Abstract
A large number of studies have analyzed the putative functions of the prion protein (PrPC) in mammals. Although its sequence conservation over a wide range of different animals may indicate that this protein could have a key role in prion diseases, an absolutely accepted involvement has not been found so far. We have recently reported that PrPC regulates Nanog mRNA expression, the first non-redundant function of PrPC in embryonic stem cells (ESC), which translates into control of pluripotency and early differentiation. Contrary to what is believed, the other two members of the prion protein family, Doppel and Shadoo, cannot replace the absence of PrPC, causing the appearance of a new embryoid body (EB) population in our in vitro culture. The similarities between EB and an early post-implantation embryo suggest that this might also occur in vivo, enhancing the importance of this finding. On the other hand, our data may support the hypothesis of a relationship between the loss of PrPC function and neuronal degeneration in prion diseases. A reduction in brain stem cells pluripotency after PrPC is misfolded into the pathological conformation (PrPSc) could lead to a delay or a disappearance of the normal brain damage recovery.
Key words: prion protein, differentiation, pluripotency, embryoid bodies, primordial germ cells, integrins, prion diseases
Although an important function for the cellular prion protein (PrPC) has been proposed, it still remains unclear. PrPC plays a major role not only in the central nervous system, but also in other tissues. In addition to the brain, it has been detected in several non-neuronal tissues, including lymphoid cells, lung, kidney, heart, gastrointestinal tract, muscle, mammary glands, etc.1,2 Recently, there is new evidence that it may also have possible implications in tissue morphogenesis,3,4 tissue and stem cells regeneration5 and embryogenesis.6 However, our understanding of its physiology remains poorly detailed. Regarding the embryo development, it is known to be a highly intricate matrix of genes that require a precise timepoint coordination to achieve the adequate biochemical signaling. Until now, several groups of genes have been considered to play an essential role in this process, but those that are involved in pluripotency and differentiation are of particular interest. The maintenance or the loss of stemness is one of the main activities the cell has to carefully manage, bearing in mind that the embryo is a differentiating system.
Current knowledge places Sox2, Oct3/4, Nanog and Stat3 among the most important genes involved in the maintenance of stem cell pluripotency.7–9 PrPC has also been related to this property since it is expressed at almost 1.5 times higher in embryonic stem cells (ESC) than in somatic cells,7 reaching a maximum of 6.2 times in the first two weeks of differentiation.8 The discovery of its essentiality in supporting self-renewal in haematopoietic stem cells9 confirms this point. An involvement in long term proliferation of cancer cells (glioblastoma, breast, prostate and gastric cancer) has been described as well,10,11 similar to that exhibited by ESC, thus there was a need to study how PrPC is able to regulate this process.
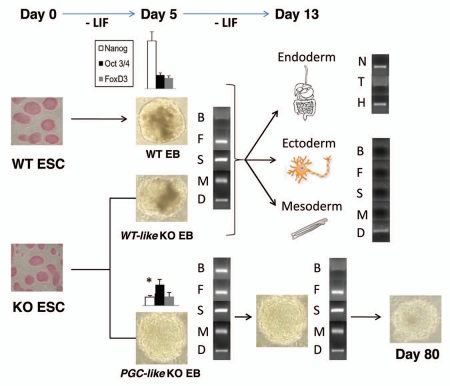
In our work, we demonstrated that PrPC influences the expression of genes involved in ESC self-renewal. Particulary, we found that PrPC transcription regulates the mRNA expression of the important pluripotent marker gene Nanog in early ESC differentiation12 (Fig. 1). Furthermore, the absence of PrPC also provoked the appearance of a subpopulation of embryoid bodies (EBs) in the PrPC-null (KO) culture at that time, which retained the expression of primordial germ cell (PGC) markers for a longer period (Fig. 1) and maintained a high pluripotency level.12
Figure 1.
PrPC function during early ESC differentiation. Scheme showing gene expression and morphological differences during differentiation of WT and PrPC-null (KO) ESC. In the KO line, two EB populations (WT-like KO and PGC-like KO EBs) appeared when Leukemia Inhibitory Factor (LIF) was removed from the medium. The WT-like KO EBs developed in a similar way to WT EBs, showing a different Nanog expression on Day 5 of differentiation than the PGC-like KO EBs. This led to a complete differentiation into the three embrionary layers by Day 13, since all the early ectoderm (Nestin [N]), mesoderm (Brachiury [T]) and endoderm (Hnf3 [H]) markers were present and the majority of the PGC makers analysed (Bmp4 [B], Fragilis [F], Stella [S], Mvh4 [M] and Dazl [D]) were absent. On the other side, the PGC-like KO EBs expressed all the PGC makers analysed during the entire early differentiation, and this population retained its morphology even longer (80 days).
Even though Nanog is not included between the minimal combinations of genes required to obtain iPS from fibroblasts,13,14 it is a crucial protein. Several studies report how the overexpression of this gene releases ESC pluripotency from the dependence of Stat3 stimulation, also reducing and retarding the ESC differentiation.15,16 On the contrary, the ablation leads to a failure in the specification of the early embryo pluripotent cells and to a parietal and visceral ESC segregation.15,16 Nanog is also important because it is a member of the feedback cluster that exists along with Oct3/4 and FoxD3,17,18 both described to play a key role in pluripotency.19,20 These data enhance the value of the relationship between Prnp and Nanog in animal development, moreover considering that it seems to take place in foetal and neonates gonads.12
On the other hand, we have described that this association was partially mediated by integrin-β5.12 Integrins are heterodimeric transmembrane proteins that are involved in cell adhesion and signal transmission mechanisms, forming part of processes such as cell death, migration, differentiation and proliferation.21,22 This result was supported by some studies, which described that integrins, e.g., integrin-β1, were related to Nanog expression21 and several others that showed how PrPC was also involved in integrins regulation, e.g., in the development of embryonic cell adhesion via E-cadherin.23 Thus, our findings suggest a complex interaction matrix in which integrins play a significant role. Further experiments should clarify if this role may be linked to the facilitation of protein interaction or to signal modulation as a feedback control.
In our in vitro experiments, we included the derivation of ESC into EBs because of their documented similarities with an early post-implantation embryo.24 Unexpectedly, a new EB population appeared from Day 5 in the KO line culture, persisting even longer (80 days) 12 (Fig. 1). It might suggest the possibility of preexistence populations of ESCs in the theoretical pure initial culture, since a variety of ESC based on low or high Nanog levels has been previously described in reference 17. The Prnp knockout could have promoted the differentiation of one of those specific KO ESCs when LIF was removed from the medium, being revealed on Day 5 as a special EB subpopulation which expressed PGC markers (PGC-like KO EB)12 (Fig. 1).
In a prion context, this is the first non-redundant function described for the PrPC, since the other members of the prion family (Doppel and Shadoo) are not able to compensate the effects derived from PrPC disappearance.12 Although many studies have tried to elucidate the physiological role of the protein, their conclusions were contradictory or not conclusive.25 Furthermore, KO animals, in which Doppel is not artifactually upregulated, did not show any significant alterations.26,27 This unfinished exploration is impeding the resolution of an important question, since PrPC changes its conformation to acquire pathological properties (PrPSc),28 leading perhaps to a loss of PrPC functionality. The consequence could be a role in the pathogenesis of prion diseases, for instance, reducing post-injury regeneration. Stella et al. show a delay in muscle reparation due to the absence of PrPC, maybe via tumor necrosis factor (TNF)α, a molecule described to mediate either neuronal cell death or neuroprotection,29 not only in neurons4 but also in muscle.3 Furthermore, it was demonstrated that the differentiation of neural precursor cells (NPCs) was delayed when the PrPC was not functional, although it did not affect their final morphology or the final tissue morphology.5 In this scenario, additional defects in differentiation, late activation or prolonged proliferation in the “repairing” cells could critically worsen the pathological circumstances, preventing an improvement. Thus the discovery of a reduced Nanog expression in the KO EBs12 might imply a critical decrease in the NPCs quantity and pluripotency in vivo in a prion infected brain. Accordingly, it could lead to a lower response against the lesions caused by the common harmful agents, e.g., reactive oxygen species, and to a possible explanation for the progressive tissue degeneration in these unique pathologies.
In conclusion, PrPC has a key function during early embryogenesis and adult regeneration, with this last feature suggesting an explanation for prion pathogenesis. The response against external damage could be diminished as a consequence of the PrPC conformational change that occurs in prion diseases. Hence, it remarked the relevance of the PrPC function studies, which allow us to increase our comprehension of the prion disease behaviour. Importantly, our work also proposes the timing when the putative PrPC role might take place. All KO systems are perfectly viable probably because of a compensation phenomenon, thus these studies have to be carried out at the moment when these compensatory pathways are still not established, in our particular case, during early differentiation.
Acknowledgments
Elia Alamillo and Danielle A. Padilla de Beer for the English correction. This work was funded by Grant AGL2009-11358 from the Spanish Ministry of Science and Innovation.
Abbreviations
- EB
embryoid body
- PrPC
prion protein
- PGC
primordial germ cell
- NPCs
neural precursor cells
- LIF
leukemia inhibitory factor
- KO
knock out
- ESC
embryonic stem cells
- WT
wild type
References
- 1.Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M. A cellular form of prion protein (PrPC) exists in many non-neuronal tissues of sheep. J Gen Virol. 1995;76:2583–2587. doi: 10.1099/0022-1317-76-10-2583. [DOI] [PubMed] [Google Scholar]
- 2.Castilla J, Gutierrez-Adan A, Brun A, Pintado B, Parra B, Ramirez MA, et al. Different behavior toward bovine spongiform encephalopathy infection of bovine prion protein transgenic mice with one extra repeat octapeptide insert mutation. J Neurosci. 2004;24:2156–2164. doi: 10.1523/JNEUROSCI.3811-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stella R, Massimino ML, Sandri M, Sorgato MC, Bertoli A. Cellular prion protein promotes regeneration of adult muscle tissue. Mol Cell Biol. 2010;30:4864–4876. doi: 10.1128/MCB.01040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pradines E, Loubet D, Mouillet-Richard S, Manivet P, Launay JM, Kellermann O, et al. Cellular prion protein coupling to TACE-dependent TNFalpha shedding controls neurotransmitter catabolism in neuronal cells. J Neurochem. 2009;110:912–923. doi: 10.1111/j.1471-4159.2009.06176.x. [DOI] [PubMed] [Google Scholar]
- 5.Steele AD, Emsley JG, Ozdinler PH, Lindquist S, Macklis JD. Prion protein (PrPc) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc Natl Acad Sci USA. 2006;103:3416–3421. doi: 10.1073/pnas.0511290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young R, Passet B, Vilotte M, Cribiu EP, Beringue V, Le Provost F, et al. The prion or the related Shadoo protein is required for early mouse embryogenesis. FEBS Lett. 2009;583:3296–3300. doi: 10.1016/j.febslet.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Grskovic M, Chaivorapol C, Gaspar-Maia A, Li H, Ramalho-Santos M. Systematic identification of cis-regulatory sequences active in mouse and human embryonic stem cells. PLoS Genet. 2007;3:145. doi: 10.1371/journal.pgen.0030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hailesellasse Sene K, Porter CJ, Palidwor G, Perez-Iratxeta C, Muro EM, Campbell PA, et al. Gene function in early mouse embryonic stem cell differentiation. BMC Genomics. 2007;8:85. doi: 10.1186/1471-2164-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang CC, Steele AD, Lindquist S, Lodish HF. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc Natl Acad Sci USA. 2006;103:2184–2189. doi: 10.1073/pnas.0510577103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Y, Zhao L, Liang J, Liu J, Shi Y, Liu N, et al. Cellular prion protein promotes invasion and metastasis of gastric cancer. Faseb J. 2006;20:1886–1888. doi: 10.1096/fj.06-6138fje. [DOI] [PubMed] [Google Scholar]
- 11.Mehrpour M, Codogno P. Prion protein: From physiology to cancer biology. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Miranda A, Pericuesta E, Ramirez MA, Gutierrez-Adan A. Prion protein expression regulates embryonic stem cell pluripotency and differentiation. PLoS One. 2011;6:18422. doi: 10.1371/journal.pone.0018422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 16.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 17.Kalmar T, Lim C, Hayward P, Munoz-Descalzo S, Nichols J, Garcia-Ojalvo J, et al. Regulated fluctuations in nanog expression mediate cell fate decisions in embryonic stem cells. PLoS Biol. 2009;7:1000149. doi: 10.1371/journal.pbio.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan G, Li J, Zhou Y, Zheng H, Pei D. A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. FASEB J. 2006;20:1730–1732. doi: 10.1096/fj.05-5543fje. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Labosky PA. Regulation of embryonic stem cell self-renewal and pluripotency by Foxd3. Stem Cells. 2008;26:2475–2484. doi: 10.1634/stemcells.2008-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, Fukuhara Y, et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells. 2007;25:3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 22.Lee ST, Yun JI, Jo YS, Mochizuki M, van der Vlies AJ, Kontos S, et al. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials. 2010;31:1219–1226. doi: 10.1016/j.biomaterials.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 23.Malaga-Trillo E, Solis GP, Schrock Y, Geiss C, Luncz L, Thomanetz V, et al. Regulation of embryonic cell adhesion by the prion protein. PLoS Biol. 2009;7:55. doi: 10.1371/journal.pbio.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin J, Guo X, Cui GH, Zhou YC, Zhou DR, Tang AF, et al. Cluster characterization of mouse embryonic stem cell-derived pluripotent embryoid bodies in four distinct developmental stages. Biologicals. 2009;37:235–244. doi: 10.1016/j.biologicals.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Westergard L, Christensen HM, Harris DA. The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim Biophys Acta. 2007;1772:629–644. doi: 10.1016/j.bbadis.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bueler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 27.Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol. 1994;8:121–127. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- 28.Padilla D, Beringue V, Espinosa JC, Andreoletti O, Jaumain E, Reine F, et al. Sheep and goat BSE propagate more efficiently than cattle BSE in human PrP transgenic mice. PLoS Pathog. 2011;7:1001319. doi: 10.1371/journal.ppat.1001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szelenyi J. Cytokines and the central nervous system. Brain Res Bull. 2001;54:329–338. doi: 10.1016/s0361-9230(01)00428-2. [DOI] [PubMed] [Google Scholar]