·97). Serum IGF-I trended to a significant negative association with BMI (r = −0·23; P = 0·05) and was negatively associated with SAT (r = 0·29; P = 0·01) but not VAT (r = −0·15; P = 0·21) in univariate analyses.
In analyses limited to obese subjects only, IGF-I similarly was not associated with total testosterone (r = −0·04; P = 0·78) but trended to a significant positive relationship with FT (r = +0·28; P = 0·06). IGF-I was also associated with SHBG (r = −0·38; P = 0·008) but was not associated with estradiol (r = −0·19; P = 0·20), LH (r = +0·16; P = 0·27) or FSH (r = +0·10; P = 0·51). IGF-I remained significantly associated with SAT in a negative manner (r = −0·30; P = 0·04) but was not associated with BMI (r = −0·21; P = 0·16) or VAT (r = −0·08; P = 0·61) in obese subjects.
Assessment of reproductive hormone and metabolic indices in study subjects stratified by GH
We next stratified obese subjects into GHS or relative GHD based on peak stimulated GH of ≤4·2 µg/l. Using this criterion, 38% of obese subjects demonstrated inadequate peak stimulated GH secretion. Normal weight subjects, obese subjects with GHS and obese subjects with relative GHD were not significantly different in age, race or ethnicity (Table S1). While BMI, VAT and SAT were higher in obese compared to normal weight subjects, these indices were not significantly different between obese GHS and GHD subjects (Table S1). Mean testosterone and FT levels were highest in normal weight control subjects, lowest in the obese GHD subjects and intermediate in the obese GHS subjects (P for overall trend = 0·0004 by anova) (Fig. 1b). In addition, the obese GHD subjects demonstrated increased cardiovascular disease risk parameters, including lower HDL cholesterol, higher diastolic blood pressure and triglyceride levels and increased cIMT (Table S1).
Assessment of peak stimulated GH and metabolic indices in study subjects stratified by FT
Obese subjects were also stratified into eugonadal or hypogonadal based on fasting morning FT of <0·31 nmol/l (<9 ng/dl). Using this criterion, 28% of obese subjects had low FT secretion. Normal weight, obese eugonadal and obese hypogonadal subjects were not significantly different in age, race or ethnicity (Table S2). While BMI, VAT and SAT were higher in obese compared to normal weight subjects, these indices were not significantly different between obese eugonadal or hypogonadal subjects (Table S2). Mean total testosterone and FT levels were lower among obese hypogonadal subjects as expected. Mean peak stimulated GH levels were highest in normal weight control subjects, lowest in the obese hypogonadal subjects and intermediate in the obese eugonadal subjects (P for overall trend <0·0001 by anova). In addition, the obese hypogonadal subjects demonstrated increased cardiovascular disease risk parameters, including higher triglycerides, fasting glucose, fasting insulin and increased cIMT (Table S2).
Association of FT and peak stimulated GH to cIMT
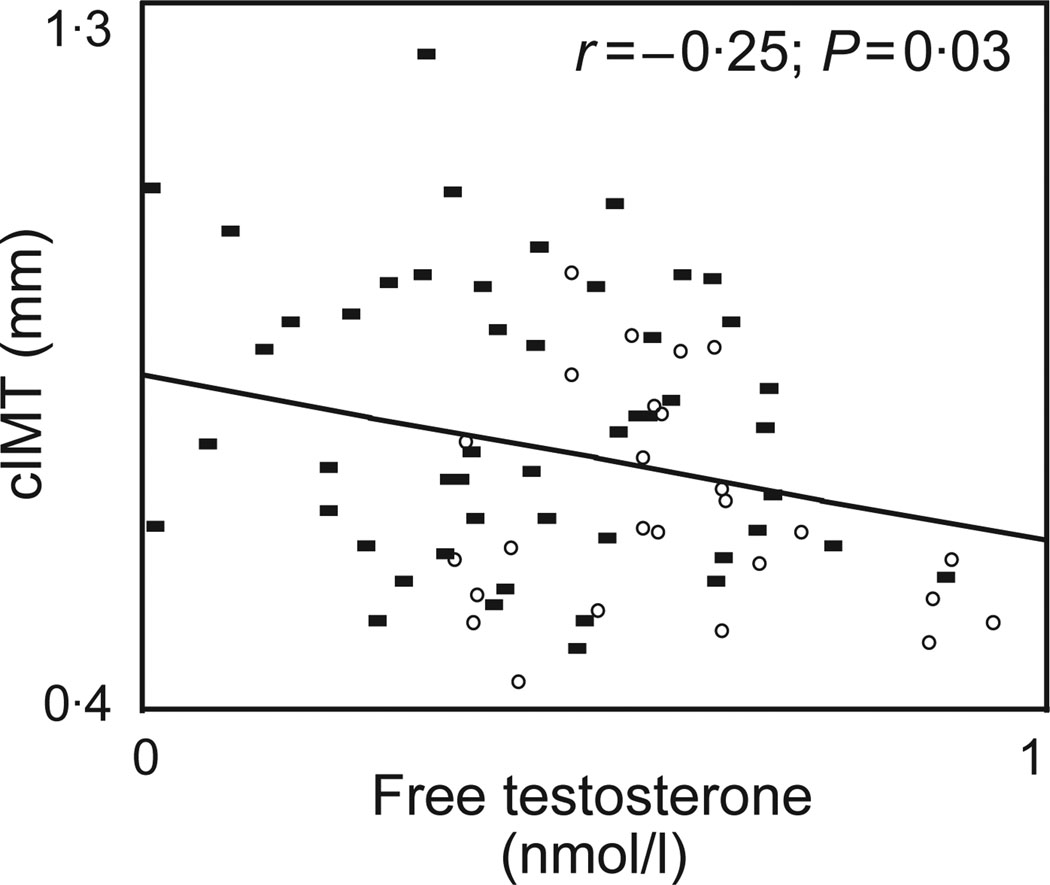
Both peak stimulated GH (r = −0·33; P = 0·004) and FT (r = −0·25, P = 0·03) (Fig. 2) were significantly associated with cIMT in univariate regression analysis. To determine whether reduced testosterone contributes independently to increased cIMT in obesity, controlling for reduced GH, we performed standard least squares multivariate regression modelling for cIMT. In a simple model controlling for age, race, FT and peak stimulated GH, a significant association between peak stimulated GH and cIMT (β = −0·003; P = 0·007; overall R2 for the model = 0·29; overall P for the model <0·0001) was observed. However, FT was not associated with cIMT in this model (P = 0·82). The substitution of total testosterone, estradiol or SHBG for FT did not significantly affect the association between peak GH and cIMT (data not shown). With the addition of traditional cardiovascular disease risk factors including tobacco use, systolic and diastolic blood pressure, LDL cholesterol, fasting glucose as well as VAT in an expanded model, peak stimulated GH remained significantly associated with cIMT (β = −0·003; P = 0·04; overall R2 for the model = 0·38; overall P for the model = 0·0003) where as FT was not associated with cIMT (P = 0·35) (Table 4). In addition, the use of a calculated free testosterone index (FTI = total testosterone/SHBG), in lieu of the directly measured FT by equilibrium dialysis, did not significantly alter the results of the multivariate modelling. Peak stimulated GH remained significantly associated with cIMT (β = −0·002; P = 0·04; overall R2 for the model = 0·41; overall P for the model = 0·0001) after controlling for traditional cardiovascular disease risk factors as before while FTI was not associated with cIMT (P = 0·06).
Fig. 2.
Univariate regression analysis of free testosterone by equilibrium dialysis and carotid intima-media thickness (cIMT). Normal weight subjects are depicted by open circles while obese subjects are depicted by closed squares.
Table 4.
Multivariate regression analysis for carotid intima-media thickness controlling for age, race, tobacco use, systolic and diastolic blood pressure, VAT, LDL cholesterol, fasting glucose, free testosterone (FT) and peak stimulated growth hormone (GH)
| β | Standard error | P | |
|---|---|---|---|
| Age | 0·006 | 0·002 | 0·001 |
| Race | −0·02 | 0·02 | 0·30 |
| Tobacco use | 0·003 | 0·001 | 0·04 |
| Systolic blood pressure | 0·0006 | 0·002 | 0·76 |
| Diastolic blood pressure | 0·0003 | 0·003 | 0·91 |
| VAT | 0·00002 | 0·0002 | 0·94 |
| LDL cholesterol | 0·02 | 0·02 | 0·44 |
| Fasting glucose | 0·03 | 0·01 | 0·08 |
| FT | 0·11 | 0·12 | 0·35 |
| Peak stimulated GH | −0·003 | 0·001 | 0·04 |
VAT, visceral adipose tissue.
Overall R2 for the model is 0·38 (P = 0·0003).
In a separate, independent model controlling for age, race, tobacco, FT, insulin, IGF-I and C-reactive protein, peak stimulated GH was significantly associated with cIMT (β = −0·003; P = 0·04; overall R2 for the model = 0·31; overall P for the model = 0·01) whereas FT was not associated with cIMT (P = 0·91). In another separate independent model controlling for age, race, tobacco, total testosterone, SHBG and estradiol, peak stimulated GH was significantly associated with cIMT (β = −0·003; P = 0·007; overall R2 for the model = 0·34; overall P for the model = 0·0001) whereas total testosterone (P = 0·35), SHBG (P = 0·54) and estradiol (P = 0·63) were not associated with cIMT.
Association of IGF-I to cIMT
Similar to peak stimulated GH, IGF-I was also negatively associated with cIMT on univariate analyses (r = −0·27; P = 0·02). However, after controlling for age, race and FT on multivariate modelling, IGF-I was no longer associated with cIMT (β = −0·008; P = 0·12; overall R2 for the model = 0·22; overall P for the model = 0·002).
Discussion
In the present study, we demonstrate a significant relationship between reduced secretion of total and FT and peak stimulated GH in otherwise healthy normal weight and obese subjects. We further demonstrate this relationship is most likely mediated by adiposity rather than a direct effect of reduced testosterone on GH secretion. We also demonstrate that the significant relationship between reduced peak stimulated GH secretion of obesity and cIMT is independent of total or FT as well as traditional cardiovascular disease risk markers.
Previous studies have demonstrated both GH1–5 and testosterone 6,7 are reduced in obesity. Our study similarly demonstrates reduced GH secretion after standard GHRH–arginine stimulation test and reduced FT by equilibrium dialysis among obese men. However, previous studies have not examined the interrelationship between reduced testosterone and GH in healthy obese men. In the paediatric literature, low testosterone associated with pre-to early puberty is known to be associated with reduced peak stimulated GH. Using the arginine–insulin tolerance test, Marin et al. demonstrated 61% of otherwise healthy prepubertal boys have an inadequate GH stimulation test defined as a peak stimulated GH<7µg/l.8 Furthermore, treatment of prepubertal to early pubertal boys with testosterone increases the peak stimulated GH on GH stimulation test9,10 demonstrating a role for potential interactions between the two hypothalamic-pituitary axes. In adult hypogonadal men, testosterone treatment can increase GH secretion and IGF-I levels.11 In elderly men, who are known to have low GH secretion, testosterone treatment can also increase pulsatile release of GH12 and may do so by attenuating the feed back suppression of IGF-I on GH secretion.26 Even in healthy non-obese men, testosterone treatment increases GHRH induced GH secretion13 and IGF-I levels.27 However, to our knowledge, the relationship between testosterone and peak stimulated GH has yet to be evaluated in otherwise healthy obese subjects. In this study, we demonstrate a strong relationship between reduced total and FT and reduced GH secretion associated with obesity. However, using multivariate regression modelling, we demonstrate this association is most likely mediated by BMI, and more specifically, visceral adiposity, rather than a direct effect of testosterone.
While the mechanisms of reduced stimulated GH and testosterone secretion in obesity are not fully understood, a central hypothalamic-pituitary process appears to be responsible for the reduction of both hormonal axes. Obesity is associated with a decrease in spontaneous GH pulse amplitude without a significant change in pulse frequency.1–3 Similarly, Giagulli et al. demonstrated an altered gonadostat with decreased LH pulse amplitude with intact LH pulse frequency in severely obese men (BMI > 40 kg/m2).28 In regard to the decrease in GH pulse amplitude associated with obesity, investigators have proposed a variety of hypotheses including increased somatostatin release,29 decreased GHRH release or responsiveness to GHRH,30,31 and alteration of normal hypothalamic/pituitary regulation because of hyperinsulinaemia, hyperglycaemia, hyperlipidaemia and elevated free fatty acids.2,32 These causes may similarly play a role in the reduced testosterone secretion in obese men. Our data is also consistent with a centrally mediated process with decreased GH responsiveness to GHRH–arginine and inappropriately normal LH and FSH, in the setting of reduced FT, in otherwise healthy obese men, suggesting a relative hypogonadotropic hypogonadism with respect to the low testosterone levels in obesity. Further studies investigating the mechanism of this hypogonadotropic hypogonadism, including investigation of the role of gene products, such as kisspeptin will be necessary.
We have previously demonstrated a significant association between reduced GH secretion and increased cardiovascular disease risk, including increased cIMT, in normal weight and obese men and women.14 At the time, we demonstrated this relationship was independent of traditional cardiovascular disease risk factors such as age, gender, race, tobacco use, blood pressure, cholesterol and fasting glucose. In this study, we investigated this relationship further by examining the role of testosterone. Reduced testosterone in men is associated with increased cardiovascular disease risk including unfavourable lipid profile19 and FT is inversely associated with cIMT in overweight and obese men as shown by De Pergola et al.33 and confirmed by our own data. We initially hypothesized that the strong relationship between reduced GH secretion and cIMT was mediated, in part, via reduced testosterone. However, multivariate modelling demonstrates that total or FT or other reproductive hormones did not appear to be confounders in the relationship between peak stimulated GH and cIMT. This relationship remained strong even with the addition of traditional cardiovascular disease risk markers including age, race, blood pressure, cholesterol, fasting glucose and measures of adiposity to testosterone. These results suggest the mechanisms by which reduced GH and testosterone increase cardiovascular disease risk are independent of each other. The specific mechanisms by which reduced peak stimulated GH may be related to premature vascular disease in obesity are not known, but may include endothelial dysfunction and unfavourable lipid and inflammatory profile among other possibilities. Assessment of the patho-physio-logical mechanisms contributing to the relationship between reduced GH and increased cIMT was beyond the scope of the current study, and further mechanistic studies are necessary in this regard.
The strength of our study lies in the large number of subjects, obese and normal weight, who were carefully and simultaneously phenotyped for GH, using the GH-releasing hormone–arginine stimulation test, for gonadal function using equilibrium dialysis to measure FT, and for cardiovascular disease risk using cIMT. We show for the first time that the relationship of reduced peak GH to increased cIMT in obesity is not related to prevalent relative hypogonadism in this population. Moreover, our data suggest that excess visceral adiposity mediates both low GH and low testosterone. However, this was a cross-sectional study and therefore causality cannot be definitively established. Interventional studies are necessary to further analyse the relationship between GH, testosterone and cardio-metabolic end points in obesity. FT was measured by equilibrium dialysis using a well known assay from the Mayo Clinic Medical Laboratories with an inter-assay CV which ranged from 10% to 19%. While it is possible that the relatively higher CV for the FT assay may have contributed to some degree of imprecision in the multivariate assessment of the relationship of FT to peak GH, controlling for VAT, we do not believe this is likely to have significantly affected the results in a major way. The FT assay was sensitive and precise enough to demonstrate a significant effect of obesity on FT as well as significant relationship between FT and visceral adipose tissue. In addition, the use of a calculated index of FT, the free testosterone index (FTI = total testosterone/SHBG), in lieu of free testosterone did not significantly affect the results. In addition, the study was limited to men and further evaluation of the role of reproductive hormones and peak stimulated GH in cardiovascular disease should also be pursued in women. Further assessment of the specific mechanisms by which reduced GH contributes to increased cIMT in obesity are needed as are studies investigating the patho-physiology of reduced GH and gonadotrophin secretion in obesity.
In summary, we demonstrated a very strong relationship between reduced testosterone and reduced GH secretion in obesity. This relationship appears to be mediated by adiposity rather than a direct effect of testosterone itself. Furthermore, we demonstrate a significant relationship between reduced growth hormone secretion and carotid intima-media thickness, in which testosterone or other reproductive hormones and traditional cardiovascular disease risk markers did not appear to be confounders.
Supplementary Material
Acknowledgments
Grant Support:
National Institutes of Health grant 1R01HL085268-01A1 to SG, K24DK064545-06 to SG, grant UL1RR025758 to the Harvard Catalyst.
Footnotes
Disclosure Statement
The authors have nothing to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1 Baseline characteristics of study subjects stratified by GH status (n = 76). Data are presented as mean ± SEM. Relative GH deficiency determined as peak stimulated GH ≤4·2 µg/l on GHRH-arginine stimulation test
Table S2 Baseline characteristics of study subjects stratified by free testosterone status (n = 76). Data are presented as mean ± SEM. Relative hypogonadism of obesity is determined as fasting free testosterone <0·31 nmol/l (9 ng/dl)
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. Journal of Clinical Endocrinology & Metabolism. 1991;73:1081–1088. doi: 10.1210/jcem-73-5-1081. [DOI] [PubMed] [Google Scholar]
- 2.Riedel M, Hoeft B, Blum WF, et al. Pulsatile growth hormone secretion in normal-weight and obese men: differential metabolic regulation during energy restriction. Metabolism. 1995;44:605–610. doi: 10.1016/0026-0495(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 3.Van Dam EW, Roelfsema F, Helmerhorst FH, et al. Low amplitude and disorderly spontaneous growth hormone release in obese women with or without polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2002;87:4225–4230. doi: 10.1210/jc.2002-012006. [DOI] [PubMed] [Google Scholar]
- 4.Bonert VS, Elashoff JD, Barnett P, et al. Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: diagnostic caveat for adult GH deficiency. Journal of Clinical Endocrinology & Metabolism. 2004;89:3397–3401. doi: 10.1210/jc.2003-032213. [DOI] [PubMed] [Google Scholar]
- 5.Makimura H, Stanley T, Mun D, et al. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. Journal of Clinical Endocrinology & Metabolism. 2008;93:4254–4260. doi: 10.1210/jc.2008-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidell JC, Bjorntorp P, Sjostrom L, et al. Visceral fat accumulation in men is positively associated with insulin, glucose, and c-peptide levels, but negatively with testosterone levels. Metabolism. 1990;9:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 7.Pasquali R, Casimirri F, Balestra V, et al. The relative contribution of androgens and insulin in determining abdominal body fat distribution in premenopausal women. Journal of Endocrinological Investigation. 1991;14:839–846. doi: 10.1007/BF03347939. [DOI] [PubMed] [Google Scholar]
- 8.Marin G, Domene HM, Barnes KM, et al. The effects of estrogen priming and puberty on the growth hormone response to standardized treadmill exercise and arginine-insulin in normal girls and boys. Journal of Clinical Endocrinology & Metabolism. 1994;79:537–541. doi: 10.1210/jcem.79.2.8045974. [DOI] [PubMed] [Google Scholar]
- 9.Gonc EN, Yordam N, Kandemir N, et al. Comparison of stimulated growth hormone levels in primed versus unprimed provocative tests. Effect of various testosterone doses on growth hormone levels. Hormone Research. 2001;56:32–37. doi: 10.1159/000048087. [DOI] [PubMed] [Google Scholar]
- 10.Molina S, Paoli M, Camacho N, et al. Is testosterone and estrogen priming prior to clonidine useful in the evaluation of the growth hormone status of short peripubertal children? Journal of Pediatric Endocrinology & Metabolism. 2008;21:257–266. doi: 10.1515/jpem.2008.21.3.257. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Merriam GR, Sherins RJ. Chronic sex steroid exposure increases mean plasma growth hormone concentration and pulse amplitude in men with isolated hypogonadotropic hypogonadism. Journal of Clinical Endocrinology & Metabolism. 1987;64:651–656. doi: 10.1210/jcem-64-4-651. [DOI] [PubMed] [Google Scholar]
- 12.Gentili A, Mulligan T, Godschalk M, et al. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. Journal of Clinical Endocrinology & Metabolism. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. [DOI] [PubMed] [Google Scholar]
- 13.Devesa J, Lois N, Arce V, et al. The role of sexual steroids in the modulation of growth hormone (GH) secretion in humans. Journal of Steroid Biochemistry and Molecular Biology. 1991;40:165–173. doi: 10.1016/0960-0760(91)90179-9. [DOI] [PubMed] [Google Scholar]
- 14.Makimura H, Stanley T, Mun D, et al. Reduced growth hormone secretion is associated with increased carotid intima-media thickness in obesity. Journal of Clinical Endocrinology & Metabolism. 2009;94:5131–5138. doi: 10.1210/jc.2009-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannsson G, Marin P, Lonn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. Journal of Clinical Endocrinology & Metabolism. 1997;82:727–734. doi: 10.1210/jcem.82.3.3809. [DOI] [PubMed] [Google Scholar]
- 16.Franco C, Brandberg J, Lonn L, et al. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. Journal of Clinical Endocrinology & Metabolism. 2005;90:1466–1474. doi: 10.1210/jc.2004-1657. [DOI] [PubMed] [Google Scholar]
- 17.Colangelo LA, Ouyang P, Liu K, et al. Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32:1049–1051. doi: 10.2337/dc08-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vikan T, Schirmer H, Njolstad I, et al. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type. European Journal of Endocrinology. 2010;162:747–754. doi: 10.1530/EJE-09-0943. [DOI] [PubMed] [Google Scholar]
- 19.Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207:318–327. doi: 10.1016/j.atherosclerosis.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2009;210:232–236. doi: 10.1016/j.atherosclerosis.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 21.Corneli G, Di Somma C, Baldelli R, et al. The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. European Journal of Endocrinology. 2005;153:257–264. doi: 10.1530/eje.1.01967. [DOI] [PubMed] [Google Scholar]
- 22.Biller BM, Samuels MH, Zagar A, et al. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. Journal of Clinical Endocrinology & Metabolism. 2002;87:2067–2079. doi: 10.1210/jcem.87.5.8509. [DOI] [PubMed] [Google Scholar]
- 23.Rietschel P, Hadigan C, Corcoran C, et al. Assessment of growth hormone dynamics in human immunodeficiency virus-related lipodystrophy. Journal of Clinical Endocrinology & Metabolism. 2001;86:504–510. doi: 10.1210/jcem.86.2.7175. [DOI] [PubMed] [Google Scholar]
- 24.Johnsen S, Dolan SE, Fitch KV, et al. Carotid intima medial thickness in human immunodeficiency virus-infected women: effects of protease inhibitor, use, cardiac risk factors, and the metabolic syndrome. Journal of Clinical Endocrinology & Metabolism. 2006;91:4916–4924. doi: 10.1210/jc.2006-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan R, Kaufhold J, Hemphill LC, et al. Anisotropic edge-preserving smoothing in carotid B-mod ultrasound for improved segmentation and intima-media thickness (IMT) measurement. Computers in Cardiology. 2000;27:37–40. [Google Scholar]
- 26.Veldhuis JD, Keenan DM, Bailey JN, et al. Testosterone supplementation in older men restrains insulin-like growth factor’s dose-dependent feedback inhibition of pulsatile growth hormone secretion. Journal of Clinical Endocrinology & Metabolism. 2009;94:246–254. doi: 10.1210/jc.2008-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobbs CJ, Plymate SR, Rosen CJ, et al. Testosterone administration increases insulin-like growth factor-I levels in normal men. Journal of Clinical Endocrinology & Metabolism. 1993;77:776–779. doi: 10.1210/jcem.77.3.7690364. [DOI] [PubMed] [Google Scholar]
- 28.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. Journal of Clinical Endocrinology & Metabolism. 1994;79:997–1000. doi: 10.1210/jcem.79.4.7962311. [DOI] [PubMed] [Google Scholar]
- 29.Cordido F, Casanueva F, Dieguez C. Cholinergic receptor activation by pyridostigmine restores growth hormone (GH) responsiveness to GH-releasing hormone administration in obese subjects: evidence for hypothalamic somatostatinergic participation in the blunted GH release of obesity. Journal of Clinical Endocrinology & Metabolism. 1989;68:290–293. doi: 10.1210/jcem-68-2-290. [DOI] [PubMed] [Google Scholar]
- 30.Williams T, Berelowitz M, Joffe SN, et al. Impaired growth hormone responses to growth hormone-releasing factor in obesity. A pituitary defect reversed with weight reduction. New England Journal of Medicine. 1984;311:1403–1407. doi: 10.1056/NEJM198411293112203. [DOI] [PubMed] [Google Scholar]
- 31.Kopelman PG, Noonan K, Goulton R, et al. Impaired growth hormone response to growth hormone releasing factor and insulin-hypoglycaemia in obesity. Clinical Endocrinology (Oxford) 1985;23:87–94. doi: 10.1111/j.1365-2265.1985.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 32.Cordido F, Fernandez T, Martinez T, et al. Effect of acute pharmacological reduction of plasma free fatty acids on growth hormone (GH) releasing hormone-induced GH secretion in obese adults with and without hypopituitarism. Journal of Clinical Endocrinology & Metabolism. 1998;83:4350–4354. doi: 10.1210/jcem.83.12.5310. [DOI] [PubMed] [Google Scholar]
- 33.De Pergola G, Pannacciulli N, Ciccone M, et al. Free testosterone plasma levels are negatively associated with the intima-media thickness of the common carotid artery in overweight and obese glucose-tolerant young adult men. International Journal of Obesity and Related Metabolic Disorders. 2003;27:803–807. doi: 10.1038/sj.ijo.0802292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.