Abstract
The effects of late pregnancy on adipose tissue metabolism have been examined in fed and fasted rats. Lumbar fat was excised from 19-day pregnant and age-matched virgin rats which had been given unrestricted access to food (“fed”) or fasted for 48 hr before sacrifice.
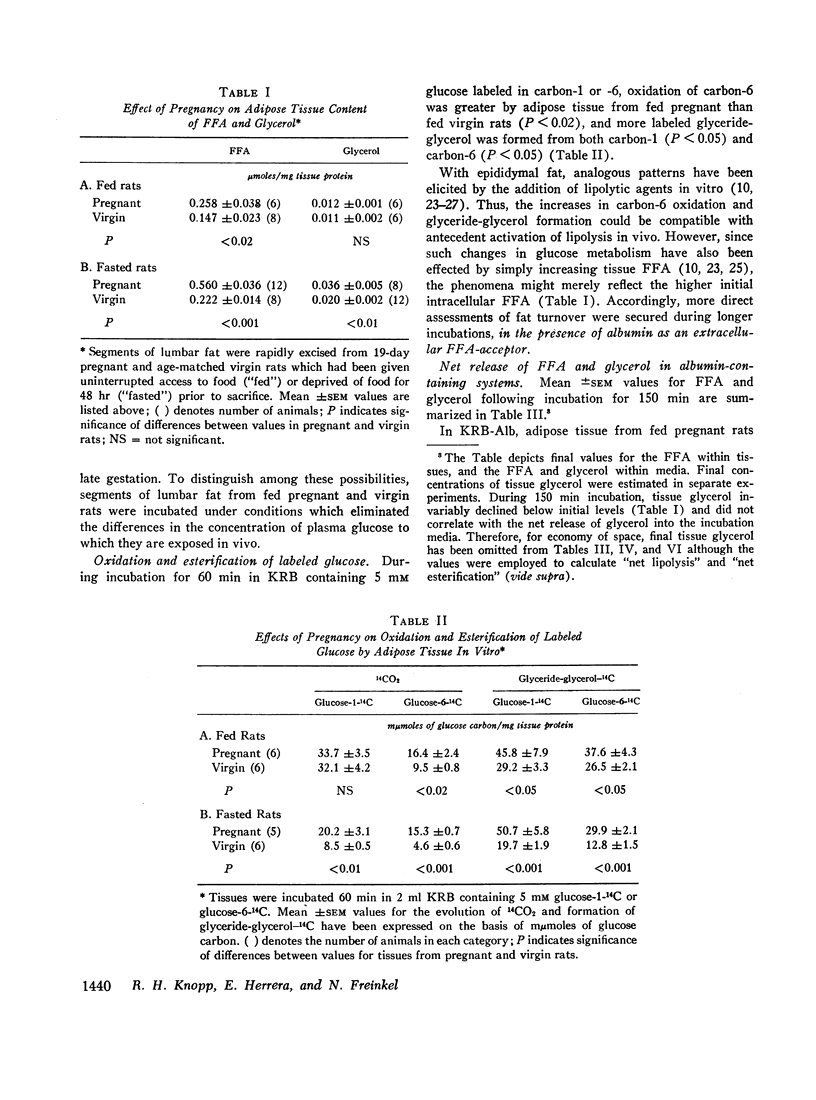
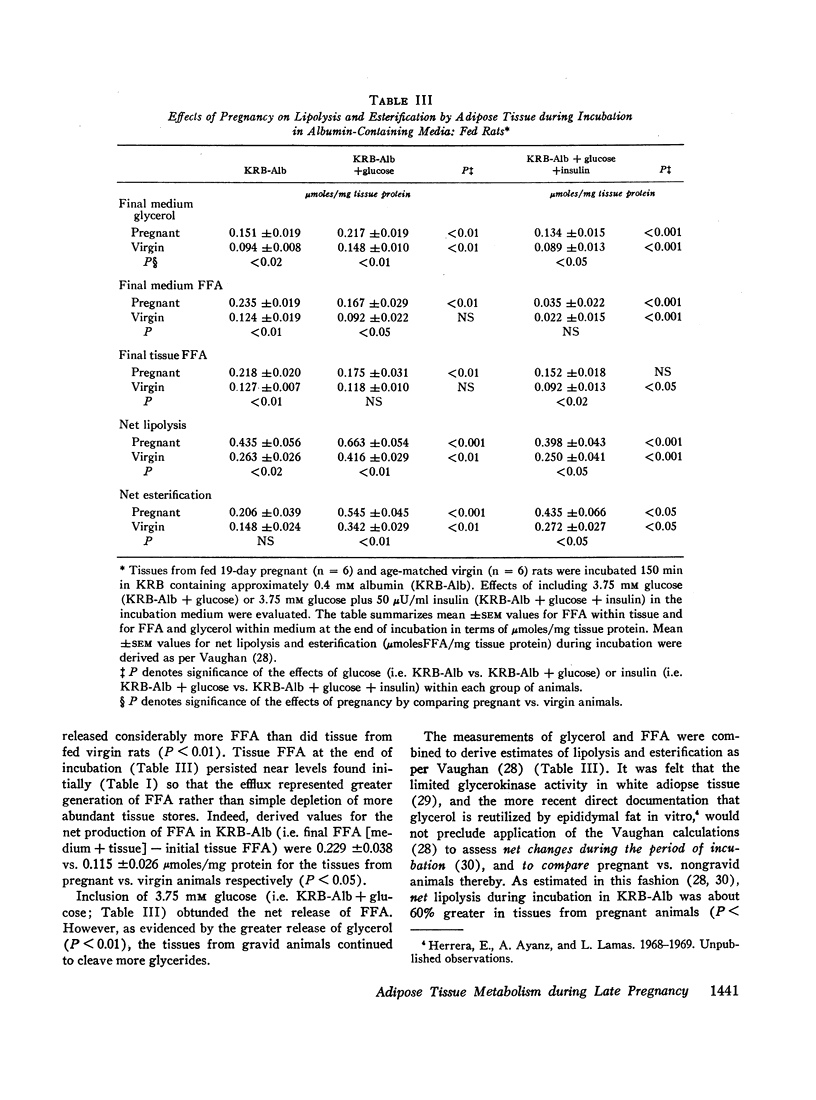
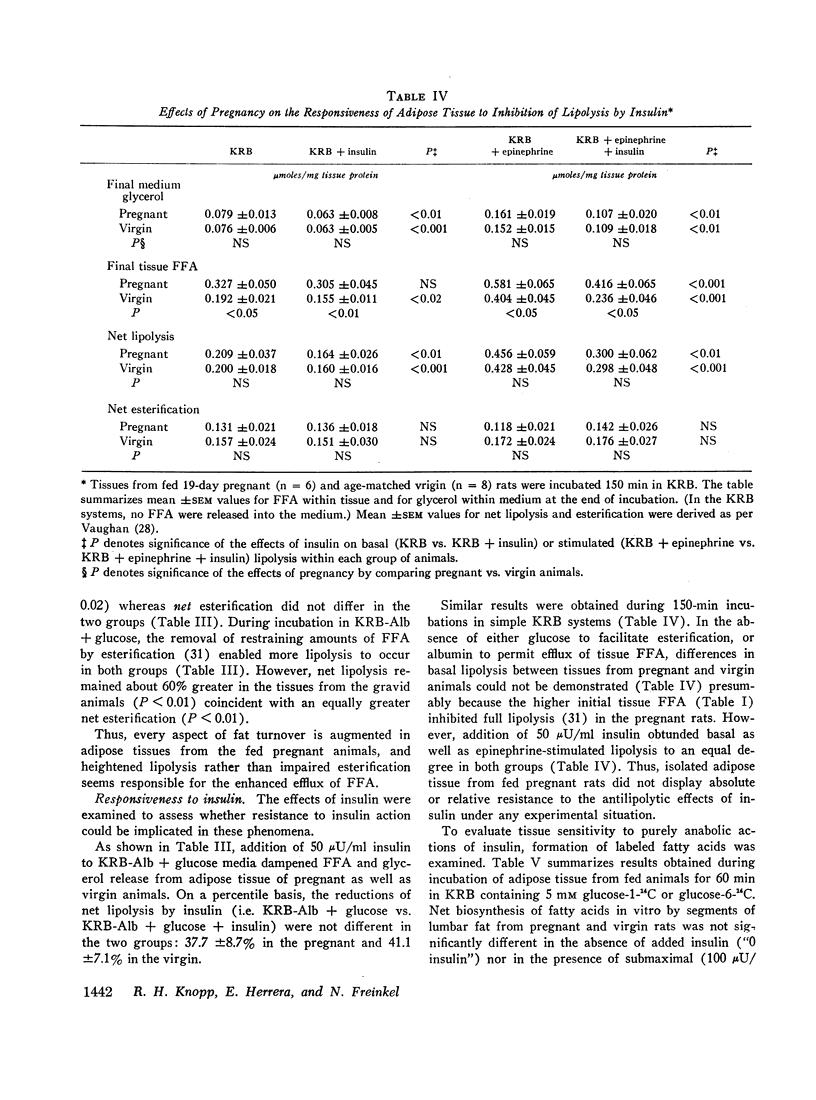
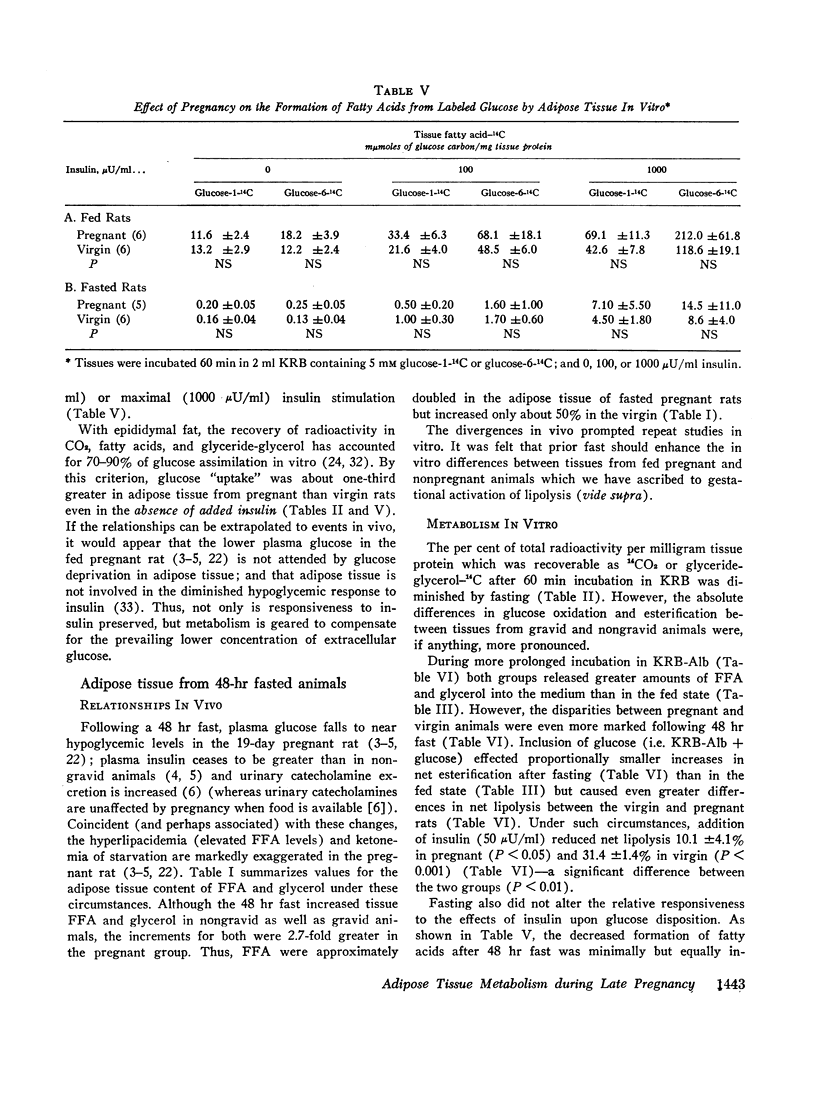
In the fed state, adipose tissue from pregnant rats displayed an increased content of free fatty acids (FFA). This coincided with augmented cleavage of preformed glycerides during incubation in vitro as evidenced by greater net production of FFA and glycerol, and altered disposition of labeled glucose. The enhanced lipolysis was independent of the availability of glucose and was not accompanied by impaired responsiveness to the antilipolytic or to the lipogenic actions of added insulin. In the presence of glucose and albumin, esterification as well as lipolysis was greater in adipose tissue from pregnant than nongravid animals. All the differences were exaggerated by prior fasting.
These properties of adipose tissue during late gestation have been ascribed to a primary activation of lipolysis rather than impaired esterification or resistance to insulin. It has been suggested that the hormones of pregnancy may be responsible. Although increased intake of food and heightened availability of insulin may offset the net lipolytic effects in the fed state, a heightened turnover of adipose stores is always present. Thus, the pregnant animal appears better poised to mobilize preformed fat whenever exogenous nutrients are withheld.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALL E. G., JUNGAS R. L. On the action of hormones which accelerate the rate of oxygen consumption and fatty acid release in rat adipose tissue in vitro. Proc Natl Acad Sci U S A. 1961 Jul 15;47:932–941. doi: 10.1073/pnas.47.7.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALL E. G., MARTIN D. B., COOPER O. Studies on the metabolism of adipose tissue. I. The effect of insulin on glucose utilization as measured by the manometric determination of carbon dioxide output. J Biol Chem. 1959 Apr;234(4):774–780. [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Measurement of adipose-tissue metabolites in vivo. Biochem J. 1969 Apr;112(2):195–202. doi: 10.1042/bj1120195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAHILL G. F., Jr, LEBOEUF B., FLINN R. B. Studies on rat adipose tissue in vitro. VI. Effect of epinephrine on glucose metabolism. J Biol Chem. 1960 May;235:1246–1250. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Clark C. M., Jr, Cahill G. F., Jr, Soeldner J. S. Effects of exogenous insulin on the rate of fatty acid synthesis and glucose C-14 utilization in the twenty-day rat fetus. Diabetes. 1968 Jun;17(6):362–368. doi: 10.2337/diab.17.6.362. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- DUNCOMBE W. G. THE COLORIMETRIC MICRO-DETERMINATION OF NON-ESTERIFIED FATTY ACIDS IN PLASMA. Clin Chim Acta. 1964 Feb;9:122–125. doi: 10.1016/0009-8981(64)90004-x. [DOI] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FREINKEL N., COHEN A. K., ARKY R. A., FOSTER A. E. ALCOHOL HYPOGLYCEMIA. II. A POSTULATED MECHANISM OF ACTION BASED ON EXPERIMENTS WITH RAT LIVER SLICES. J Clin Endocrinol Metab. 1965 Jan;25:76–94. doi: 10.1210/jcem-25-1-76. [DOI] [PubMed] [Google Scholar]
- FREINKEL N. Extrathyroidal actions of pituitary thyrotropin: effects on the carbohydrate, lipid and respiratory metabolism of rat adipose tissue. J Clin Invest. 1961 Mar;40:476–489. doi: 10.1172/JCI104275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREINKEL N. Further observations concerning the action of pituitary thyrotropin on the intermediate metabolism of sheep thyroid tissue in vitro. Endocrinology. 1960 Jun;66:851–859. doi: 10.1210/endo-66-6-851. [DOI] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- GREIG M., COYLE M. G., COOPER W., WALKER J. Plasma progesterone in mother and foetus in the second half of human pregnancy. J Obstet Gynaecol Br Emp. 1962 Oct;69:772–779. doi: 10.1111/j.1471-0528.1962.tb01279.x. [DOI] [PubMed] [Google Scholar]
- HAGEN J. H., BALL E. G. Studies on the metabolism of adipose tissue. IV. The effect of insulin and adrenaline on glucose utilization, lactate production, and net gas exchange. J Biol Chem. 1960 Jun;235:1545–1549. [PubMed] [Google Scholar]
- Herrera E. M., Knopp R. H., Freinkel N. Urinary excretion of epinephrine and norepinephrine during fasting in late pregnancy in the rat. Endocrinology. 1969 Feb;84(2):447–450. doi: 10.1210/endo-84-2-447. [DOI] [PubMed] [Google Scholar]
- Herrera E., Knopp R. H., Freinkel N. Carbohydrate metabolism in pregnancy. VI. Plasma fuels, insulin, liver composition, gluconeogenesis, and nitrogen metabolism during late gestation in the fed and fasted rat. J Clin Invest. 1969 Dec;48(12):2260–2272. doi: 10.1172/JCI106192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- LEBOEUF B., CAHILL G. F., Jr Studies on rat adipose tissue in vitro. VIII. Effect of preparations of pituitary adrenocorticotropic and growth hormones on glucose metabolism. J Biol Chem. 1961 Jan;236:41–46. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYNN W. S., MACLEOD R. M., BROWN R. H. Effects of epinephrine, insulin, and corticotrophin on the metabolism of rat adipose tissue. J Biol Chem. 1960 Jul;235:1904–1911. [PubMed] [Google Scholar]
- Leake N. H., Burt R. L. Effect of HPL and pregnancy on glucose uptake in rat adipose tissue. Am J Obstet Gynecol. 1969 Jan 1;103(1):39–43. doi: 10.1016/s0002-9378(16)34337-x. [DOI] [PubMed] [Google Scholar]
- Leake N. H., Burt R. L. Response of rat adipose tissue to insulin during pregnancy. Am J Obstet Gynecol. 1966 Sep 1;96(1):131–133. doi: 10.1016/s0002-9378(16)34652-x. [DOI] [PubMed] [Google Scholar]
- MCKAY D. G., KAUNITZ H. STUDIES OF THE GENERALIZED SHWARTZMAN REACTION INDUCED BY DIET. VI. EFFECTS OF PREGNANCY ON LIPID COMPOSITION OF SERUM AND TISSUES. Metabolism. 1963 Nov;12:990–995. [PubMed] [Google Scholar]
- Rodbell M. Modulation of lipolysis in adipose tissue by fatty acid concentration in fat cell. Ann N Y Acad Sci. 1965 Oct 8;131(1):302–314. doi: 10.1111/j.1749-6632.1965.tb34798.x. [DOI] [PubMed] [Google Scholar]
- SCOW R. O., CHERNICK S. S., BRINLEY M. S. HYPERLIPEMIA AND KETOSIS IN THE PREGNANT RAT. Am J Physiol. 1964 Apr;206:796–804. doi: 10.1152/ajplegacy.1964.206.4.796. [DOI] [PubMed] [Google Scholar]
- Samaan N., Yen S. C., Friesen H., Pearson O. H. Serum placental lactogen levels during pregnancy and in trophoblastic disease. J Clin Endocrinol Metab. 1966 Dec;26(12):1303–1308. doi: 10.1210/jcem-26-12-1303. [DOI] [PubMed] [Google Scholar]
- Turtle J. R., Kipnis D. M. The lipolytic action of human placental lactogen on isolated fat cells. Biochim Biophys Acta. 1967 Dec 5;144(3):583–593. doi: 10.1016/0005-2760(67)90047-1. [DOI] [PubMed] [Google Scholar]
- VAUGHAN M. Effect of hormones on glucose metabolism in adipose tissue. J Biol Chem. 1961 Aug;236:2196–2199. [PubMed] [Google Scholar]
- VAUGHAN M., STEINBERG D. EFFECT OF HORMONES ON LIPOLYSIS AND ESTERIFICATION OF FREE FATTY ACIDS DURING INCUBATION OF ADIPOSE TISSUE IN VITRO. J Lipid Res. 1963 Apr;4:193–199. [PubMed] [Google Scholar]
- VAUGHAN M. The production and release of glycerol by adipose tissue incubated in vitro. J Biol Chem. 1962 Nov;237:3354–3358. [PubMed] [Google Scholar]
- WINEGRAD A. I., RENOLD A. E. Studies on rat adipose tissue in vitro. I. Effects of insulin on the metabolism of glucose, pyruvate, and acetate. J Biol Chem. 1958 Aug;233(2):267–272. [PubMed] [Google Scholar]


