Abstract
A highly facile, straightforward synthesis of 1-(3-indolyl)-tetrahydroisoquinolines was developed using either simple copper or iron catalysts. N-protected and unprotected tetrahydroisoquinolines (THIQ) could be used as starting materials. Extension of the substrate scope of the pronucleophile from indoles to pyrroles and electron-rich arenes was realized. Additionally, methoxyphenylation is not limited to THIQ but can be carried out on isochroman as well, again employing iron and copper catalysis.
Introduction
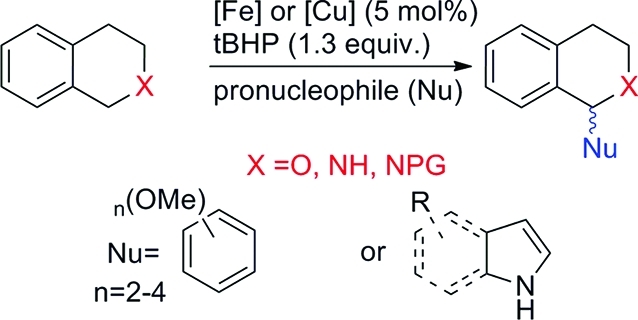
Functionalized, nitrogen-containing heterocycles constitute a widespread structural motif in biologically active compounds(1) and an invaluable template for chiral auxiliaries(2) in asymmetric synthesis.(3) In particular, tetrahydroisoquinolines (THIQs) and indoles are common structural motifs in important compounds such as natural products(4) as well as pharmaceuticals(5) and display significant antitumor activities.(6) 1-Arylated THIQs have been demonstrated to have interesting pharmacological properties,(7) including anti-HIV(8) and neuroprotective activity.(9) Due to the broad application possibilities of these compounds, methods for their ever more efficient preparation are continuously sought after. In this respect, transition-metal-catalyzed cross-coupling reactions of various reactive functional groups have been developed as powerful methods for constructing C–C bonds.(10) However, it would be even more desirable to use C–H bonds directly for C–C bond formation. Such transformations are usually termed C–H activation reactions.(11) In the case of sp3 C–H bonds, it was found that C–H bonds adjacent to a nitrogen could be efficiently activated, and several protocols were reported.(12) Such reactions display much better atom efficiency in comparison to traditional cross-coupling reactions, since prefunctionalization of building blocks for later C–C bond formation can be avoided.3b,13 This also helps to make synthetic schemes shorter and more efficient. However, it would be even more efficient to use the C–H bonds of two substrates to be coupled directly with each other for C–C bond formation. This was realized in cross dehydrogenative coupling (CDC) reactions, which are defined as the cross-coupling of two different C–H bonds of pronucleophiles and proelectrophiles.(14) Using THIQ as substrate it was reported that N-arylated THIQs can be coupled to a series of nucleophiles, such as NaCN, nitroalkanes, terminal alkynes, malonates, malononitriles, pyrroles, 2-naphthol, and phenylboronic acids, as well as Morita–Baylis–Hillman (MBH) adducts. Such reactions have been reported by Murahashi et al.,(16) Li et al.,(17) and others.(18)
One inspiration for the research published within this contribution was a recent report by the group of Li on the CDC between functionalized indoles and position 1 of N-arylated THIQs. The presence of tert-butyl hydroperoxide (tBHP as a solution in decane) as oxidant proved to be necessary, but no additional solvent was needed using copper(I) bromide as catalyst.(19) However, there is one significant limitation of this protocol, the removal of the N-phenyl group, which would allow further functionalization of the THIQ on its amine group. Although the removal of phenyl from amines was reported, it required conditions which are only tolerated by a small set of organic compounds (100 equiv of Li/NH3/THF/–40 °C, 3 h).(20) Alternatively, the authors used the p-methoxyphenyl group (PMP), which should be removable in principle; however, using standard deprotection conditions(21) we were unable to deprotect the indolated THIQs. Using a generally removable protecting group (PG) instead would open the possibility to perform further transformations after the initial C–C bond forming step. This would be highly desirable if a cheap and readily available catalyst could be used for the CDC reaction. Copper catalysts are highly attractive, since they are significantly cheaper and environmentally more benign compared to noble metal catalysts such as Pd, Ru, and Rh. An early example of a C–C bond forming process using Cu catalysts was the Ullmann coupling reaction.(22) In recent years copper catalysis has regained much attention and several interesting contributions have been reported.(23) An even more attractive metal for catalytic systems would be iron, since it is environmentally and economically superior to copper and there are already many literature reports where iron catalysts show promising properties in many synthetically valuable transformations.17e,24 Also, our group contributed to this field by disclosing an improved protocol for the direct indolation and methoxyarylation of protected THIQs and isochroman by employing cheap iron salts as catalysts.(25) Even more importantly, removable protecting groups such as the Boc group could be used instead of phenyl or PMP, and these were easily cleaved under very mild reaction conditions in most cases in quantitative yield.
Within this contribution, we report a method for the indolation, pyrrolation, and methoxyphenylation of THIQs and isochroman under copper catalysis in the absence of any protecting group on THIQ. Additionally, iron- and copper-catalyzed protocols for the transformations of protected THIQs and isochroman were investigated. Advantages and limitations of the catalytic systems will be discussed in detail.
Results and Discussion
Initially, various protected THIQs were synthesized according to literature protocols5b,25 to identify protected THIQs suitable for the proposed transformations. They were then submitted to standard indolation conditions(19) using copper(I) bromide as catalyst and tBHP as oxidizing agent at 50 °C. Copper(I) bromide was chosen, since indolation of N-phenyl-THIQ was reported in the presence of this catalyst (Table 1).
Table 1. Optimization of the Catalyst System and Choice of PG.
| entry | R | cat. | oxidant | yield (%) |
|---|---|---|---|---|
| 1 | Ac (1a) | Cu(NO3)2c | tBHP | 54 |
| 2 | Ac (1a) | CuBr | tBHP | 47 |
| 3 | Piv (1b) | Cu(NO3)2c | tBHP | 26 |
| 4 | Piv (1b) | CuBr | tBHP | 21 |
| 5 | Bn (1c) | Cu(NO3)2c | tBHP | 60 |
| 6 | Bn (1c) | CuBr | tBHP | 36 |
| 7 | Bz (1d) | Cu(NO3)2c | tBHP | 40 |
| 8 | Bz (1d) | CuBr | tBHP | 10 |
| 9 | CBz (1e) | Cu(NO3)2c | tBHP | 60 |
| 10 | CBz (1e) | CuBr | tBHP | 41 |
| 11 | Tos (1f) | Cu(NO3)2c | tBHP | traces |
| 12 | Tos (1f) | CuBr | tBHP | traces |
| 13 | 2-Py (1g) | Cu(NO3)2c | tBHP | 61 |
| 14 | 2-Py (1g) | CuBr | tBHP | 44 |
| 15 | Boc (1h) | Cu(NO3)2c | tBHP | 79 |
| 16 | Boc (1h) | CuBr | tBHP | 72 |
| 17 | Boc (1h) | CuCN | tBHP | (65) |
| 18 | Boc (1h) | Cu(OAc)2 | tBHP | (74) |
| 19 | Boc (1h) | CuI | tBHP | (61) |
| 20 | Boc (1h) | CuBr | tBHP | (73) |
| 21 | Boc (1h) | CuCl | tBHP | (74) |
| 22 | Boc (1h) | CuF2 | tBHP | (76) |
| 23 | Boc (1h) | CuCl | nca | |
| 24 | Boc (1h) | CuCl2 | nca | |
| 25 | Boc (1h) | Cu(NO3)2c | H2O2 (30%) | tracesb |
| 26 | Boc (1h) | Cu(NO3)2c | mCPBA | tracesb |
| 27 | Boc (1h) | Cu(NO3)2c | DBPO | tracesb |
| 28 | Boc (1h) | tBHP | nc |
nc = no conversion.
1 equiv of catalyst was employed.
Monitoring according to GC/MS, conversion in parentheses according to HPLC.
Cu(NO3)2·3H2O employed.
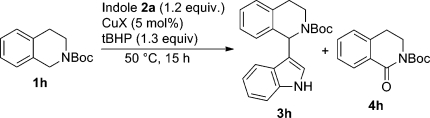
The starting material carrying the Boc protecting group (1h) afforded the best product yield (79%, entry 15), followed by Ac-protected 1a (47%, entry 2), CBz-protected 1e (41%, entry 10), and benzyl-protected 1c (Bn, 36%, entry 6). The benzoyl-protected substrate 1d (Bz, 10%, entry 8) was less efficient. In the case of Piv-protected 1b (21%, entry 2) the significantly lower yield can be attributed to steric hindrance. Heterocyclic substituents such as 2-pyridinyl (1g) gave the indolated product in 44% yield (entry 14); however, this group requires a two-step protocol for cleavage.12a,27 Only the tosyl PG (substrate 1f) seemed to be ineffective in this transformation (entry 16). In summary, the copper-catalyzed indolation is not limited to protective groups carrying a carbonyl function as was the case for our previously disclosed iron-catalyzed protocol.(25) Since the Boc group (substrate 1h) turned out to be the most suitable PG using copper(I) bromide as catalyst, this substrate was selected for a subsequent round of parameter optimizations, starting with the catalyst species. Interestingly, copper(II) nitrate (entry 11) showed the best conversion (82%), closely followed by copper(II) acetate (entry 18) and copper halide salts (entries 19–22). In the case of copper(I) iodide (entry 19) significant byproduct formation (oxidized THIQ 4h and other unidentified byproduct, Scheme 1) could be monitored by HPLC, resulting in a decrease in conversion to the desired product, whereas cleaner conversions were achieved when employing copper(I) chloride (74%, entry 21) or copper(II) fluoride (76%, entry 22). Such a benzylic oxidation was already reported in the literature under related conditions (82 °C, excess tBHP, iron(III) chloride hexahydrate).(28) Double substitution at the C3 and N1 positions of the indole with 1h was not observed, as was reported in a similar CDC reaction of N,N-dimethylanilines with indoles.(29)
Scheme 1. Copper-Catalyzed Indolation and Benzylic Oxidation of Boc-NTHIQ.
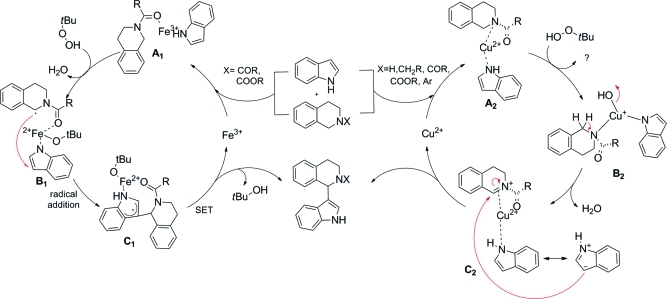
To confirm that copper(II) nitrate is the catalyst of choice in combination with Boc as the best PG, the other protected THIQs were also submitted to the indolation process using copper(II) nitrate as catalyst. Generally, all yields obtained with copper(II) nitrate were slightly higher in comparison to the corresponding transformation catalyzed by copper(I) bromide, and again the best yield of the desired product (79%, entry 15) was obtained using 1h as starting material. CBz (1e, 60%, entry 10), Bn (1c, 60%, entry 5), and 2-pyridinyl (1g, 61%, entry 13) also performed well. With Bz as the PG (1d), the yield could be significantly increased from 10% (entry 8) to 40% (entry 7). Again, Tos-protected starting material 1f was not accepted in this transformation (entry 11). Finally, the role of the oxidant was examined: tBHP without catalyst (entry 28) or stoichiometric amounts of copper in absence of an oxidant failed to perform the reaction, independent of the oxidation state of the copper salt (entries 23 and 24). Hence, it seems that the oxidizing agent is not only required to reoxidize the copper salt from copper(I) to copper(II) in the catalytic cycle. However, this additional role of the oxidizing agent has not yet been elucidated. This result supports the proposed mechanism of Li et al., which suggests that a peroxide is required as oxidant to convert copper to the oxy-copper species B2, which then coordinates to the nitrogen of THIQ and forms the iminium-type intermediate C2 by elimination of water, which is then attacked by a nucleophile to form the desired product (Scheme 2).(30) In contrast to the iron-catalyzed transformation, a carbonyl group adjacent to the nitrogen of the THIQ is not mandatory for the copper-catalyzed reaction, indicating a complexation of the copper with the nitrogen to form species A2 rather than with the oxygen of the carbonyl group. In the copper(II) nitrate catalyzed transformation addition of TEMPO, a radical scavenger, did not result in a decrease in product formation, in contrast to our results when employing iron salts as catalysts.(25) This indicates that the product is formed by an ionic mechanism rather than through a radical pathway. Other oxidants such as hydrogen peroxide (entry 25) and mCPBA (entry 26) only showed traces of the desired product. Thus, tBHP seems to be mandatory as the oxidant in this reaction. In Scheme 2 the proposed mechanism for the iron- and copper catalyzed transformations are displayed.
Scheme 2. A Comparison: Tentative Mechanisms of the Iron/Copper-Catalyzed Indolation of (N-PG)THIQ.
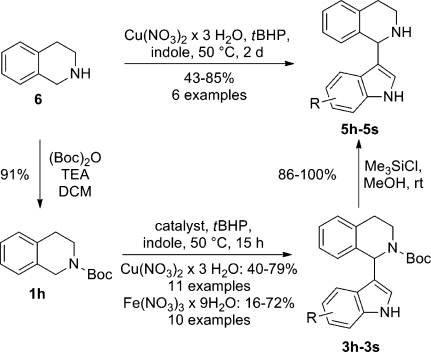
After we established optimized reaction conditions, a set of various functionalized indoles was reacted with N-Boc THIQ. The results of the copper-catalyzed variant are compared with the results of our previously disclosed iron-catalyzed protocol in Table 2.
Table 2. Indolation of Boc-THIQ 1h.
| yield of indolation (%) |
deprotection |
|||||||
|---|---|---|---|---|---|---|---|---|
| entry | R | X/Y | product | Fea | Cub | entry | product | yield (%)e |
| 1 | H | CH/CH | 3h | 54 | 79 | 13 | 5h | 100c |
| 89d | ||||||||
| 2 | 1-CH3 | CH/CH | 3i | 65 | 70 | 14 | 5i | 100c |
| 97d | ||||||||
| 3 | 2-CH3 | CH/CH | 3j | 23 | 67 | 15 | 5j | 100c |
| 4 | 5-NH2 | CH/C | 3k | 16 | nc | 16 | np | |
| 5 | 5-OCH3 | CH/C | 3l | 43 | 52 | 17 | 5l | 99c |
| 6 | 5-NO2 | CH/C | 3m | 66 | 59 | 18 | 5m | 100c |
| 7 | 5-COOMe | CH/C | 3n | 5 | 50 | 19 | np | |
| 8 | 5-Cl | CH/C | 3o | 72 | 66 | 20 | 5o | 100c |
| 9 | 6-Cl | CH/CH | 3p | 56 | 75 | 21 | 5p | 86c |
| 10 | 7-NO2 | C/CH | 3q | 70 | 46 | 22 | 5q | 99c |
| 11 | H | N/CH | 3r | 42 | 44 | 23 | 5r | 100c |
| 12 | 6-Cl | N/N | 3s | nc | 40 | 24 | np | |
Fe(NO3)3·9H2O.
Cu(NO3)2·3H2O.
Conditions: TMSCl, MeOH, room temperature.
Conditions: ethylene glycol, 250 °C, microwave, 30 s.
yYield after deprotection of the Boc PG; np = not performed.
It was found that, depending on the indole derivative applied, either iron or copper gave better results, whereas the latter metal was favored in most cases. Simple indole (Table 2, entry 1) gave good yields in both the copper- and iron-catalyzed processes (3h, 79% and 54% respectively). N-Methylindole (Table 2, entry 2) was also well tolerated by both catalysts; however, copper performed slightly better than the iron catalyst (3i, 70% vs 65% yield). A significant difference in substrate conversion was observed when employing 2-methylindole: in the copper-catalyzed reaction the desired product 3j was obtained in 67% yield (Table 2, entry 3). This good yield is especially remarkable, since it shows that sterically hindered indoles can also be applied in this transformation; this was not the case for the iron catalyst, where only 23% of 3j could be obtained. This result is in line with our proposed mechanism for the iron-catalyzed indolation process, where the iron species coordinates to the carbonyl group of the Boc PG as well as to the nitrogen of the indole.(25) Thus, complexation might be hindered due to the methyl group at position C-2 of indole, resulting in a decreased yield. On the other hand, a free amine functionality (Table 2, entry 4) was not tolerated at all in copper catalysis and only 16% of 3k could be isolated when using iron(III) nitrate as catalyst. In copper catalysis both electron-withdrawing (3m–o) and electron-donating (3l) substituents are well tolerated at position C-5 of indole. Substituents at positions 6 (Table 2, entry 9) and 7 (Table 2, entry 10) were also tolerated (3p,q). The picture completely changes when looking at the iron-catalyzed yields: with 5-nitro (Table 2, entry 6), 5-chloro (Table 2, entry 8), and 7-nitro substituents (Table 2, entry 10) even better yields were obtained in comparison to the case for the copper-catalyzed reactions (3m, 66% vs 59%; 3o, 72% vs 66%; 3q, 70% vs 46%). In these cases, the oxidized N-Boc THIQ byproduct 4h that always formed was formed to a lesser extent compared to the case for the copper-catalyzed protocol. Since iron has a high affinity toward oxygen,(31) it was not surprising that the 5-methoxy-substituted product 3l was obtained in a notably lower yield (Table 2, entry 5), and the ester substituent (Table 2, entry 7) was even less tolerated as a functional group (3n). In addition to indole, also other ring systems could be applied as coupling partners, such as 7-azaindole (3r, 42% and 44%) and 6-chlorodeazapurine (Table 2, entry 12). In the case of 7-azaindole (Table 2, entry 11) comparably mediocre yields were achieved with copper as well as with iron catalysis. Using copper(II) nitrate as catalyst 40% of the desired product derived from 6-chlorodeazapurine 3s was isolated. On the other hand, the desired product was only observed in traces on TLC when using iron(III) nitrate. Benzo[b]furan, benzo[b]thiophene, and benzimidazole gave no conversion.
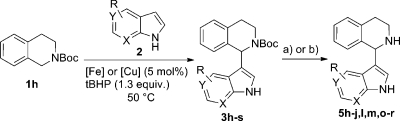
Having established two protocols for the indolation of N-Boc-THIQ, we finally attempted to cleave the Boc protecting group in order to obtain the free NH 1-indolated NH-THIQs. Initially, standard acidic deprotection conditions(32) were used however decomposition of the starting material 3h was observed. Only in refluxing AcOH was a mediocre yield of 40% of 5h obtained, in addition to decomposition products. In the search for milder conditions we found that using TMSCl in MeOH at room temperature gave excellent results (Table 2).(33) In most cases quantitative yields of the deprotected products were obtained, independent of the nature of the substituents on the indole core. The deprotection in the presence of 6-Cl-indole toward compound 5p gave the lowest but still satisfactory yield of 86% (Table 2, entry 21). A second alternative thermal deprotection of the Boc group under microwave irradiation(34) would be an operationally simple alternative strategy to classical deprotection. It was found that starting materials 3h,i could be deprotected in excellent yield to afford 5h (89%, Table 2, entry 13) and 5i (97%, Table 2, entry 14) on heating to 250 °C under microwave irradiation. This method proved to be very time efficient, since 30 s of hold time was sufficient for complete deprotection. Unfortunately, in other cases decomposition of the starting materials was observed and the protocol was hence not generally applicable.
Overall, a three-step procedure for the synthesis of 1-indolated THIQs was developed which delivers high yields due to the high-yielding introduction and cleavage of a versatile protecting group. However, CDC of unprotected THIQ and substituted indoles would represent an even more atom efficient transformation. In such a process, two reaction steps (installation and cleavage of the protecting group) could be circumvented, which also saves time and material resources otherwise needed for purification of the intermediate products. To our delight, indolation of THIQ 6 toward products 5 could be achieved in good yields (Table 3) even without N-protection using copper(II) nitrate as catalyst. When standard conditions were applied for indolation from the above series of experiments on Boc-protected precursors, THIQ (1.0 equiv of indole, 0.8 equiv of THIQ) could be indolated in approximately 50% yield, independent of the functional group of the indole (Table 3, entries 4, 7, 9, 11, 14, 16, and 17). 3,4-Dihydroisoquinoline (DHIQ) was also formed as a byproduct via oxidative dehydrogenation to approximately the same extent as the desired products.(35) Changing the ratio of substrates to 2 equiv of THIQ and 1.0 equiv of indole resulted in an increased yield of the desired product 5h of 74% (Table 3, entry 3). A further improved yield of 5h of 85% (Table 3, entry 2) was achieved when 4 equiv of 6 was employed. These results indicate that DHIQ byproduct formation occurs at approximately the same reaction rate as the desired product formation. An even higher excess of THIQ (8 equiv) did not improve the yield further (Table 3, entry 1). Also in other cases an excess of 2 equiv of THIQ (Table 3, entries 10, 12, and 18) gave better yields. It has to be pointed out, that 1-methyl- (Table 3, entry 6), 3-methyl- (Table 3, entry 8), and 7-nitroindoles (Table 3, entry 15) are ineffective substrates in this particular transformation. The failure of 1-methylindole might indicate that a free indole NH group is mandatory for complexation with the copper catalyst and that this step is important for initiating the reaction. In the case of 3-methylindole, the C2 position of the indole is probably not sufficiently electron rich to undergo this type of transformation (Table 3, entry 8). On the other hand, 7-nitroindole competes with the free NH group for complexation with the copper-species, and therefore no desired product is formed.(36)
Table 3. Copper-Catalyzed Indolation of THIQ.
| entry | R | X | amt of THIQ (equiv) | yield (%) |
|---|---|---|---|---|
| 1 (5h) | H | CH | 8.0 | 84 |
| 2 (5h) | H | CH | 4.0 | 85 |
| 3 (5h) | H | CH | 2.0 | 74 |
| 4 (5h) | H | CH | 0.8 | 48 |
| 5 (5h) | H | CH | 0.4 | 53 |
| 6 (5i) | 1-Me | CH | 0.8 | 1 |
| 7 (5j) | 2-Me | CH | 0.8 | 43 |
| 8 | 3-Me | CH | 0.8 | ncb |
| 9 (5l) | 5-OMe | CH | 0.8 | 48 |
| 10 (5l) | 5-OMe | CH | 2.0 | 71 |
| 11 (5m) | 5-NO2 | CH | 0.8 | 53a |
| 12 (5m) | 5-NO2 | CH | 2.0 | 62a |
| 13 (5n) | 5-COOMe | CH | 2.0 | 58 |
| 14 (5p) | 6-Cl | CH | 0.8 | 46 |
| 15 (5q) | 7-NO2 | CH | 0.8 | 2 |
| 16 (5r) | N | 0.8 | 43 | |
| 17 (5t) | 7-Me | CH | 0.8 | 48 |
| 18 (5t) | 7-Me | CH | 2.0 | 68 |
Reaction time: 2 days.
nc = no conversion.
Also, iron catalysis was investigated for this transformation but did not give any indolated product. Thus, protection of nitrogen with a carbonyl PG is mandatory for this metal, since only THIQs with a PG containing a carbonyl group showed successful indolation, due to coordination of the iron species with the oxygen of the carbonyl group.(25)
On comparison of the two outlined methods, reaction of the unprotected THIQ is superior with regard to atom efficiency, even though an excess of THIQ is required in order to obtain high yields (Scheme 3). The same is true regarding time efficiency, since two reaction steps can be avoided. Still, the iron- and copper-catalyzed protocols can be useful alternatives in cases where the free NH of THIQ is not tolerated. Two such examples were encountered: i.e., 1-methyl- (Table 2, entry 2) and 7-nitroindole (Table 2, entry 10). In these cases the protocol employing Fe(NO3)3 as catalyst gave 57% and 63% yields over three steps and the Cu(NO3)2-catalyzed method gave 63% and 41% yields, respectively, again over three steps. Generally, when the overall yield over three steps is compared to the yield of the direct protocol, higher yields are obtained in the direct method when 2 equiv of THIQ is applied. When only 1 equiv of this substrate is used, the three-step protocol including protection and deprotection compares favorably. Hence, it can be concluded that all three protocols (copper-catalyzed indolation of unprotected THIQ, copper-catalyzed indolation of N-protected THIQ, and iron-catalyzed indolation of N-protected THIQ) can be of value, depending on the synthetic problem. The protection–deprotection pathway might be of special value when more elaborate and, hence, more expensive THIQ derivatives are used as substrates.
Scheme 3. Indolation of Unprotected THIQ vs Protection–Deprotection Pathway.
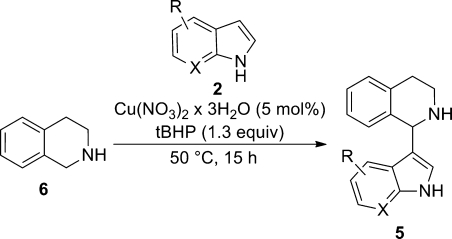
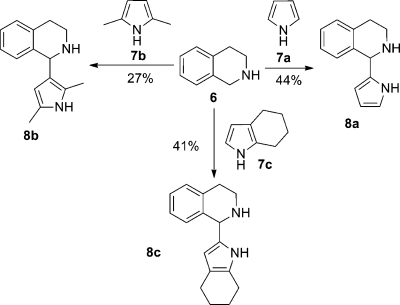
After we established the principal reactivity on indole, it was further investigated whether pyrroles can also undergo a CDC with THIQ. Thus, pyrrole 7a was reacted with 2 equiv of THIQ 6, which afforded the desired product 8a in 44% yield (Scheme 4). The new carbon–carbon bond was formed in this case between C1 of THIQ and C2 of pyrrole, so again the more electron rich position of the pronucleophile reacts.
Scheme 4. Copper-Catalyzed Pyrrolation of THIQ.
Conditions: Cu(NO3)2·3H2O (5 mol %), tBHP (1.3 equiv), 50 °C, 15 h.
The yield dropped dramatically when the pyrrolation was performed on Boc-protected 1h: only traces of the desired product 8d were observed employing copper catalysis and only 11% of the desired product could be isolated in the iron-catalyzed reaction (see the Supporting Information).
Thus, the scope of the pyrrolation was investigated on 6: also 4,5,6,7-tetrahydroindole gave the desired product in 41% yield. Electron-poor pyrroles such as 2-acetylpyrrole did not show any conversion according to GC/MS. Notably, also 1-methylpyrrole was ineffective, which is in line with results obtained for 1-methylindole (Table 3, entry 6); we understand this as further evidence of the importance of a free NH group in the pronucleophile. Interestingly, the reaction was also successful when using 2,5-dimethylpyrrole 7b as substrate, this time leading to C–C bond formation at C3 of pyrrole, affording product 8b. However, the yield dropped to 27% (Scheme 4).
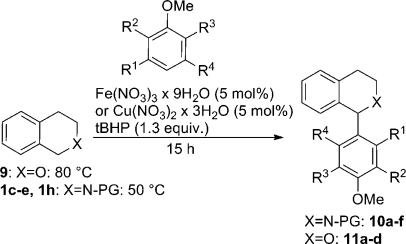
On the basis of the above results, we became interested in extending the substrate scope of the transformation to other coupling partners in addition to (aza)indoles and pyrroles. Since the pronucleophile needs to be electron rich, high electron density arenes were submitted to the reaction conditions with THIQs. Gratifyingly, Boc-protected 1h (Table 4, entry 1), Bz-protected 1d (Table 4, entry 2), and CBz-protected 1e (Table 4, entry 3) were found to be suitable substrates for methoxyarylation with 1,3,5-trimethoxybenzene. In marked contrast to the indolation reactions, unprotected THIQ 6, Bn-protected 1c (Table 4, entry 4) and PMP-protected THIQ (Table 4, entry 5) were inefficient under both copper and iron catalysis.
Table 4. Scope of Methoxyphenylation on N-PG THIQ and Isochroman.
| yield (%) |
|||||||
|---|---|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | R4 | X | Fe | Cu |
| 1 (10a) | OMe | H | H | OMe | NBoc | 81 | 76 |
| 2 (10b) | OMe | H | H | OMe | NBz | 54a | 46a |
| 3 (10c) | OMe | H | H | OMe | NCBz | 58a | 51a |
| 4 | OMe | H | H | OMe | NBn | ncb | nc |
| 5 | OMe | H | H | OMe | NPMP | nc | nc |
| 6 | OMe | H | H | OMe | NH | nc | nc |
| 7 (10d) | OMe | H | OMe | H | NBoc | 47 | 23 |
| 8 (10e) | OMe | OMe | H | OMe | NBoc | 52 | 46 |
| 9 (10f) | H | H | H | OMe | NBoc | 34a | 41 |
| 10 (11a) | OMe | H | H | OMe | O | 55 | 51 |
| 11 (11b) | OMe | OMe | H | OMe | O | 17 | 39 |
| 12 (11c) | H | H | H | OMe | O | 12a | 23 |
| 13 (11d) | OMe | H | OMe | H | O | 15 | 32 |
36 h of reaction time.
nc = no conversion according to GC/MS.
The best yield in this methoxyarylation reaction was obtained using 1,3,5-trimethoxybenzene as the most electron rich aryl coupling partner and Boc-protected 1h as substrate, where a good yield of 81% in iron catalysis and 76% (Table 4, entry 1) in copper catalysis was obtained. Decreasing the electron density on the aryl coupling partner as in 1,3-dimethoxybenzene also showed product formation of 10f but, as could be expected, with a lower yield of 34% (copper) and 41% (iron) (Table 4, entry 9). Also, a less favorable arrangement of three methoxy groups led to decreased product yield: 1,2,4-trimethoxybenzene led to 47% (Table 4, entry 7, Fe) and 23% (Table 4, entry 7, Cu) 10d, respectively. It has to be noted that only arylation product deriving from a reaction at position 5 of 1,2,4-trimethoxybenzene was observed. Principally, position 3 would be activated to a similar extent but is sterically less favored. The presence of four methoxy groups in the aryl coupling partner gave, especially in the Cu-catalyzed protocol, again a higher yield (10e, Table 4, entry 8). Generally, the Fe-catalyzed variant performed better in most cases.
Next, we investigated possible extensions to other proelectrophiles; therefore, isochroman 9 was tested as the starting material as well (Table 4, entries 10–13). Attempts to use 9 as starting material for indolations unfortunately failed, leading rather to bis-indolation via C–H bond oxidation and C–O cleavage, which is in line with recent reports by Li et al.(37) However, 1,3,5-trimethoxybenzene also reacted with 9 as coupling partner to afford the desired product 11a in 55% and 51% yields, respectively (Table 4, entry 10). This confirms that this arylation reaction is not limited to THIQ but also isochroman can be used as substrate; however, lower yields were obtained for both metal catalysts in comparison to the case for THIQ substrates. Still, this is an interesting extension to existing protocols, since it demonstrates that the transformation is limited neither to THIQ starting materials nor to indole or pyrrole coupling partners, consequently broadening the substrate scope considerably. Comparable less electron rich 1,3-dimethoxybenzene also showed product formation, but again with lower yields of 12% (11c, entry 12, Fe) and 23% (11c, entry 12, Cu). With regard to 1,2,4-trimethoxybenzene (11d, entry 13) and 1,2,3,5-tetramethoxybenzene (11b, entry 11) the same trends in yield were observed as for the corresponding reactions on THIQ 1h. For both starting materials 1h and 9 anisole is no longer sufficiently activated and no arylated product was formed.
Conclusion
In summary, an alternative copper- and iron-catalyzed method for the indolation, pyrrolation, and methoxyphenylation of THIQs and for the methoxyphenylation of isochroman was developed. Most importantly, cleavable N-protecting groups can be applied using the outlined protocols, which represents a significant extension of this methodology in comparison to previous reports. The Boc group can be removed under mild conditions with TMSCl or within seconds in excellent yield using high-temperature microwave conditions. It could be demonstrated that unprotected THIQ could be directly functionalized with indoles and pyrroles in a copper-catalyzed reaction. This emphasizes that copper and iron catalyses often complement each other.
Experimental Section
General notes and instrumentation are provided in the Supporting Information. Protected THIQs and 4,5,6,7-tetrahydro-1H-indole were synthesized according to literature procedures.(26)
General Procedure for Indolation of N-Protected THIQ
A mixture of protected THIQ (0.857 mmol, 1.0 equiv), catalyst (Cu(NO3)2·3H2O, 10.4 mg; Fe(NO3)3·9H2O, 17.3 mg; 42.9 μmol, 0.05 equiv), and indole (1.03 mmol, 1.2 equiv) was placed into a 5 mL glass vial in air. tBHP (203 μL, 5.5 M in decane, 1.3 equiv) was added dropwise at 0 °C in air, and the dark greenish slurry was stirred for 10 min at 0 °C. The neat mixture was then slowly heated to 50 °C, whereupon the color changed to dark brown, and stirring was continued for 15 h in a heating block. The reaction was monitored by TLC and/or GC-MS. The dark brown slurry was diluted with DCM (3 mL) and directly subjected to flash column chromatography (100 g of SiO2), applying the solvent mixture used for TLC.
1-[1-(1H-Indol-3-yl)-3,4-dihydroisoquinolin-2(1H)-yl]ethanone (3a):
yield for Cu 134 mg (54%) and for Fe traces; pale yellow solid; mp 208–211 °C; TLC Rf (PE/EtOAc 1/1) = 0.18; GC/MS (EI+) m/z (rel intensity) 290 (M+, 50), 273 (14), 248 (18), 247 (100), 232 (29), 230 (14), 218 (28), 217 (28), 132 (16), 131 (24), 130 (50), 117 (25), 115 (27), 103 (21), 89 (12), 77 (12); 1H NMR (200 MHz, DMSO- d6) mixture of rotamers 1:0.17, δ 2.10 (s, 3H), 2.25 (s, 0.51H), 2.74–2.90 (m, 1.30H), 3.02 (ddd, 2J = 17.2 Hz, 3J = 11.6 Hz, 3J = 5.8 Hz, 1H), 3.24–3.45 (m, 1.30H), 3.77 (dd, 2J = 13.7 Hz, 3J = 5.4 Hz, 1H), 4.26 (bs, 0.16H), 6.40 (bs, 0.17H), 6.58 (d, 3J = 2.0 Hz, 1H), 6.89–7.27 (m, 8H), 7.34 (d, 3J = 7.9 Hz, 1.35H), 7.57 (d, 3J = 7.8 Hz, 1H), 10.93 (s, 1H), 11.10 (s, 0.17H); 13C NMR (50 MHz, DMSO-d6): mixture of rotamers 1:0.17, δ 21.4 (q), 28.4 (t), 39.1 (t, overlap with DMSO signal), 47.9 (d), 111.4 (d), 117.1 (s), 118.7 (d), 119.2 (d), 121.3 (d), 125.4 (d), 125.7 (d), 126.1 (s), 126.5 (d), 128.2 (d), 128.8 (d), 134.4 (s), 136.2 (s), 136.3 (s), 167.7 (s).
2,2-Dimethyl-1-(1-(1H-indol-3-yl)-3,4-dihydroisoquinolin-2(1H)-yl)-propan-1-one (3b):
yield for Cu 73 mg (26%) and for Fe traces; off white powder; mp 184–186 °C; TLC Rf (PE/EtOAc 2/1) = 0.27; GC/MS (EI+) m/z (rel intensity) 332 (M+, 27), 248 (19), 247 (100), 232 (35), 231 (14), 218 (25), 217 (23), 144 (11), 132 (26), 130 (28), 117 (10), 115 (17), 57 (24); 1H NMR (200 MHz, CDCl3) δ 1.24 (s, 9H), 2.73 (ddd, 2J = 16.4 Hz, 3J = 4.1 Hz, 3J = 0.9 Hz, 1H), 3.04 (ddd, 2J = 17.0 Hz, 3J = 12.4 Hz, 3J = 6.1 Hz, 1H), 3.44 (ddd, 2J = 13.9 Hz, 3J = 12.6 Hz, 3J = 4.2 Hz, 1H), 4.05 (ddd, 2J = 13.6 Hz, 3J = 6.2 Hz, 3J = 1.4 Hz, 1H), 6.56 (d, 4J = 2.3 Hz, 1H), 6.97 (ddd, 3J = 8.0 Hz, 3J = 7.1 Hz, 4J = 1.1 Hz, 1H), 7.04–7.16 (m, 5H), 7.16–7.21 (m, 1H), 7.25 (d, 3J = 8.0 Hz, 1H), 7.50 (d, 3J = 7.9 Hz, 1H), 8.06 (s, 1H); 13C NMR (50 MHz, CDCl3) δ 28.4 (3C, q), 29.2 (t), 39.1 (s), 39.6 (t), 50.4 (d), 110.9 (d), 119.1 (s), 119.8 (d), 120.4 (d), 122.2 (d), 125.6 (d), 125.9 (d), 126.4 (s), 126.6 (d), 128.6 (d), 128.7 (d), 133.7 (s), 136.4 (s), 136.5 (s), 175.7 (s).
2-Phenylmethyl-1-(1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (3c):
yield for Cu 181 mg (60%) and for Fe 20 mg (7%); pale yellow solid; mp 55–60 °C; TLC Rf (PE/Et2O 1/1) = 0.45; GC/MS (EI+) m/z (rel intensity) 338 (M+, 75), 337 (56), 247 (32), 245 (16), 231 (22), 219 (50), 218 (100), 217 (35), 130 (11), 106 (15), 91 (31); HRMS (ESI+): exact mass calculated for C24H22N2 339.1856, found: 339.1852 [M + H]+; 1H NMR (200 MHz, DMSO-d6) δ 2.32–2.47 (m, 1H), 2.70–2.88 (m, 1H), 2.92–3.12 (m, 2H), 3.26 (d, 2J = 13.7 Hz, 1H), 3.81 (d, 2J = 13.7 Hz, 1H), 4.88 (s, 1H), 6.75–7.39 (m, 13H), 7.45 (d, 3J = 7.8 Hz, 1H), 10.97 (s, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 28.8 (t), 47.1 (t), 58.2 (t), 61.2 (d), 111.4 (d), 116.4 (s), 118.2 (d), 120.2 (d), 120.9 (d), 125.3 (d), 125.49 (d), 125.53 (d), 125.9 (s), 126.5 (d), 127.9 (d), 128.0 (d, 2C), 128.1 (d), 128.4 (d, 2C), 134.3 (s), 136.9 (s), 138.7 (s), 139.6 (s).
(1-(1H-Indol-3-yl)-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)-methanone (3d):
yield for Cu 120 mg (40%) and for Fe 67 mg (22%); brown solid; mp 226–228 °C; TLC Rf (PE/EtOAc 2/1) = 0.29; GC/MS (EI+) m/z (rel intensity) 352 (M+, 40), 248 (20), 247 (100), 232 (32), 218 (21), 217 (21), 130 (18), 115 (12), 105 (56), 77 (44); HRMS (ESI+) exact mass calculated for C24H20N2O 353.1648, found 353.1639 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 2.75 (d, 2J = 14.6 Hz, 1H), 2.97–3.11 (m, 1H), 3.47 (dt, 2J = 13.4 Hz, 3J = 3.3 Hz, 1H), 3.66 (dd, 2J = 13.2 Hz, 3J = 5.7 Hz, 1H), 6.68 (s, 1H), 7.13 (t, 3J = 7.3 Hz, 1H), 7.18–7.54 (m, 12H), 7.85 (d, 3J = 7.6 Hz, 1H), 8.34 (bs, 1H); 1H NMR (200 MHz, DMSO-d6) δ 2.75 (d, 2J = 16.0 Hz, 1H), 2.86–3.10 (m, 1H), 3.20–3.60 (m, 2H), 6.64 (bs, 1H), 6.89–7.51 (m, 13H), 7.65 (d, 3J = 7.9 Hz, 1H), 11.03 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 29.5 (t), 40.6 (t), 49.0 (d), 111.1 (d), 118.6 (s), 120.0 (d), 120.3 (d), 122.3 (d), 125.7 (d), 126.1 (d), 126.4 (2d), 126.9 (d), 127.3 (s), 128.5 (4C, d), 128.8 (d), 128.9 (d), 129.4 (d), 133.8 (s), 135.7 (s), 136.4 (s), 136.6 (s), 169.9 (s); 13C NMR (50 MHz, APT, DMSO-d6) δ 28.6 (t), (CH2 group at 40.6 ppm in CDCl3 overlaps with DMSO signal), 48.4 (d), 111.6 (d), 116.8 (s), 118.9 (d), 119.0 (d), 121.4 (d), 125.8 (d), 125.9 (s), 126.1 (d), 126.8 (d), 128.2 (d), 128.5 (4C, d), 128.9 (d), 129.3 (d), 134.0 (s), 135.6 (s), 136.4 (2C overlapping, s), 168.6 (s).
Phenylmethyl-1-(1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3e):
yield for Cu 171 mg (60%) and for Fe 135 mg (41%); white powder; mp 62–64 °C; TLC Rf (PE/EtOAc 5/1) = 0.55; GC/MS (EI+) m/z (rel intensity) 382 (M+, 3), 292 (7), 291 (40), 248 (20), 247 (100), 218 (8), 217 (7), 130 (12), 103 (5), 91 (6); HRMS (ESI+) exact mass calculated for C25H22N2O2 383.1754, found 383.1722 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 2.73 (dd, 1H, 2J = 16.7 Hz, 3J = 2.7 Hz, 1H), 2.90–3.36 (m, 2H), 3.80–4.30 (m, 1H), 5.10–5.46 (m, 2H), 6.48–6.84 (m, 2H), 6.88–7.82 (m, 13H), 8.19 (bs, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 28.6 (t), 37.4 (t), 51.5 (d), 67.1 (t), 111.0 (d), 118.5 (s), 119.8 (d), 120.2 (d), 122.2 (d), 125.0 (d), 125.8 (d, 2C), 126.4 (s), 126.7 (d), 127.7 (d), 128.0 (d), 128.4 (d), 128.5 (d, 2C), 129.0 (d), 134.6 (s), 136.0 (s), 136.3 (s), 136.7 (s), 154.9 (s).
1-(1H-Indol-3-yl)-2-(pyridin-2-yl)-1,2,3,4-tetrahydroisoquinoline (3g):
yield for Cu 171 mg (61%); pale yellow powder; mp 177–178 °C; TLC Rf (PE/EtOAc 2/1) = 0.51; GC/MS (EI+) m/z (rel intensity) 325 (M+ 40), 248 (19), 247 (100), 232 (24), 230 (15), 218 (24), 217 (27), 195 (12), 130 (17), 117 (14), 115 (15); HRMS (ESI+) exact mass calculated for C22H19N3 326.1652, found 326.1652 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 2.80 (dt, 2J = 16.1 Hz, 3J = 3.9 Hz, 1H), 3.12 (ddd, 2J = 16.3 Hz, 3J = 10.7 Hz, 3J = 5.6 Hz, 1H), 3.63 (ddd, 2J = 13.6 Hz, 3J = 10.7 Hz, 3J = 4.4 Hz, 1H), 3.98 (td, 2J = 13.6 Hz, 3J = 4.3 Hz, 1H), 6.58 (dd, 3J = 7.0 Hz, 3J = 5.0 Hz, 1H), 6.65 (d, 4J = 2.2 Hz, 1H), 6.76 (d, 3J = 8.6 Hz, 1H), 7.04 (t, 3J = 7.1 Hz, 1H), 7.10–7.23 (m, 5H), 7.27–7.35 (m, 2H), 7.47 (ddd, 3J = 8.8 Hz, 3J = 7.2 Hz, 4J = 2.0 Hz, 1H), 7.70 (d, 3J = 7.8 Hz, 1H), 8.06 (s, 1H), 8.28 (dd, 3J = 4.9 Hz, 3J = 1.9 Hz, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 26.9 (t), 38.9 (t), 52.2 (d), 106.5 (d), 111.0 (d), 112.0 (d), 119.2 (s), 119.5 (d), 120.1 (d), 122.0 (d), 124.4 (d), 125.6 (d), 126.5 (d), 126.6 (s), 128.3 (d), 128.8 (d), 135.4 (s), 136.5 (s), 137.4 (s), 137.5 (d), 148.1 (d), 157.9 (s).
1,1-Dimethylethyl-1-(1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3h):
yield for Cu 235 mg (79%) and for Fe 160 mg (54%); off white powder; mp 137–139 °C; TLC Rf (PE/Et2O 2/1) = 0.18; GC/MS (EI+) m/z (rel intensity) 248 (46), 247 (91), 245 (100), 230 (18), 218 (53), 130 (52), 121 (46), 117 (38), 108 (50), 103 (21); HRMS (ESI+) exact mass calculated for C22H24N2O2 371.1735. found 371.1730 [M + Na]+; 1H NMR (400 MHz, DMSO-d6) δ 1.46 (s, 9H), 2.77 (d, 2J = 15.8 Hz, 1H), 2.84–2.96 (m, 1H), 3.07 (dt, 2J = 12.8 Hz, 3J = 4.3 Hz, 1H), 3.94 (bs, 1H), 6.37–6.85 (m, 2H), 6.96 (t, 3J = 7.4 Hz, 1H), 7.08 (t, 3J = 7.6 Hz, 1H), 7.15 (bs, 2H), 7.22 (d, 3J = 3.1 Hz, 2H), 7.36 (d, 3J = 8.1 Hz, 1H), 7.58 (bs, 1H), 10.95 (s, 1H); 1H NMR (200 MHz, CDCl3) δ 1.55 (s, 9H), 2.63–2.85 (m, 1H), 2.92–3.27 (m, 1H), 4.05 (bs, 1H), 6.60 (bs, 1H), 6.75 (bs, 1H), 7.06–7.25 (m, 6H), 7.35 (d, 3J = 7.7 Hz, 1H), 7.80 (bs, 1H), 8.14 (bs, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 28.4 (3C, q), 28.5 (t), 37.6 (t), 50.5 (d), 79.7 (s), 111.1 (d), 118.6 (s), 119.5 (d), 119.9 (d), 122.0 (d), 125.0 (d), 125.6 (d), 126.4 (s), 126.5 (d), 128.3 (s), 128.3 (d), 129.0 (d), 134.8 (s), 136.3 (s), 136.4 (s), 154.3 (s).
1,1-Dimethylethyl-1-(1-methyl-1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3i):
yield for Cu 218 mg (70%) and for Fe 203 mg (65%); white powder; mp 70–72 °C; TLC Rf (PE/EtOAc 3/1) = 0.78; GC/MS (EI+) m/z (rel intensity) 262 (48), 261 (100), 259 (33), 245 (12), 232 (22), 217 (20), 157 (6), 130 (22), 103 (7), 77 (5); HRMS (ESI+) exact mass calculated for C23H26N2O2 363.2067, found 363.2072 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.58 (s, 9H), 2.68–2.88 (m, 1H), 2.96–3.30 (m, 2H), 3.70 (s, 3H), 3.88–4.32 (m, 1H), 6.49 (s, 1H), 6.74 (bs, 1H), 7.08–7.36 (m, 7H), 7.82 (bs, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 28.4 (t), 28.6 (3C, q), 32.6 (q), 37.5 (t), 50.4 (d), 79.5 (s), 109.1 (d), 117.4 (s), 119.2 (d), 120.4 (d), 121.8 (d), 125.6 (d), 126.5 (d), 127.0 (s), 128.4 (d), 129.0 (d), 129.4 (d), 135.0 (s), 136.5 (s), 137.1 (s), 154.2 (s).
1,1-Dimethylethyl-1-(2-methyl-1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3j):
yield for Cu 209 mg (67%) and for Fe 72 mg (23%); pale yellow powder; mp 81–84 °C; TLC Rf (PE/EtOAc 2/1) = 0.69; GC/MS (EI+) m/z (rel intensity) 262 (62), 261 (100), 245 (61), 244 (37), 219 (21), 218 (44), 131 (34), 130 (85), 103 (18), 77 (20); HRMS (ESI+) exact mass calculated for C23H26N2O2 385.1886, found 385.1896 [M + Na]+; 1H NMR (200 MHz, CDCl3) δ 1.49 (s, 9H), 2.24 (s, 3H), 2.78 (dd, 2J = 16.7 Hz, 3J = 3.0 Hz, 1H), 3.00–3.80 (m, 2H), 4.14 (dd, 2J = 11.8 Hz, 3J = 3.5 Hz, 1H), 6.66 (s, 1H), 6.83–6.94 (m, 1H), 6.98–7.13 (m, 4H), 7.17–7.26 (m, 3H), 7.91 (bs, 1H); 1H NMR (200 MHz, DMSO-d6) δ 1.44 (s, 9H), 2.19 (s, 3H), 2.74–3.28 (m, 3H), 4.01 (dd, 2J = 13.3 Hz, 3J = 3.3 Hz, 1H), 6.50 (s, 1H), 6.67–6.78 (m, 1H), 6.81–7.02 (m, 3H), 7.05–7.15 (m, 1H), 7.15–7.28 (m, 3H), 10.95 (s, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 12.6 (q), 28.5 (3C, q), 28.6 (t), 38.3 (t), 51.0 (d), 79.7 (s), 110.1 (d), 113.3 (s), 119.3 (d), 119.5 (d), 120.9 (d), 126.3 (d), 126.5 (d), 128.3 (d), 128.5 (s), 129.0 (d), 133.9 (s), 134.9 (s), 134.9 (s), 137.0 (s), 154.2 (s); 13C NMR (50 MHz, APT, DMSO-d6) δ 11.9 (q), 27.9 (t), 28.0 (3C, q), 37.8 (t), 50.3 (d), 78.9 (s), 110.4 (d), 111.6 (s), 118.1 (d), 118.4 (d), 119.9 (d), 126.0 (d), 126.4 (d), 127.7 (s), 127.8 (d), 128.8 (d), 134.2 (s), 134.4 (s), 134.8 (s), 136.7 (s), 153.3 (s).
1,1-Dimethylethyl-1-(5-amino-1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3k):
yield for Cu no conversion and for Fe 50 mg (16%); dark brown solid; mp 96–98 °C; TLC Rf (PE/Et2O 1/1) = 0.31; GC/MS (EI+) m/z (rel intensity) 263 (48), 262 (30), 258 (40), 246 (75), 245 (100), 233 (13), 218 (17), 132 (23), 130 (20); HRMS (ESI+) exact mass calculated for C22H25N3O2 386.1839, found 386.1854 [M + Na]+; 1H NMR (400 MHz, CDCl3) δ 1.48 (s, 9H), 2.82 (dd, 2J = 16.4 Hz, 3J = 2.9 Hz, 1H), 3.09–3.22 (m, 1H), 3.41 (dt, 2J = 13.3 Hz, 3J = 3.6 Hz, 1H), 4.07 (dd, 2J = 13.6 Hz, 3J = 5.7 Hz, 1H), 6.66 (s, 1H), 5.13 (s, 1H), 6.68 (d, 3J = 8.6 Hz, 1H), 6.78 (t, 4J = 2.7 Hz, 1H), 7.03 (d, 3J = 7.7 Hz, 1H), 7.10 (t, 3J = 7.3 Hz, 1H), 7.14 (d, 3J = 8.6 Hz, 1H), 7.19–7.24 (m, 2H), 7.86 (s, 1H), NH2 group gives a broad signal between 2 and 5 ppm; 13C NMR (101 MHz, APT, CDCl3) δ 28.4 (q, 3C), 28.5 (t), 39.2 (t), 53.8 (d), 80.2 (s), 101.3 (d), 111.7 (d), 113.8 (d), 116.0 (s), 123.7 (d), 126.3 (d), 126.5 (d), 128.4 (s), 128.9 (d), 128.9 (d), 130.8 (s), 134.8 (s), 137.4 (s), 139.9 (s), 155.5 (s).
1,1-Dimethylethyl-1-(5-methoxy-1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3l):
yield for Cu 174 mg (52%) and for Fe 140 mg (43%); off-white powder; mp 69–72 °C; TLC Rf (PE/EtOAc 5/1) = 0.27; GC/MS (EI+) m/z (rel intensity) 278 (59), 277 (100), 261 (53), 249 (22), 248 (24), 233 (12), 218 (10), 204 (10), 132 (10), 130 (14); HRMS (ESI+) exact mass calculated for C23H26N2O3 401.1836, found 401.1822 [M + Na]+; 1H NMR (200 MHz, DMSO-d6) δ 1.46 (s, 9H), 2.63–3.19 (m, 3H), 3.69 (s, 3H), 3.88 (bs, 1H), 6.52 (bs, 2H), 6.73 (dd, 3J = 8.8 Hz, 4J = 2.1 Hz, 1H), 7.01–7.32 (m, 6H), 10.78 (s, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 27.8 (t), 28.0 (3C, q), 37.0 (t), 50.1 (d), 55.0 (q), 78.8 (s), 100.7 (d), 111.5 (d), 112.2 (d), 116.8 (s), 125.6 (d), 126.4 (s), 126.5 (d), 127.9 (d), 128.9 (d), 131.4 (s), 134.4 (s), 136.0 (s), 153.1 (s), 153.4 (s).
1,1-Dimethylethyl-1-(5-nitro-1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3m):
yield for Cu 200 mg (59%) and for Fe 222 mg (66%); bright yellow solid; mp 219–221 °C; TLC Rf (PE/EtOAc 3/1) = 0.22; GC/MS (EI+) m/z (rel intensity) 393 (43), 292 (100), 276 (10), 263 (24), 246 (34), 217 (30), 189 (8), 146 (6), 132 (6), 130 (12); HRMS (ESI+) exact mass calculated for C22H23N3O4 416.1581, found 416.1589 [M + Na]+; 1H NMR (200 MHz, DMSO-d6) δ 1.50 (s, 9H), 2.63–3.07 (m, 3H), 3.76–4.09 (m, 1H), 6.62 (s, 1H), 6.80 (s, 1H), 7.09–7.29 (m, 4H), 7.55 (d, 3J = 9.0 Hz, 1H), 8.02 (dd, 3J = 9.0 Hz, 4J = 2.2 Hz, 1H), 8.73 (s, 1H), 11.50–11.86 (m, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 27.7 (t), 27.9 (q, 3C), 37.0 (t), 49.9 (d), 79.5 (s), 112.1 (d), 116.4 (d), 116.8 (d), 119.9 (s), 125.3 (s), 125.7 (d), 126.8 (d), 128.0 (d), 128.9 (d), 129.0 (d), 134.5 (s), 135.1 (s), 139.6 (s), 140.6 (s), 153.3 (s).
1,1-Dimethylethyl-1-(5-(methoxycarbonyl)-1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3n):
yield for Cu 175 mg (50%) and for Fe 17 mg (5%); pale yellow powder; mp 221–224 °C; TLC Rf (PE/Et2O 1/1) = 0.18; GC/MS (EI+) m/z (rel intensity) 306 (45), 305 (100), 289 (12), 276 (18), 247 (8), 244 (8), 217 (15), 189 (6), 144 (8), 130 (16); HRMS (ESI+) exact mass calculated for C24H26N2O4 429.1785, found 429.1771 [M + Na]+; 1H NMR (200 MHz, CDCl3) δ 1.62 (s, 9H), 2.60–2.83 (m, 1H), 2.90–3.18 (m, 2H), 3.90 (s, 3H), 3.93–4.27 (m, 1H), 6.58 (d, 4J = 1.9 Hz, 1H), 6.61–6.89 (m, 1H), 7.08–7.24 (m, 4H), 7.34 (d, 3J = 8.6 Hz, 1H), 7.91 (dd, 3J = 8.6 Hz, 4J = 1.2 Hz, 1H), 8.58 (bs, 1H); 8.63 (s, 1H), 1H NMR (200 MHz, DMSO-d6) δ 1.52 (s, 9H), 2.64–3.10 (m, 3H), 3.82 (s, 3H), 3.90 (bs, 1H), 6.58 (bs, 1H), 6.63 (bs, 1H), 7.07–7.30 (m, 4H), 7.45 (d, 3J = 8.6 Hz, 1H), 7.75 (d, 3J = 8.6 Hz, 1H), 8.49 (s, 1H), 11.34 (s, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 27.6 (t), 27.9 (3C, q), (CH2 group at 36.2 ppm in CDCl3 overlaps with DMSO signal), 50.4 (d), 51.4 (q), 79.5 (s), 111.5 (d), 118.6 (s), 120.3 (s), 121.9 (d), 122.4 (d), 125.57 (s), 125.61 (d), 126.7 (d), 127.0 (d), 127.9 (d), 129.1 (d), 134.5 (s), 135.6 (s), 139.0 (s), 153.4 (s), 167.1 (s); 13C NMR (50 MHz, APT, CDCl3) δ 28.1 (t), 28.4 (q, 3C), 36.2 (t), 51.2 (d), 51.6 (q), 80.7 (s), 111.0 (d), 120.1 (s), 121.5 (s), 122.7 (d), 123.7 (d), 125.6 (d), 126.0 (s), 126.4 (d), 126.8 (d), 128.2 (d), 129.1 (d), 134.9 (s), 135.8 (s), 139.2 (s), 154.2 (s), 168.2 (s).
1,1-Dimethylethyl-1-(5-chloro-1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3o):
yield for Cu 215 mg (66%) and for Fe 236 mg (72%); pale pink powder; mp 202–204 °C; TLC Rf (PE/EtOAc 3/1) = 0.56; GC/MS (EI+) m/z (rel intensity) 284 (13), 283 (38), 282 (42), 281 (100), 279 (14), 265 (9), 253 (21), 252 (22), 217 (30), 216 (11), 164 (8), 130 (26), 103 (9); HRMS (ESI+): exact mass calculated for C22H23N2O2Cl 405.1340; found 405.1350 [M + Na]+; 1H NMR (200 MHz, DMSO-d6) δ 1.49 (s, 9H), 2.65–3.09 (m, 3H), 3.91 (bs, 1H), 6.50 (s, 1H), 6.63 (bs, 1H), 7.09 (dd,3J = 8.6 Hz, 4J = 1.9 Hz, 1H), 7.13–7.26 (m, 4H), 7.38 (d, 3J = 8.6 Hz, 1H), 7.66 (d, 4J = 1.8 Hz, 1H), 11.15 (s, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 27.7 (t), 28.0 (q, 3C), (CH2-group at 37.7 ppm in CDCl3 overlaps with DMSO signal), 50.1 (d), 79.3 (s), 113.1 (d), 117.0 (s), 118.3 (d), 121.2 (d), 123.4 (s), 125.6 (d), 126.7 (d), 126.8 (d), 127.0 (s), 127.9 (d), 129.0 (d), 134.4 (s), 134.8 (s), 135.6 (s), 163.4 (s); 13C NMR (50 MHz, APT, CDCl3) δ 28.3 (t), 28.6 (3C, q), 37.7 (t), 51.0 (d), 79.3 (s), 112.0 (d), 118.9 (s), 119.7 (d), 122.6 (d), 125.5 (s), 125.7 (d), 126.1 (d), 126.8 (d), 127.5 (s), 128.3 (d), 129.1 (d), 134.7 (s), 135.0 (s), 135.8 (s), 154.2 (s)
1,1-Dimethylethyl-1-(6-chloro-1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3p):
yield for Cu 245 mg (75%) and for Fe 184 mg (56%); white powder; mp 83–85 °C; TLC Rf (PE:EtOAc=3:1) = 0.63; GC/MS (EI+) m/z (rel intensity) 284 (14), 283 (33), 282 (40), 281 (100), 279 (22), 265 (22), 253 (35), 252 (25), 217 (30), 216 (16), 130 (27), 103 (12); HRMS (ESI+): exact mass calculated for C22H23N2O2Cl 405.1340; found 405.1339 [M + Na]+; 1H NMR (200 MHz, CDCl3) δ 1.52 (s, 9H), 2.63–2.81 (m, 1H), 2.90–3.20 (m, 2H), 4.07 (bs, 1H), 6.52–6.75 (m, 2H), 7.05 (dd, 3J = 8.6 Hz, 4J = 1.8 Hz, 1H), 7.10–7.23 (m, 4H), 7.31 (d, 4J = 1.7 Hz, 1H), 7.68 (bs, 1H), 8.19 (bs, 1H); 1H NMR (200 MHz, DMSO-d6) δ 1.45 (s, 9H), 2.66–3.12 (m, 3H), 3.90 (bs, 1H), 6.50 (s, 1H), 6.68 (bs, 1H), 7.00 (dd, 3J = 8.5 Hz, 4J = 1.9 Hz, 1H), 7.06–7.27 (m, 4H), 7.41 (d, 4J = 1.8 Hz, 1H), 7.54 (d, 3J = 7.5 Hz, 1H), 11.09 (s, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 27.8 (t), 28.0 (3C, q), CH2 group at 37.5 ppm in CDCl3 overlaps with DMSO signal, 50.2 (d), 79.1 (s), 111.2 (d), 117.3 (s), 119.0 (d), 120.3 (d), 124.8 (s), 125.7 (d), 126.0 (s), 126.2 (d), 126.6 (d), 127.9 (d), 128.9 (d), 134.4 (s), 135.8 (s), 136.7 (s), 153.4 (s); 13C NMR (50 MHz, APT, CDCl3) δ 28.4 (t), 28.5 (3C, q), 37.5 (t), 50.5 (d), 79.8 (s), 110.9 (d), 119.2 (s), 120.5 (d), 121.2 (d), 125.1 (s), 125.4 (d), 125.7 (d), 126.7 (d), 128.2 (s), 128.3 (d), 129.1 (d), 134.9 (s), 135.9 (s), 136.8 (s), 154.4 (s).
1,1-Dimethylethyl-1-(7-nitro-1H-indol-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3q):
yield for Cu 155 mg (46%) and for Fe 235 mg (70%); bright yellow powder; mp 88–91 °C; TLC Rf (PE/EtOAc 5/1) = 0.45; GC/MS (EI+) m/z (rel intensity) 293 (56), 292 (100), 291 (17), 290 (38), 264 (15), 263 (24), 246 (20), 244 (16), 217 (32), 216 (20), 189 (13), 188 (10), 132 (21), 130 (27), 121 (16), 108 (26), 103 (13); 1H NMR (200 MHz, DMSO-d6) δ 1.46 (s, 9H), 2.66–3.13 (m, 3H), 3.92 (d, 2J = 12.1 Hz, 1H), 6.60 (s, 1H), 6.73 (bs, 1H), 7.06–7.29 (m, 5H), 8.01–8.13 (m, 2H), 11.80 (s, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 27.7 (t), 28.0 (q, 3C), 37.0 (t), 49.8 (d), 79.3 (s), 118.7 (d), 118.9 (d), 119.2 (s), 125.8 (d), 126.9 (d), 127.6 (d), 127.9 (d), 128.2 (d), 128.5 (s), 129.1 (d), 130.3 (s), 132.6 (s), 134.5 (s), 135.2 (s), 153.5 (s).
1,1-Dimethylethyl-1-(1H-pyrrolo[2,3-b]pyridin-3-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3r):
yield for Cu 133 mg (44%) and for Fe 126 mg (42%); white powder; mp 84–86 °C; TLC Rf (PE/Et2O 1/1) = 0.11; GC/MS (EI+) m/z (rel intensity) 249 (56), 248 (100), 245 (36), 244 (57), 219 (50), 190 (10), 144 (14), 132 (24), 130 (28), 119 (34), 109 (27), 103 (18), 95 (16); HRMS (ESI+) exact mass calculated for C21H23N3O2 350.1863; found 350.1871 [M + H]+; 1H NMR (200 MHz, DMSO-d6) δ 1.45 (s, 9H), 2.68–3.15 (m, 3H), 3.92 (d, 2J = 11.2 Hz, 1H), 6.50 (s, 1H), 6.77 (bs, 1H), 7.03 (dd, 3J = 7.9 Hz, 3J = 4.7 Hz, 1H), 7.09–7.28 (m, 4H), 7.82 (bs, 1H), 8.20 (dd, 3J = 4.6 Hz, 4J = 1.4 Hz, 1H), 11.54 (s, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 27.8 (t), 28.0 (q, 3C), 37.1 (t), 50.4 (d), 79.1 (s), 115.3 (d), 115.9 (s), 118.2 (s), 125.1 (d), 125.7 (d), 126.7 (d), 127.1 (d), 127.9 (d), 128.9 (d), 134.5 (s), 135.4 (s), 142.7 (d), 148.4 (s), 153.4 (s).
1,1-Dimethylethyl-1-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (3s)
yield for Cu 145 mg (44%) and for Fe no conversion; white powder; mp 87–91 °C; TLC Rf (PE/Et2O 1/5) = 0.28; GC/MS (EI+) m/z (rel intensity) 286 (14), 285 (35), 284 (41), 283 (100), 254 (26), 219 (11), 191 (11), 166 (6), 130 (14), 103 (8); HRMS (ESI+) exact mass calculated for C20H21N4O2Cl 385.1426; found 385.1423 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.42 (s, 9H), 2.75 (d, 2J = 16.2 Hz, 1H), 2.94–3.38 (m, 2H), 4.10 (bs, 1H), 6.81 (bs, 1H), 6.86 (bs, 1H), 7.12–7.28 (m, 4H), 8.64 (s, 1H), 11.02 (s, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 28.0 (t), 28.4 (3C, q), 38.2 (t), 50.6 (d), 80.2 (s), 115.6 (s), 119.0 (s), 126.0 (d), 127.0 (d), 128.1 (d), 129.3 (d), 134.6 (s), 135.8 (s), 150.3 (d), 152.4(s), 152.6 (s), 155.0 (s).
Deprotection of the Boc PG. Method A: Microwave-Assisted Deprotection
3h (150 mg, 0.450 mmol) or 3i (50 mg, 0.138 mmol) was suspended in ethylene glycol (3h, 20 mL; 3i, 10 mL) and microwaved at 250 °C for 30 s (hold time). The reaction mixture was diluted with water and extracted three times with diethyl ether. The combined organic layers were washed twice with brine, dried over sodium sulfate, filtered, and evaporated. The crude product was purified by flash column chromatography (100 g of SiO2, PE/EtOAc 100/0 → 0/100 (30 min), EtOAc/EtOH 95/5 → 60/40 (30 min)). In the case of 3i the reaction mixture was diluted with water, chilled with liquid nitrogen, and lyophilized to afford the desired product as a pale brown solid.
Deprotection of the Boc PG. Method B: General Procedure for TMSCl-Promoted Boc Deprotection
TMSCl (272 mg, 2.50 mmol, 5.0 equiv) was added dropwise to an argon-degassed solution of the corresponding Boc-protected amine (0.5 mmol, 1.0 equiv) in dry MeOH (4 mL) at room temperature, and the reaction mixture was stirred for 4 to 24 h under argon. After the reaction mixture was cooled to 0 °C, it was poured into ice cold 2 N aqueous NaOH. The suspension was extracted three times with EtOAc, and the combined organic layers were washed once with 2 N NaOH. The combined organic layers were then dried over sodium sulfate, filtered, and evaporated to afford the desired product in quantitative yields.
Method C: General Procedure for Indolation of THIQ
THIQ (200 mg, 1.50 mmol, 1.0 equiv), catalyst (18.1 mg, 75 μmol, 0.05 equiv), and the corresponding indole (1.80 mmol, 1.2 equiv) were placed into a 5 mL glass vial in air and stirred for 10 min at 0 °C. tBHP (390 μL, 5.5 M in decane, 1.3 equiv) was added dropwise at 0 °C in air and the dark greenish slurry was stirred for 1 h at 0 °C. The neat mixture was then slowly heated to 50 °C (within 1 h), whereupon the color changed to black and stirring was continued for 15 h in a heating block. The reaction was monitored by GC-MS. The mixture was diluted with DCM (3 mL) and directly subjected to flash column chromatography, using PE/EtOAc as eluent to afford the desired product.
1-(1H-Indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5h):
method A 95 mg (89%), method B 124 mg (100%), method C 179 mg (48%); brown solid; mp 44–46 °C; TLC Rf (EtOAc/EtOH 5/1) = 0.20; GC/MS (EI+) m/z (rel intensity) 248 (M+, 38), 247 (100), 231 (15), 219 (22), 218 (32), 217 (19), 130 (20); HRMS (ESI+) exact mass calculated for C17H16N2 249.1398, found 249.1386 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 2.02 (bs, 1H), 2.80–3.37 (m, 4H), 5.51 (s, 1H), 6.88 (d, 3J = 1.9 Hz, 1H), 6.95–7.23 (m, 6H), 7.33 (d, 3J = 8.0 Hz, 1H), 7.49 (d, 3J = 7.8 Hz, 1H), 8.34 (bs, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 29.4 (t), 41.5 (t), 53.6 (d), 111.3 (d), 118.6 (s), 119.1 (d), 119.3 (d), 121.8 (d), 124.1 (d), 125.6 (d), 126.1 (d), 126.2 (s), 127.8 (d), 128.8 (d), 134.9 (s), 136.5 (s), 138.1 (s).
1-(1-Methyl-1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5i):
method A 35 mg (97%), method B 131 mg (100%), method C 4 mg (1%); pale yellow solid; mp 55–58 °C; TLC Rf (EtOAc/EtOH/TEA 10/1/1) = 0.57; GC/MS (EI+) m/z (rel intensity) 262 (M+, 60), 261 (100), 245 (6), 233 (14), 232 (24), 217 (12), 131 (22), 130 (29), 115 (11), 108 (14); 1H NMR (200 MHz, CDCl3) δ 2.52 (s, 1H), 2.83–3.17 (m, 3H), 3.19–3.35 (m, 1H), 3.73 (s, 3H), 5.52 (s, 1H), 6.79 (s, 1H), 6.97–7.36 (m, 7H), 7.51 (d, 3J = 7.9 Hz, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 29.7 (t), 32.7 (q), 41.5 (t), 53.6 (d), 109.3 (d), 118.1 (s), 119.1 (d), 119.4 (d), 121.7 (d), 125.6 (d), 126.1 (d), 126.9 (s), 127.9 (d), 128.5 (d), 128.9 (d), 135.2 (s), 137.2 (s), 138.3 (s).
1-(2-Methyl-1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5j):
method B 131 mg (100%), method C 170 mg (43%); pale yellow solid; mp 68–70 °C; TLC Rf (EtOAc/EtOH 10/1) = 0.14; GC/MS (EI+) m/z (rel intensity) 262 (M+, 86), 261 (100), 247 (24), 245 (60), 244 (37), 233 (22), 230 (21), 218 (38), 217 (41), 130 (46); HRMS (ESI+) exact mass calculated for C18H18N2 263.1543, found 263.1548 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.94 (bs, 1H), 2.35 (s, 3H), 2.79–3.00 (m, 1H), 3.03–3.46 (m, 3H), 5.43 (s, 1H), 6.79–7.28 (m, 8H), 7.99 (bs, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 11.9 (q), 29.8 (t), 43.3 (t), 53.6 (d), 110.2 (d), 113.6 (s), 118.8 (d), 119.2 (d), 120.8 (d), 125.9 (d), 126.0 (d), 127.3 (s), 127.4 (d), 128.8 (d), 133.3 (s), 134.9 (s), 135.4 (s), 138.5 (s).
1-(5-Methoxy-1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5l):
method B 137 mg (99%), method C 202 mg (48%); light brown solid; mp 74–76 °C; TLC Rf (EtOAc/EtOH 5/1) = 0.11; GC/MS (EI+) m/z (rel intensity) 278 (M+, 62), 277 (100), 261 (49), 249 (26), 248 (26), 234 (10), 233 (8), 217 (7), 132 (9), 130 (12); HRMS (ESI+) exact mass calculated for C18H18N2O 279.1492; found 279.1499 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.98 (bs, 1H), 2.79–3.36 (m, 4H), 3.76 (s, 3H), 5.47 (s, 1H), 6.79–6.88 (m, 2H), 6.91 (d, 3J = 2.3 Hz, 1H), 6.95–7.18 (m, 4H), 7.22 (d, 3J = 8.7 Hz, 1H), 8.18 (bs, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 27.8 (t), 40.5 (t), 52.9 (d), 55.6 (q), 100.8 (d), 112.1 (d), 112.2 (d), 115.0 (s), 126.1 (d), 126.2 (d), 126.6 (s), 126.8 (d), 128.1 (d), 128.7 (d), 131.5 (s), 133.6 (s), 135.8 (s), 153.8 (s).
1-(5-Nitro-1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5m):
method B 146 mg (100%), method C 233 mg (53%); shining dark yellow solid; mp 83–85 °C; TLC Rf (EtOAc/EtOH 5/1) = 0.21; GC/MS (EI+) m/z (rel intensity) 293 (M+, 43), 292 (100), 276 (12), 263 (22), 246 (31), 218 (14), 217 (27), 130 (12); HRMS (ESI+) exact mass calculated for C17H15N3O2 294.1237; found 294.1239 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.93 (bs, 1H), 2.80–3.34 (m, 4H), 5.51 (s, 1H), 6.92 (d, 3J = 7.6 Hz, 1H), 6.97–7.23 (m, 4H), 7.32 (d, 3J = 9.0 Hz, 1H), 8.06 (dd, 3J = 9.0 Hz, 4J = 2.1 Hz, 1H), 8.51 (d, 4J = 2.0 Hz, 1H), 8.91 (bs, 1H); 13C NMR (50 MHz, CDCl3) δ 29.4 (t), 41.6 (t), 53.5 (d), 111.2 (d), 117.1 (d), 117.7 (d), 121.9 (s), 125.8 (d), 125.9 (s), 126.6 (d), 127.0 (d), 127.5 (d), 129.3 (d), 135.1 (s), 137.2 (s), 139.6 (s), 141.5 (s).
1-(5-Methoxycarbonyl-1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5n):
method C 321 mg (58%); pale yellow solid; mp 195–197 °C; TLC Rf (EtOAc/EtOH 5/1) = 0.17; GC/MS (EI+) m/z (rel intensity) 306 (M+, 41), 305 (100), 289 (8), 276 (16), 253 (20), 244 (8), 217 (14), 191 (16), 144 (8), 130 (12); HRMS (ESI+) exact mass calculated for C19H18N2O2 307.1441, found 307.1446 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.77 (bs, 1H), 2.79–3.33 (m, 4H), 3.90 (s, 3H), 5.55 (s, 1H), 6.98–7.20 (m, 5H), 7.36 (d, 3J = 8.6 Hz, 1H), 7.90 (dd, 3J = 8.6 Hz, 4J = 1.5 Hz, 1H), 8.37 (bs, 2H); 1H NMR (200 MHz, DMSO-d6) δ 2.57–3.21 (m, 5H), 3.78 (s, 3H), 5.31 (s, 1H), 6.79 (d, 3J = 7.6 Hz, 1H), 6.97 (dt, 3J = 7.3 Hz, 4J = 1.9 Hz, 1H), 7.03–7.17 (m, 2H), 7.19 (d, 3J = 1.9 Hz, 1H), 7.42 (d, 3J = 8.6 Hz, 1H), 7.69 (dd, 3J = 8.6, 4J = 1.4 Hz, 1H), 8.19 (d, 4J = 1.1 Hz, 1H), 11.30 (s, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 25.8 (t), 40.0 (t), 51.8 (q), 51.9 (d), 111.5 (d), 113.0 (s), 121.6 (d), 121.6 (s), 123.2 (d), 125.8 (s), 126.8 (d), 127.8 (d), 128.0 (d), 128.1 (d), 128.7 (d), 132.1 (s), 132.9 (s), 138.9 (s), 168.1 (s).
1-(5-Chloro-1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5o):
method B 141 mg (100%, reaction time 7 h); pale yellow solid; mp 148–151 °C; TLC Rf (EtOAc/Et3N 11/1) = 0.29; GC/MS (EI+) m/z (rel intensity) 284 (19), 283 (38), 282 (M+, 57), 281 (100), 254 (12), 253 (19), 252 (27), 217 (30), 131 (14), 130 (26), 109 (17); HRMS (ESI+): exact mass calculated for C17H15N2Cl 283.0997, found 283.0998 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.93 (bs, 1H), 2.79–3.34 (m, 4H), 5.43 (s, 1H), 6.91 (s, 1H), 6.94 (d, 3J = 8.0 Hz, 1H), 6.99–7.19 (m, 4H), 7.23 (d, 3J = 8.7 Hz, 1H), 7.46 (d, 4J = 1.8 Hz, 1H), 8.36 (s, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 29.4 (t), 41.7 (t), 53.6 (d), 112.3 (d), 118.7 (s), 118.8 (d), 122.3 (d), 125.1 (s), 125.4 (d), 125.7 (d), 126.4 (d), 127.3 (s), 127.6 (d), 129.0 (d), 134.9 (s), 134.9 (s), 137.7 (s).
1-(6-Chloro-1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5p):
method B 122 mg (86%, reaction time 4 h), method C 197 mg (46%); off white powder; mp 146–148 °C; TLC Rf (EtOAc/EtOH 5/1) = 0.15; GC/MS (EI+) m/z (rel intensity) 284 (18), 283 (38), 282 (M+, 56), 281 (100), 265 (10), 253 (22), 252 (28), 217 (29), 132 (19), 131 (19), 130 (35), 123 (13), 109 (29); HRMS (ESI+) exact mass calculated for C17H15N2Cl 283.0997, found 283.1000 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 2.23 (s, 1H), 2.79–3.37 (m, 4H), 5.44 (s, 1H), 6.87 (s, 1H), 6.93 (d, 3J = 7.6 Hz, 1H), 6.96–7.09 (m, 2H), 7.13–7.21 (m, 2H), 7.25 (d, 4J = 1.7 Hz, 1H), 7.32 (d, 3J = 8.5 Hz, 1H), 8.61 (s, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 20.3 (t), 32.8 (t), 45.1 (d), 102.6 (d), 109.7 (s), 110.8 (d), 111.7 (d), 116.6 (s), 117.1 (d), 117.2 (d), 117.9 (d), 118.8 (s), 119.4 (d), 120.3 (d), 126.4 (s), 129.1 (s), 129.5 (s).
1-(7-Nitro-1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5q):
method B 145 mg (99%, reaction time 6 h), method C: 7 mg (2%); shining dark yellow solid; mp 58–61 °C; TLC Rf (EtOAc/MeOH 11/1) = 0.35; GC/MS (EI+) m/z (rel intensity) 293 (M+, 57), 292 (100), 264 (15), 263 (23), 246 (18), 217 (26), 189 (10), 132 (10), 131 (8), 130 (14), 109 (11); 1H NMR (200 MHz, CDCl3) δ 1.87 (bs, 1H), 2.79–3.36 (m, 4H), 5.48 (s, 1H), 6.90 (d, 3J = 7.5 Hz, 1H), 6.96–7.22 (m, 5H), 7.84 (d, 3J = 7.7 Hz, 1H), 8.13 (d, 3J = 8.1 Hz, 1H), 9.86 (bs, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 29.6 (t), 42.0 (t), 53.8 (d), 119.0 (d), 119.3 (d), 121.1 (s), 125.7 (d), 126.1 (d), 126.5 (d), 127.5 (d), 128.4 (d), 129.1 (d), 130.0 (s), 130.2 (s), 132.9 (s), 135.1 (s), 137.6 (s).
1-(1H-pyrrolo[2,3-b]pyridin-3-yl)-1,2,3,4-tetrahydroisoquinoline (5r):
method B 124 mg (100%), method C 160 mg (43%); light brown solid; mp 172–174 °C; TLC Rf (EtOAc/EtOH 5/1) = 0.11; GC/MS (EI+) m/z (rel intensity) 249 (M+, 36), 248 (100), 232 (7), 220 (18), 219 (30), 218 (11), 131 (8), 130 (10), 119 (12); HRMS (ESI+) exact mass calculated for C16H15N3 250.1339; found 250.1352 [M + H]+; 1H NMR (200 MHz DMSO-d6) δ 2.63–3.24 (m, 4H), 3.70 (s, 1H), 5.30 (s, 1H), 6.80 (d, J = 7.6 Hz, 1H), 6.85–7.02 (m, 2H), 7.03–7.18 (m, 2H), 7.27 (d, 4J = 1.3 Hz, 1H), 7.66 (dd, 3J = 7.8 Hz, 4J =1.3 Hz, 1H), 8.15 (d, 3J = 3.6 Hz, 1H), 11.48 (s, 1H); 13C NMR (50 MHz, APT, DMSO-d6) δ 29.0 (t), 41.7 (t), 53.9 (d), 114.8 (d), 116.8 (s), 118.4 (s), 124.8 (d), 125.2 (d), 125.8 (d), 127.0 (d), 128.0 (d), 128.7 (d), 135.0 (s), 138.5 (s), 142.3 (d), 148.9 (s).
1-(7-Methyl-1H-indol-3-yl)-1,2,3,4-tetrahydroisoquinoline (5t):
method C 189 mg (48%); pale brown solid; mp 141–144 °C; TLC Rf (EtOAc/EtOH 5/1) = 0.12; GC/MS (EI+) m/z (rel intensity) 262 (M+, 56), 261 (100), 245 (11), 233 (14), 232 (21), 217 (12), 132 (9), 131 (12), 130 (20), 115 (10); 1H NMR (200 MHz, CDCl3) δ 2.08 (bs, 1H), 2.48 (s, 3H), 2.81–3.37 (m, 4H), 5.51 (s, 1H), 6.87 (d, 3J = 2.3 Hz, 1H), 6.94–7.22 (m, 6H), 7.30–7.41 (m, 1H), 8.26 (bs, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 16.6 (q), 29.6 (t), 41.7 (t), 53.9 (d), 117.0 (d), 119.8 (d), 120.5 (s), 122.6 (d), 123.7 (d), 125.6 (d), 125.9 (s), 126.1 (d), 127.9 (d), 128.9 (d), 135.0 (s), 136.1 (s), 138.2 (s).
General Procedure for Pyrrolation of THIQ
THIQ (999 mg, 7.50 mmol, 2.0 equiv), catalyst (45.3 mg, 0.188 mmol, 0.05 equiv), and the corresponding pyrrole (3.75 mmol, 1.0 equiv) were placed in a 5 mL glass vial in air and cooled to 0 °C and stirred for 10 min. tBHP (975 μL, 5.5 M in decane, 1.3 equiv) was added dropwise at 0 °C in air and the dark greenish slurry stirred for 1 h at 0 °C. The neat mixture was then slowly heated to 50 °C (within 1 h), upon which the mixture turned black, and stirred for 15 h in a heating block. The reaction was monitored by GC-MS. The mixture was diluted with DCM (3 mL) and directly subjected to flash chromatography, using EtOAc/EtOH as eluent. Due to polar unidentified impurities, the desired product was further purified by preparative HPLC.
1-(1H-Pyrrol-2-yl)-1,2,3,4-tetrahydroisoquinoline (8a):
327 mg (44%); white solid; mp 117–118 °C; TLC Rf (EtOAc/EtOH 5/1) = 0.11; GC/MS (EI+) m/z (rel intensity) 198 (M+, 50), 197 (100), 182 (99), 169 (36), 168 (95), 167 (40), 132 (46), 131 (28), 130 (60), 115 (21), 103 (21), 77 (22); HRMS (ESI+) exact mass calculated for C13H14N2 199.1230, found 199.1223 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 2.07 (s, 1H), 2.76 (dt, 2J = 16.4 Hz, 3J = 5.3 Hz, 1H), 2.84–3.26 (m, 3H), 5.14 (s, 1H), 6.06 (dd, 3J = 3.3 Hz, 3J = 2.5 Hz, 1H), 6.14 (dd, 3J = 5.7 Hz, 3J = 2.8 Hz, 1H), 6.68 (dd, 3J = 4.1 Hz, 3J = 2.5 Hz, 1H), 6.95–7.04 (m, 1H), 7.05–7.23 (m, 3H), 9.11 (s, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 29.2 (t), 41.9 (t), 55.1 (d), 107.4 (d), 107.9 (d), 117.6 (d), 125.6 (d), 126.5 (d), 127.6 (d), 129.1 (d), 133.7 (s), 134.8 (s), 137.0 (s).
1-(2,5-Dimethyl-1H-pyrrol-3-yl)-1,2,3,4-tetrahydroisoquinoline (8b):
230 mg (27%); off-white crystals; mp 127–129 °C; TLC Rf (EtOAc/EtOH/TEA 10/1/1) = 0.53; GC/MS (EI+) m/z (rel intensity) 226 (M+, 100), 225 (96), 209 (79), 208 (56), 196 (38), 194 (32), 182 (39), 167 (28), 132 (24), 130 (36), 94 (78), 91 (28); HRMS (ESI+) exact mass calculated for C15H18N2 227.1543; found 227.1544 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.84 (s, 1H), 2.13 (s, 3H), 2.15 (s, 3H), 2.71–3.15 (m, 3H), 3.32 (dt, 2J = 15.9 Hz, 3J = 7.0 Hz, 1H), 5.01 (s, 1H), 5.51 (d, 4J = 2.3 Hz, 1H), 6.90 (d, 3J = 6.9 Hz, 1H), 6.98–7.13 (m, 3H), 7.74 (s, 1H); 13C NMR (50 MHz, CDCl3) δ 11.1 (q), 12.9 (q), 29.9 (t), 42.6 (t), 54.1 (d), 105.9 (d), 122.5 (s), 123.2 (s), 125.1 (s), 125.5 (d), 125.6 (d), 127.7 (d), 128.7 (d), 135.0 (s), 139.8 (s).
1-(4,5,6,7-tetrahydro-1H-indol-2-yl)-1,2,3,4-tetrahydroisoquinoline (8c):
387 mg (41%); pale yellow solid; mp 115–117 °C; TLC Rf (EtOAc/EtOH 5/1) = 0.14; GC/MS (EI+) m/z (rel intensity) 252 (M+, 46), 251 (52), 236 (56), 223 (44), 222 (39), 194 (31), 180 (39), 132 (43), 131 (65), 130 (100), 105 (32), 103 (31), 93 (72), 77 (31); HRMS (ESI+) exact mass calculated for C17H20N2 253.1699, found 253.1692 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.68–1.88 (m, 4H), 2.21 (s, 1H), 2.44–2.55 (m, 4H), 2.68–3.30 (m, 4H), 5.10 (s, 1H), 5.76 (d, 4J = 2.3 Hz, 1H), 7.03–7.21 (m, 4H), 8.27 (s, 1H); 13C NMR (50 MHz, APT, CDCl3) δ 22.7 (t), 22.8 (t), 23.4 (t), 23.8 (t), 29.3 (t), 41.6 (t), 55.1 (d), 107.1 (d), 116.1 (s), 125.5 (d), 126.4 (d), 126.8 (s), 127.8 (d), 129.0 (d), 131.8 (s), 134.9 (s), 137.1 (s).
1,1-Dimethylethyl-1-(1H-pyrrol-2-yl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (8d):
55 mg (11%); yellow oil; TLC Rf (PE/EtOAc 5/1) = 0.53; GC/MS (EI+) m/z (rel intensity) 298 (M+, 8), 242 (49), 198 (30), 197 (100), 182 (42), 168 (26), 167 (15), 132 (10), 130 (18), 57 (20); 1H NMR (200 MHz, CDCl3) δ 1.51 (s, 9H), 2.70–3.03 (m, 2H), 3.26 (bs, 1H), 3.98 (bs, 1H), 5.58 (bs, 1H), 6.02 (dd, 3J = 5.0 Hz, 3J = 2.5 Hz, 1H), 6.30 (bs, 1H), 6.73 (dd, 3J = 4.1 Hz, 3J = 2.5 Hz, 1H), 7.14–7.23 (m, 4H), 9.14 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 28.6 (q, 3C), 28.7 (t), 39.1 (t), 51.6 (d), 80.2 (s), 107.1 (d), 107.7 (d), 117.7 (d), 125.9 (d), 127.0 (d), 128.5 (d), 128.6 (d), 134.1 (s), 134.3 (s), 135.2 (s), 156.2 (s).
General Procedure for Methoxyarlyation of N-Protected THIQ
2-Protected 1,2,3,4-tetrahydroisoquinoline (PG = Boc, 200 mg; PG = Bz, 203 mg; PG = Cbz; 229 mg; 0.857 mmol, 1.0 equiv), catalyst (Cu(NO3)2·3H2O, 10.4 mg; Fe(NO3)3·9H2O, 17.3 mg; 42.9 μmol, 0.05 equiv), and the methoxybenzene derivative (1.03 mmol, 1.2 equiv) were placed into a 5 mL glass vial. tBHP (203 μL, 5.5 M in decane, 1.3 equiv) was added dropwise at 0 °C in air and the reaction mixture stirred for 10 min at 0 °C. The neat mixture was then slowly heated to 50 °C and stirred for 15 h in a heating block. The reaction was monitored by TLC and/or GC-MS. The reaction mixture was diluted with DCM (3 mL) and directly subjected to flash column chromatography. The desired product was obtained after MPLC, where the solvent mixture used for TLC was also used for column chromatography (100 g of SiO2). If necessary, the product was finally purified by preparative HPLC.
1,1-Dimethylethyl-1-(2,4,6-trimethoxyphenyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (10a):
yield for Cu 260 mg (76%) and for Fe 278 mg (81%); white solid; mp 109–111 °C; TLC Rf (PE/Et2O 1/1) = 0.10; GC/MS (EI+) m/z (rel intensity) 348 (24), 299 (60), 298 (100), 282 (26), 268 (90), 239 (14), 180 (18), 179 (20), 151 (16), 132 (9); HRMS (ESI+): exact mass calculated for C23H29NO5 400.2118, found 400.2122 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.28 (s, 9H), 2.73–3.01 (m, 2H), 3.49 (ddd, 2J = 12.6 Hz, 3J = 10.6 Hz, 3J = 4.5 Hz, 1H), 3.69 (s, 6H), 3.81 (s, 3H), 4.30–4.45 (m, 1H), 6.12 (s, 2H), 6.45 (s, 1H), 6.80 (d, 3J = 7.2 Hz, 1H), 6.95–7.16 (m, 3H); 13C NMR (50 MHz, APT, CDCl3) δ 28.3 (3C, q), 30.5 (t), 39.4 (t), 49.4 (d), 55.2 (q), 55.5 (2C, q), 79.1 (s), 90.5 (2C, d), 114.0 (s), 125.4 (d), 125.8 (d), 126.3 (d), 127.9 (d), 135.2 (s), 137.2 (s), 155.4 (s), 158.8 (2C, s), 160.1 (s).
Phenyl-(1-(2,4,6-trimethoxyphenyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone (10b):
yield for Cu 159 mg (46%) and for Fe 187 mg (54%); white solid; mp 67–69 °C; TLC Rf (PE/Et2O 1/1) = 0.09; GC/MS (EI+) m/z (rel intensity) 403 (M+, 7), 373 (26), 372 (100), 299 (4), 298 (13), 282 (6), 105 (10), 77 (9); HRMS (ESI+) exact mass calculated for C25H25NO4 404.1856, found 404.1852 [M + H]+; 1H NMR (400 MHz, DMSO-d6, 80 °C) δ 2.86 (bs, 1H), 3.61 (s, 6H), 3.63–3.74 (m, 1H), 3.78 (s, 3H), 4.07 (bs, 1H), 6.21 (s, 2H), 6.64 (bs, 1H), 6.72 (d, 3J = 7.5 Hz, 1H), 7.04 (t, 3J = 7.4 Hz, 1H), 7.10 (t, 3J = 7.2 Hz, 1H), 7.13–7.26 (m, 3H), 7.31–7.44 (m, 3H); 13C NMR (101 MHz, DMSO-d6, 80 °C) δ 29.5 (t), 49.2 (s), 54.8 (q), 55.4 (q, 2C), 91.4 (d, 2C), 112.4 (s), 125.1 (d), 125.4 (d, 2C), 126.0 (d, 2C), 127.4 (d), 127.5 (d, 2C overlapping), 128.3 (d), 134.3 (s), 136.5 (s), 137.3 (s), 158.4 (s), 159.9 (s, 2C), 169.2 (s).
Phenylmethyl-1-(2,4,6-trimethoxyphenyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (10c):
yield for Cu 190 mg (51%) and for Fe 215 mg (58%); pale yellow solid; mp 115–116 °C; TLC Rf (PE/Et2O 1/1) = 0.32; GC/MS (EI+) m/z (rel intensity) 433 (M+, 1), 342 (3), 299 (20), 298 (100), 283 (5), 282 (17), 90 (6); HRMS (ESI+) exact mass calculated for C26H27NO5 434.1962, found 434.1966 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 2.75–3.06 (m, 2H), 3.29–3.79 (m, 7H), 3.83 (s, 3H), 4.46 (d, 2J = 11.0 Hz, 1H), 5.03 (d, 2J = 16.7 Hz, 1H), 5.09 (d, 2J = 17.4 Hz, 1H), 6.08 (s, 2H), 6.56 (s, 1H), 6.79 (d, 3J = 7.3 Hz, 1H), 6.96–7.41 (m, 8H); 13C NMR (50 MHz, APT, CDCl3) δ 30.4 (t), 39.9 (t), 49.3 (d), 55.2 (q), 55.4 (q, 2C), 66.8 (t), 90.8 (d, 2C), 113.3 (s), 125.5 (d), 125.9 (d), 126.2 (d), 127.5 (d), 127.8 (d), 127.9 (d, 2C), 128.2 (d, 2C), 134.7 (s), 136.0 (s), 137.0 (s), 155.6 (s), 158.9 (s), 160.2 (s, 2C).
1,1-Dimethylethyl-1-(2,4,5-trimethoxyphenyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (10d):
yield for Cu 80 mg (23%) and for Fe 160 mg (47%); white solid; mp 84–86 °C; TLC Rf (PE/Et2O 3/1) = 0.13; HRMS (ESI+) exact mass calculated for C23H29NO5 400.2118, found 400.2123 [M + H]+; GC/MS (EI+) m/z (rel intensity) 399 (M+, 4), 343 (5), 299 (58), 298 (100), 284 (24), 268 (73), 253 (12), 239 (12), 168 (8), 132 (15); 1H NMR (200 MHz, CDCl3) δ 1.40 (s, 9H), 2.75–3.07 (m, 2H), 3.35–3.55 (m, 1H), 3.69 (s, 3H), 3.77 (s, 3H), 3.87 (s, 3H), 4.03–4.24 (m, 1H), 6.53 (s, 1H), 6.42 (s, 1H), 6.56 (s, 1H), 7.01–7.17 (m, 4H); 13C NMR (50 MHz, APT, CDCl3) δ 28.3 (3C, q), 29.0 (t), 39.1 (t), 53.0 (q), 56.0 (q), 56.1 (d), 56.7 (q), 79.4 (s), 97.5 (d), 113.8 (d), 124.2 (s), 125.9 (d), 126.2 (d), 127.8 (d), 128.4 (d), 134.8 (s), 136.7 (s), 142.4 (s), 148.8 (s), 151.5. (s), 154.7 (s).
1,1-Dimethylethyl-1-(2,3,4,6-tetramethoxyphenyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (10e):
yield for Cu 170 mg (46%) and for Fe 190 mg (52%); pale yellow solid; mp 116–118 °C; TLC Rf (PE/Et2O 2/1) = 0.17; GC/MS (EI+) m/z (rel intensity) 429 (M+, 8), 329 (27), 328 (100), 299 (10), 298 (49), 183 (10), 132 (20), 130 (10), 57 (12); HRMS (ESI+): exact mass calculated for C24H31NO6 430.2224, found 430.2230 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 1.33 (s, 9H), 2.74–3.03 (m, 2H), 3.40–3.61 (m, 4H), 3.75 (s, 3H), 3.75 (s, 3H), 3.88 (s, 3H), 4.36 (d, 3J = 12.3 Hz, 1H), 6.29 (s, 1H), 6.48 (s, 1H), 6.80 (d, 3J = 6.9 Hz, 1H), 6.96–7.16 (m, 3H); 13C NMR (50 MHz, APT, CDCl3) δ 28.3 (q, 3C), 30.2 (t), 39.4 (t), 49.8 (d), 55.7 (q), 55.9 (q), 59.8 (q), 60.7 (q), 79.2 (s), 91.7 (d), 118.9 (s), 125.6 (d), 125.7 (d), 126.5 (d), 128.1 (d), 135.1 (s), 136.5 (s), 137.3 (s), 152.6 (s), 152.7 (s), 153.6 (s), 155.1 (s).
1,1-Dimethylethyl-1-(2,4-dimethoxyphenyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (10f):
yield for Cu 131 mg (41%) and for Fe 108 mg (34%); white solid; mp 36–37 °C; TLC Rf (PE/Et2O 1/1) = 0.33; HRMS (ESI+) exact mass calculated for C22H27NO4 370.2013, found 370.2010 [M + H]+; GC/MS (EI+) m/z (rel intensity) 269 (46), 268 (100), 252 (22), 239 (12), 238 (32), 132 (15), 130 (10); 1H NMR (200 MHz, CDCl3) δ 1.42 (s, 9H), 2.81 (dt, 2J = 16.0 Hz, 3J = 3.9 Hz, 1H), 2.99 (ddd, 2J = 15.9 Hz, 3J = 10.3 Hz, 3J = 5.6 Hz, 1H), 3.29–3.48 (m, 1H), 3.78 (s, 3H), 3.82 (s, 3H), 3.99–4.22 (m, 1H), 6.34 (dd, 3J = 8.4 Hz, 4J = 2.4 Hz, 1H), 6.47 (s, 1H), 6.48 (s, 1H), 6.83 (d, 3J = 8.3 Hz, 1H), 7.00–7.18 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 28.3 (q, 3C), 28.8 (t), 38.6 (t), 52.6 (d), 55.15 (q), 55.19 (q), 79.3 (s), 98.4 (d), 103.4 (d), 125.1 (s), 125.9 (d), 126.2 (d), 127.9 (d), 128.5 (d), 130.0 (d), 135.0 (s), 136.8 (s), 154.7 (s), 158.0 (s), 159.9 (s).
General Procedure for Methoxyarylation of Isochroman
Isochroman 9 (200 mg, 1.49 mmol, 1.0 equiv), catalyst (Cu(NO3)2·3H2O, 18.0 mg; Fe(NO3)3·9H2O, 30.1 mg; 74.5 μmol, 0.05 equiv), and the corresponding methoxybenzene (1.79 mmol, 1.2 equiv) were placed into a 5 mL glass vial. tBHP (352 μL, 5.5 M in decane, 1.3 equiv) was added dropwise at 0 °C in air and the reaction mixture stirred for 10 min at 0 °C. The neat mixture was then slowly heated to 50 °C and stirred for 15 h in a heating block. Finally, the reaction temperature was raised to 80 °C, and the reaction mixture was stirred for another 24 h. The reaction was monitored by TLC and/or GC-MS. The reaction mixture was diluted with DCM (3 mL) and directly subjected to flash column chromatography. The desired product was obtained after MPLC, where the solvent mixture used for TLC was also used for column chromatography (100 g of SiO2). If necessary, the product was finally purified by preparative HPLC.
1-(2,4,6-Trimethoxyphenyl)isochroman (11a):
yield for Cu 230 mg (51%) and for Fe 246 mg (55%); white solid; mp 118–120 °C; TLC Rf (PE/Et2O 1/1) = 0.32; GC/MS (EI+) m/z (rel intensity) 300 (M+, 50), 282 (22), 272 (50), 269 (100), 251 (26), 241 (98), 239 (48), 208 (23), 195 (27), 181 (35), 168 (22), 139 (28); HRMS (ESI+) exact mass calculated for C18H20O4 301.1434; found 301.1442 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 2.66 (d, 2J = 15.9 Hz, 1H), 3.09–3.35 (m, 1H), 3.61 (s, 6H), 3.80 (s, 3H), 3.93 (dt, 2J = 11.2 Hz, 3J = 3.1 Hz, 1H), 4.30 (ddd, 2J = 11.1 Hz, 3J = 5.6 Hz, 3J = 1.9 Hz, 1H), 6.12 (s, 2H), 6.30 (s, 1H), 6.67 (d, 3J = 7.6 Hz, 1H), 6.92–7.12 (m, 3H); 13C NMR (50 MHz, APT, CDCl3) δ 29.0 (t), 55.2 (q), 55.9 (2C, q), 65.3 (t), 70.2 (d), 91.4 (2C, d), 111.7 (s), 124.2 (d), 125.1 (d), 125.5 (d), 127.8 (d), 133.8 (s), 139.7 (s), 159.9 (2C, s), 161.2 (s).
1-(2,3,4,6-Tetramethoxyphenyl)isochroman (11b):
yield for Cu 190 mg (39%) and for Fe 85 mg (17%); white solid; mp 55–58 °C; TLC Rf (PE/EtOAc 5/1) = 0.19; GC/MS (EI+) m/z (rel intensity) 330 (M+, 100), 315 (23), 312 (14), 299 (99), 284 (10), 271 (18), 238 (16), 198 (24), 195 (16), 183 (23); HRMS (ESI+) exact mass calculated for C19H22O5 331.1540, found 331.1544 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 2.69 (d, 2J = 16.0 Hz, 1H), 3.46 (s, 3H), 3.14–3.35 (m, 1H), 3.69 (s, 3H), 3.78 (s, 3H), 3.88 (s, 3H), 3.91–4.02 (m, 1H), 4.32 (ddd, 2J = 11.1 Hz, 3J = 5.6 Hz, 3J = 1.7 Hz, 1H), 6.25 (s, 1H), 6.30 (s, 1H), 6.69 (d, 3J = 7.3 Hz, 1H), 6.96–7.17 (m, 3H); 13C NMR (50 MHz, APT, CDCl3) δ 28.9 (t), 55.9 (q), 56.3 (q), 60.5 (q), 60.8 (q), 65.3 (t), 70.8 (d), 92.7 (d), 116.8 (s), 124.5 (d), 125.4 (d), 125.6 (d), 128.1 (d), 133.8 (s), 136.7 (s), 139.6 (s), 153.5 (s), 153.8 (s), 154.5 (s).
1-(2,4-Dimethoxyphenyl)isochroman (11c):
yield for Cu 92 mg (23%) and for Fe 48 mg (12%); colorless oil; TLC Rf (PE/Et2O 1/1) = 0.28; GC/MS (EI+) m/z (rel intensity) 270 (M+, 65), 255 (100), 239 (43), 225 (16), 209 (16), 194 (13), 178 (8), 165 (24), 104 (10), 77 (8); HRMS (ESI+) exact mass calculated for C17H18O3 271.1329; found 271.1324 [M + H]+; 1H NMR (200 MHz, CDCl3) δ 2.84 (dt, 2J = 16.3 Hz, 3J = 4.4 Hz, 1H), 3.01–3.22 (m, 1H), 3.81 (s, 3H), 3.88 (s, 3H), 3.90–4.03 (m, 1H), 4.18 (dt, 2J = 11.0 Hz, 3J = 5.0 Hz, 1H), 6.22 (s, 1H), 6.43 (dd, 3J = 8.4 Hz, 4J = 2.4 Hz, 1H), 6.55 (d, 4J = 2.4 Hz, 1H), 6.80 (d, 3J = 7.4 Hz, 1H), 6.96 (d, 3J = 8.4 Hz, 1H), 7.03–7.21 (m, 3H); 13C NMR (50 MHz, APT, CDCl3) δ 28.8 (t), 55.3 (q), 55.6 (q), 63.3 (t), 72.2 (d), 98.4 (d), 104.2 (d), 123.3 (s), 125.8 (d), 126.2 (d), 126.6 (d), 128.5 (d), 130.7 (d), 134.2 (s), 137.9 (s), 158.6 (s), 160.5 (s)
1-(2,4,5-Trimethoxyphenyl)isochroman (11d):
yield for Cu 145 mg (32%) and for Fe 67 mg (15%); white solid; mp 46–48 °C; TLC Rf (PE/EtOAc 5/1) = 0.39; GC/MS (EI+) m/z (rel intensity) 300 (M+, 100), 285 (35), 269 (39), 257 (9), 239 (26), 225 (9), 208 (12), 195 (14), 168 (25), 153 (13); HRMS (ESI+) exact mass calculated for C18H20O4 301.1434, found 301.1436 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 2.80 (dt, 2J = 16.2 Hz, 3J = 3.2 Hz, 1H), 3.21 (ddd, 2J = 16.0 Hz, 3J = 10.0 Hz, 3J = 5.8 Hz, 1H), 3.73 (s, 3H), 3.89 (s, 3H), 3.93 (s, 3H), 3.99 (dt, 2J = 11.1 Hz, 3J = 3.8 Hz, 1H), 4.27 (ddd, 2J = 11.2 Hz, 3J = 5.6 Hz, 3J = 3.1 Hz, 1H), 6.20 (s, 1H), 6.61 (s, 1H), 6.66 (s, 1H), 6.78 (d, 3J = 7.6 Hz, 1H), 7.05–7.12 (m, 1H), 7.14–7.21 (m, 2H); 13C NMR (101 MHz, APT, CDCl3) δ 28.8 (t), 56.0 (q), 56.4 (q), 56.8 (q), 64.4 (t), 72.6 (d), 97.4 (d), 113.0 (d), 122.2 (s), 125.8 (d), 126.2 (d), 126.5 (d), 128.5 (d), 133.9 (s), 138.0 (s), 143.2 (s), 149.4 (s), 151.9 (s).
Acknowledgments
We acknowledge the Austrian Science Foundation (FWF, project P21202-N17) for financial support of this work.
Supporting Information Available
Text giving experimental procedures and analytical data and figures giving NMR spectra of compounds unknown in the literature. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Ohfune Y.; Shinada T. Eur. J. Org. Chem. 2005, 5127. [Google Scholar]
- a Farina V.; Reeves J. T.; Senanayake C. H.; Song J. J. Chem. Rev. 2006, 106, 2734. [DOI] [PubMed] [Google Scholar]; b Blaser H. U. Chem. Rev. 1992, 92, 935. [Google Scholar]
- a Chrzanowska M.; Rozwadowska M. D. Chem. Rev. 2004, 104, 3341. [DOI] [PubMed] [Google Scholar]; b Campos K. R. Chem. Soc. Rev. 2007, 36, 1069. [DOI] [PubMed] [Google Scholar]
- a Higuchi K.; Kawasaki T. Nat. Prod. Rep. 2007, 24, 843. [DOI] [PubMed] [Google Scholar]; b Kochanowska-Karamyan A. J.; Hamann M. T. Chem. Rev. 2010, 110, 4489. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Cang S.; Ohta S.; Chiba H.; Johdo O.; Nomura H.; Nagamatsu Y.; Yoshimoto A. J. Antibiot. 2001, 54, 304. [DOI] [PubMed] [Google Scholar]; d DeCuypere M.; Kalabokis V. N.; Hao R.; Schroeder D.; Miller D. D.; LeDoux M. S. J. Neurosci. Res. 2008, 86, 2543. [DOI] [PubMed] [Google Scholar]
- a Dalence-Guzman M. F.; Toftered J.; Oltner V. T.; Wensbo D.; Johansson M. H. Bioorg. Med. Chem. Lett. 2010, 20, 4999. [DOI] [PubMed] [Google Scholar]; b Ludwig M.; Hoesl C. E.; Hoefner G.; Wanner K. T. Eur. J. Med. Chem. 2006, 41, 1003. [DOI] [PubMed] [Google Scholar]; c Zhang Y. Tetrahedron Lett. 2005, 46, 6483. [Google Scholar]; d Yi Chae S.; Yun Sang Y. J. Am. Chem. Soc. 2005, 127, 17000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. D.; Williams R. M. Chem. Rev. 2002, 102, 1669. [DOI] [PubMed] [Google Scholar]
- Wang S.; Onaran M. B.; Seto C. T. Org. Lett. 2010, 12, 2690. [DOI] [PubMed] [Google Scholar]
- Cheng P.; Huang N.; Jiang Z.-Y.; Zhang Q.; Zheng Y.-T.; Chen J.-J.; Zhang X.-M.; Ma Y.-B. Bioorg. Med. Chem. Lett. 2008, 18, 2475. [DOI] [PubMed] [Google Scholar]
- Gao M.; Kong D.; Clearfield A.; Zheng Q.-H. Bioorg. Med. Chem. Lett. 2006, 16, 2229. [DOI] [PubMed] [Google Scholar]
- a Nicolaou K. C.; Bulger P. G.; Sarlah D. Angew. Chem., Int. Ed. 2005, 44, 4442. [DOI] [PubMed] [Google Scholar]; b Geissler H. Transition Met. Org. Synth. 1998, 1, 158. [Google Scholar]; c Fairlamb I. J. S. Annu. Rep. Prog. Chem., Sect. B: Org. Chem. 2006, 102, 50. [Google Scholar]; d Liu C.; Jin L.; Lei A. Synlett 2010, 2527. [Google Scholar]; e Negishi E.; de Meijere A.. Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: Hoboken, NJ, 2002; Vols. 1 and 2. [Google Scholar]; f de Meijere A.; Diederich F.. Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004; Vols. 1 and 2. [Google Scholar]
- a Shilov A. E.; Shul’pin G. B. Chem. Rev. 1997, 97, 2879. [DOI] [PubMed] [Google Scholar]; b Murai S., Ed. Activation of unreactive bonds and organic synthesis. Topics in Organometallic Chemistry; Springer: New York, 1999; Vol. 3, p 272. [Google Scholar]; c Miura M.; Nomura M. Top. Curr. Chem. 2002, 219, 211. [Google Scholar]; d Labinger J. A.; Bercaw J. E. Nature 2002, 417, 507. [DOI] [PubMed] [Google Scholar]; e Godula K.; Sames D. Science 2006, 312, 67. [DOI] [PubMed] [Google Scholar]; f Kakiuchi F.; Chatani N. Adv. Synth. Catal. 2003, 345, 1077. [Google Scholar]; g Dyker G., Ed. Handbook of C-H Transformations: Applications in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2005; Vols. 1 and 2. [Google Scholar]
- a Prokopcova H.; Bergman S. D.; Aelvoet K.; Smout V.; Herrebout W.; Van der Veken B.; Meerpoel L.; Maes B. U. W. Chem. Eur. J. 2010, 16, 13063. [DOI] [PubMed] [Google Scholar]; b Pastine S. J.; Gribkov D. V.; Sames D. J. Am. Chem. Soc. 2006, 128, 14220. [DOI] [PubMed] [Google Scholar]
- a Chen X.; Engle K. M.; Wang D.-H.; Yu J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fuchibe K.; Kaneko T.; Mori K.; Akiyama T. Angew. Chem., Int. Ed. 2009, 48, 8070. [DOI] [PubMed] [Google Scholar]; c Davies H. M. L.; Nikolai J. Org Biomol Chem 2005, 3, 4176. [DOI] [PubMed] [Google Scholar]; d Bellina F.; Rossi R. Chem. Rev. 2010, 110, 1082. [DOI] [PubMed] [Google Scholar]
- For recent reviews see: ; a Zeng T.; Song G.; Moores A.; Li C.-J. Synlett 2010, 2002. [Google Scholar]; b Li C.-J. Acc. Chem. Res. 2009, 42, 335. [DOI] [PubMed] [Google Scholar]; c Yeung C. S.; Dong V. M. Chem. Rev. 2011, 111, 1215. [DOI] [PubMed] [Google Scholar]; d Li C.-J.; Yoo W.-J. Top. Curr. Chem. 2010, 292, 281. [DOI] [PubMed] [Google Scholar]; e Scheuermann C. J. Chem. Asian J. 2010, 5, 436. [DOI] [PubMed] [Google Scholar]; f Guo X.; Li Z.; Li C. Huaxue Jinzhan 2010, 22, 1434. [Google Scholar]; g Ackermann L.; Vicente R.; Kapdi A. R. Angew. Chem., Int. Ed. 2009, 48, 9792. [DOI] [PubMed] [Google Scholar]; For recent contributions to the field see: ; h Bugaut X.; Glorius F. Angew. Chem., Int. Ed. 2011, 50, 7479. [DOI] [PubMed] [Google Scholar]; i Xiong T.; Li Y.; Bi X.; Lv Y.; Zhang Q. Angew. Chem., Int. Ed. 2011, 50, 7140. [DOI] [PubMed] [Google Scholar]; j Murarka S.; Studer A. Org. Lett. 2011, 13, 2746. [DOI] [PubMed] [Google Scholar]; k Chen L.; Shi E.; Liu Z.; Chen S.; Wei W.; Li H.; Xu K.; Wan X. Chem. Eur. J. 2011, 17, 4085. [DOI] [PubMed] [Google Scholar]; l Han W.; Mayer P.; Ofial A. R. Angew. Chem., Int. Ed. 2011, 50, 2178. [DOI] [PubMed] [Google Scholar]; m Xie J.; Huang Z.-Z. Angew. Chem., Int. Ed. 2010, 49, 10181. [DOI] [PubMed] [Google Scholar]
- Murahashi S.-I.; Nakae T.; Terai H.; Komiya N. J. Am. Chem. Soc. 2008, 130, 11005. [DOI] [PubMed] [Google Scholar]
- a Li Z.; Li C.-J. J. Am. Chem. Soc. 2004, 126, 11810. [DOI] [PubMed] [Google Scholar]; b Li Z.; Li C.-J. Eur. J. Org. Chem. 2005, 3173. [Google Scholar]; c Li Z.; Li C.-J. J. Am. Chem. Soc. 2005, 127, 3672. [DOI] [PubMed] [Google Scholar]; d Li Z.; MacLeod P. D.; Li C.-J. Tetrahedron: Asymmetry 2006, 17, 590. [Google Scholar]; e Li Z.; Yu R.; Li H. Angew. Chem., Int. Ed. 2008, 47, 7497. [DOI] [PubMed] [Google Scholar]
- a Liu P.; Zhou C.-Y.; Xiang S.; Che C.-M. Chem. Commun. 2010, 46, 2739. [DOI] [PubMed] [Google Scholar]; b Sureshkumar D.; Sud A.; Klussmann M. Synlett 2009, 1558. [Google Scholar]; c Yang F.; Li J.; Xie J.; Huang Z.-Z. Org. Lett. 2010, 12, 5214. [DOI] [PubMed] [Google Scholar]; d Hari D.-P.; König B. Org. Lett. 2011, 13, 3852. [DOI] [PubMed] [Google Scholar]; e Boess E.; Sureshkumar D.; Sud A.; Wirtz C.; Fares C.; Klussmann M. J. Am. Chem. Soc. 2011, 133, 8106. [DOI] [PubMed] [Google Scholar]
- Li Z.; Li C.-J. J. Am. Chem. Soc. 2005, 127, 6968. [DOI] [PubMed] [Google Scholar]
- a Girard N.; Hurvois J.-P.; Toupet L.; Moinet C. Synth. Commun. 2005, 35, 711. [Google Scholar]; b Girard N.; Gautier C.; Malassene R.; Hurvois J.-P.; Moinet C.; Toupet L. Synlett 2004, 2005. [Google Scholar]; c Girard N.; Hurvois J.-P. Tetrahedron Lett. 2007, 48, 4097. [Google Scholar]
- a Chincholkar P. M.; Kale A. S.; Gumaste V. K.; Deshmukh A. R. A. S. Tetrahedron 2009, 65, 2605. [Google Scholar]; b Abraham C. J.; Paull D. H.; Dogo-Isonagie C.; Lectka T. Synlett 2009, 1651. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Giera D. S.; Sickert M.; Schneider C. Synthesis 2009, 3797. [Google Scholar]; d Taniyama D.; Hasegawa M.; Tomioka K. Tetrahedron: Asymmetry 1999, 10, 221. [Google Scholar]
- a Ullmann F. Ber. Dtsch. Chem. Ges. 1903, 36, 2382. [Google Scholar]; b Beletskaya I. P.; Cheprakov A. V. Coord. Chem. Rev. 2004, 248, 2337. [Google Scholar]; c Hassan J.; Sevignon M.; Gozzi C.; Schulz E.; Lemaire M. Chem. Rev. 2002, 102, 1359. [DOI] [PubMed] [Google Scholar]
- a Dollimore D.Handbook of Copper Compounds and Applications Marcel Dekker: New York, 1997; p 231. [Google Scholar]; b Nandivada H.; Lahann J.. Click Chemistry for Biotechnology and Materials Science; Wiley: New York, 2009; p 291. [Google Scholar]; c Daugulis O.; Do H.-Q.; Shabashov D. Acc. Chem. Res. 2009, 42, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhang M. Appl. Organomet. Chem. 2010, 24, 269. [Google Scholar]
- a Volla C. M. R.; Vogel P. Org. Lett. 2009, 11, 1701. [DOI] [PubMed] [Google Scholar]; b Zhang S.-Y.; Tu Y.-Q.; Fan C.-A.; Zhang F.-M.; Shi L. Angew. Chem., Int. Ed. 2009, 48, 8761. [DOI] [PubMed] [Google Scholar]; c Li P.; Zhang Y.; Wang L. Chem. Eur. J. 2009, 15, 2045. [DOI] [PubMed] [Google Scholar]; d Han W.; Ofial A. R. Chem. Commun. 2009, 6023. [DOI] [PubMed] [Google Scholar]; e Czaplik W. M.; Mayer M.; Cvengros J.; Jacobi von Wangelin A. ChemSusChem 2009, 2, 396. [DOI] [PubMed] [Google Scholar]; f Sherry B. D.; Fuerstner A. Acc. Chem. Res. 2008, 41, 1500. [DOI] [PubMed] [Google Scholar]; g Bolm C.; Legros J.; Le Paih J.; Zani L. Chem. Rev. 2004, 104, 6217. [DOI] [PubMed] [Google Scholar]; h Correa A.; Garcia Mancheno O.; Bolm C. Chem. Soc. Rev. 2008, 37, 1108. [DOI] [PubMed] [Google Scholar]; i Maiti S.; Biswas S.; Jana U. J. Org. Chem. 2010, 75, 1674. [DOI] [PubMed] [Google Scholar]; j Sun C.-L.; Li B.-J.; Shi Z.-J. Chem. Rev. 2011, 111, 1293. [DOI] [PubMed] [Google Scholar]
- Ghobrial M.; Harhammer K.; Mihovilovic M. D.; Schnürch M. Chem. Commun. 2010, 46, 8836. [DOI] [PubMed] [Google Scholar]
- a Shu X.-Z.; Xia X.-F.; Yang Y.-F.; Ji K.-G.; Liu X.-Y.; Liang Y.-M. J. Org. Chem. 2009, 74, 7464. [DOI] [PubMed] [Google Scholar]; b Morphy J. R.; Rankovic Z.; York M. Tetrahedron Lett. 2001, 42, 7509. [Google Scholar]; c Al-Hiari Y. M.; Bennett S. J.; Cox B.; Davies R. J.; Khalaf A. I.; Waigh R. D.; Worsley A. J. J. Heterocycl. Chem. 2005, 42, 647. [Google Scholar]; d Alonso E.; Ramon D. J.; Yus M. Tetrahedron 1997, 53, 14355. [Google Scholar]; e Berglund M.; Dalence-Guzman M. F.; Skogvall S.; Sterner O. Bioorg. Med. Chem. 2008, 16, 2513. [DOI] [PubMed] [Google Scholar]; f Siu J.; Baxendale I. R.; Lewthwaite R. A.; Ley S. V. Org. Biomol. Chem. 2005, 3, 3140. [DOI] [PubMed] [Google Scholar]; g Aubert C.; Huard-Perrio C.; Lasne M.-C. J. Chem. Soc., Perkin Trans. 1 1997, 2837. [Google Scholar]; h Ludwig M.; Hoesl C. E.; Hoefner G.; Wanner K. T. Eur. J. Med. Chem. 2006, 41, 1003. [DOI] [PubMed] [Google Scholar]; i Proctor G. R.; Thomson R. H. J. Chem. Soc. 1957, 2312. [Google Scholar]; m Toma G.; Fujita K.-i.; Yamaguchi R. Eur. J. Org. Chem. 2009, 4586. [Google Scholar]; j Dubs C.; Hamashima Y.; Sasamoto N.; Seidel T. M.; Suzuki S.; Hashizume D.; Sodeoka M. J. Org. Chem. 2008, 73, 5859. [DOI] [PubMed] [Google Scholar]; k De Kimpe N.; Keppens M. Tetrahedron 1996, 52, 3705. [Google Scholar]
- a Jana C. K.; Studer A. Angew. Chem., Int. Ed. 2007, 46, 6542. [DOI] [PubMed] [Google Scholar]; b Glorius F.; Spielkamp N.; Holle S.; Goddard R.; Lehmann C. W. Angew. Chem., Int. Ed. 2004, 43, 2850. [DOI] [PubMed] [Google Scholar]; c Jana C. K.; Grimme S.; Studer A. Chem. Eur. J. 2009, 15, 9078. [DOI] [PubMed] [Google Scholar]
- Nakanishi M.; Bolm C. Adv. Synth. Catal. 2007, 349, 861. [Google Scholar]
- Huang L.; Niu T.; Wu J.; Zhang Y. J. Org. Chem. 2011, 76, 1759. [DOI] [PubMed] [Google Scholar]
- Li Z.; Bohle S.; Li C.-J. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shih K.-C.; Angelici R. J. J. Org. Chem. 1996, 61, 7784. [DOI] [PubMed] [Google Scholar]; b Gast R.; Glokler J.; Hoxter M.; Kiesz M.; Frank R.; Tegge W. Anal. Biochem. 1999, 276, 227. [DOI] [PubMed] [Google Scholar]; c Goswami S.; Chakrabarty R. Chem. Lett. 2010, 39, 100. [Google Scholar]
- Dharanipragada R.; Ferguson S. B.; Diederich F. J. Am. Chem. Soc. 1988, 110, 1679. [Google Scholar]
- Chen B.-C.; Skoumbourdis A. P.; Guo P.; Bednarz M. S.; Kocy O. R.; Sundeen J. E.; Vite G. D. J. Org. Chem. 1999, 64, 9294. [Google Scholar]
- a Thaqi A.; McCluskey A.; Scott J. L. Tetrahedron Lett. 2008, 49, 6962. [Google Scholar]; b Choy J.; Jaime-Figueroa S.; Jiang L.; Wagner P. Synth. Commun. 2008, 38, 3840. [Google Scholar]; c Siro J. G.; Martin J.; Garcia-Navio J. L.; Remuinan M. J.; Vaquero J. J.. Synlett 1998, 147. [Google Scholar]
- a Choi H.; Doyle M. P. Chem. Commun. 2007, 745. [DOI] [PubMed] [Google Scholar]; b Murahashi S.; Naota T.; Taki H. J. Chem. Soc., Chem. Commun. 1985, 613. [Google Scholar]
- a Barba-Behrens N.; Mutio-Rico A. M.; Joseph-Nathan P.; Contreras R. Polyhedron 1991, 10, 1333. [Google Scholar]; b Collamati I.; Ercolani C.; Rossi G. Gazz. Chim. Ital. 1977, 107, 473. [Google Scholar]; c Ge C. H.; Zhang X. D.; Guan W.; Liu Q. T. Chin. Chem. Lett. 2005, 16, 1255. [Google Scholar]
- Guo X.; Pan S.; Liu J.; Li Z. J. Org. Chem. 2009, 74, 8848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.