Abstract
This work reports the novel microwave-assisted solvothermal synthesis and the structural, topographic, spectroscopic characterization of NaYF4:Yb,Er upconversion nanoparticles as well as their application in the labeling of HeLa cells. The nanoparticles were prepared in ethylene glycol, with rare earth acetates as precursor, NH4F and NaCl as the fluorine and sodium source. X-ray diffraction, transmission electron microscopy and luminescence spectroscopy were applied to characterize the nanoparticles. Experimental results showed that the microwave-assisted solvothermal method is an effective approach to create highly crystalline, strongly luminescent UCNPs at a lower temperature (160 °C) and within a significantly shortened reaction time (only 1 h) compared to the traditional methods. The effect of fluorine source on the optical properties of the UCNPs was investigated by using NH4F, NH4HF2, NaF, and 1-butyl-3-methyl tetrafluoroborate (BmimBF4) as a different fluorine source, respectively, while NH4F showed to be the best one making the luminescent intensity increase at least two orders of mignitude. The UCNPs with four different colors (green, yellow, orange and cyan) were successfully obtained. After being modified with amino groups and coupled with CEA-8 antibody, the obtained nanoparticles were successfully applied in the specific fluorescent immuno-labeling and imaging of HeLa cells to further verify their function as a marker in immuno-labelling.
Keywords: microwave, solvothermal, rare-earth, upconversion, cell imaging
1. Introduction
Rare-earth doped upconversion nanoparticles (UCNPs), which can convert near-infrared light (NIR) into visible light,1–3 have attracted great attention since they were proposed for bioassays at the beginning of the 1990s.4,5 They have many advantages, such as narrow emission peak, large Stokes shifts, long lifetimes, high quantum yields, superior photostability, and low toxicity.6–9 In particular, near infrared (NIR) light used for the excitation of UCNPs is safe to the human body, and can penetrate biological tissues with a high depth.10–12 In addition, the low energy radiation can avoid autofluorescence from biological samples. Thus, the signal-to-noise ratio and sensitivity of the detection can be improved.13 Now, rare-earth doped UCNPs have been established as a new class of bio-probes and widely used in cell labeling,14,15 multimodal bioimaging,16,17 photodynamic therapy,18,19 and drug delivery.20
How to fabricate the monodisperse high-quality nanocrystals with well-defined shapes, single crystallinity and pure phase, is the key for next applications.21 In the past decades, great efforts have been dedicated to the synthesis of rare-earth doped UCNPs. So far, the representative and routinely utilized chemical synthesis methods include coprecipitation, thermal decomposition, hydro/solvothermal, and microemulsion method.22 Coprecipitation is one of the most convenient techniques and does not require costly equipment and harsh operating conditions, but the synthesized nanocrystals usually need further calcination or postannealing process, during which the nanocrystals will aggregate and grow up, making it not suitable for the applications in biomedical field.23 Thermal decomposition method was first reported for the preparation of LaF3 nanoparticles, and the procedure was performed through thermal decomposition of metal trifluoroacetate or other toxic precursors at high temperature (300 °C), waterless and oxygen free conditions.24 Although it can well control the shape and size of nanoparticles, the stringent conditions still require careful handling. Hydro/solvothermal method can create controlled size and highly crystalline nanoparticles at a much lower temperature than thermal decomposition method,25 but the synthetic procedure generally requires relatively longer time. Microemulsion method can easily obtain crystals at the nanometer range in the solution composed of water, oil and surfactant, but still has some problems, such as poor dispersion of products and low yield. Above all, looking for faster and more efficient synthetic methods of UCNPs is still an active research hotspot.
Compared to the aforementioned methods, microwave-assisted methods can achieve fast and uniform heating, and are both eco-friendly and energy efficient. There are some reports on the synthesis of rare earth doped materials, such as LaPO4:Ce,Tb nanoparticles, 26 and NaYF4:Yb3+,Tm3+ microtubes by microwave hydrothermal.27 All of them have some drawbacks, such as the formation of tube-shape product of large size at micrometer level or the use of stringent conditions (high temperature, oxygen free, etc).28,29 It is known that β-NaYF4 is one of the most efficient host materials, especially for the systems doped with Yb-Er and Yb-Tm.30,31 Here, we report a novel microwave-assisted solvothermal method for the preparation of NaYF4:Yb,Er UCNPs using rare earth acetates, NH4F and NaCl as the rare earth, fluorine and sodium source, respectively. In addition, we studied the effect of fluorine source on the optical properties of the UCNPs by using NH4F, NH4HF2, NaF, and 1-butyl-3-methyl tetrafluoroborate (BmimBF4) as a different fluorine source, respectively. The obtained nanocrystals were characterized by transmission electron microscopy (TEM), X-ray diffraction (XRD) and luminescence spectroscopy. Then, a typical stöber method was used for the surface amino modification of the NaYF4:Yb,Er UCNPs. Finally, the modified UCNPs were covalently coupled with CEA-8 antibody, and used as a bio-probe for the labeling and imaging of HeLa cells. From the whole experiment, it was found that using microwave-assisted solvothermal method, the high-quality UCNPs can be synthesized successfully at a lower temperature and within a much shorter time than conventional solvothermal method, and the as-prepared nanocrystals exhibit excellent upconversion properties, which meet the demands for the biological, medical and clinical applications.
2. Experimental Section
2.1 Materials and Chemicals
Rare earth acetates including yttrium acetate (Y(CH3COO)3·4H2O), ytterbium acetate (Yb(CH3COO)3·4H2O), erbium acetate (Er(CH3COO)3·6H2O), were of 99% purity, purchased from Ji Nan Heng Hua Company of China. 1-butyl-3-methylimidazolium tetrafluoroborate (C8H15N2BF4), ammonium fluoride (NH4F), ammonium bifluoride (NH4HF2), sodium fluoride (NaF), ethanol, isopropanol, tetraethyl orthosilicate (TEOS), 3-aminopropyltrimethoxysilane (APTES), ammonia (NH3·H2O) and bovine serum albumin (BSA) were of analytical grade, supplied by the National Medicines Corporation Ltd. of China in Shenyang. Primary antibody (Ab) rabbit anti-CEA8 was purchased from Biosynthesis Biotechnology Co. Ltd. (Beijing, China). N-hydroxysuccinimide (NHS, 98%) and N-Ethyl-N′-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDC, 98%) were obtained from Acros (USA). All of the reagents were directly used without further purification. HeLa cells were supplied by China Medical University. Triple-distilled water was used throughout the experiments.
2.2 Instrumentation
The MD6C-4H Microwave Digestion System (Ying An Mei Cheng Company, Beijing China) was used for the synthesis of the UCNPs. The morphology and size of the synthesized nanoparticles were characterized by a TECNAI G2 20 transmission electron microscope (TEM, FEI Ltd., USA), using an accelerating voltage of 200 kV. Powder XRD measurements were observed at room temperature on an X’Pert Pro diffractometer (Panalytical Co., Holland) by using CuKα (1.5406 Å) radiation. The scanning rate was 8 °/min, range from 10 to 80°. Fluorescent spectra were measured on a LS-55 Luminescence Spectrometer (Perkin-Elmer Co., USA) with an external 980 nm laser as the excitation source. FT-IR spectrum characterization of the modified nanoparticles was obtained by a Spectrum One spectrometer (Perkin Elmer Co., USA). Images of cell labeling were taken on a Lecia DMIL inverted fluorescence microscope.
2.3 Microwave-assisted synthesis of NaYF4:Yb,Er UCNPs
The synthesis of NaYF4:Yb,Er nanoparticles was carried out in ethylene glycol, rare earth acetate was used as a rare earth precursor, NH4F (or NH4HF2, NaF, and BmimBF4 in the optimization experiments) and NaCl were used as the source of fluorine and sodium, respectively. First, a mixed rare earth acetates of 0.2636 g of Y(CH3COO)3·4H2O, 0.0845 g of Yb(CH3COO)3·4H2O and 0.0090 g of Er(CH3COO)3·6H2O was dissolved in a solution composed of 10 mL water and 10 mL ethylene glycol. Then, 0.1852 g of NH4F (or equivalent of NH4HF2, NaF or BmimBF4 in the optimization experiments) and 0.0585 g of NaCl were added to the mixture. The mixture was stirred under sonication to form a homogeneous solution, then transferred to an autoclave which was then sealed and treated at 160 °C for 1 h. After reaction, the autoclave was first cooled to room temperature. Then, the nanoparticles were collected with ethanol by centrifugation at 8000 rpm/min for 5 min. The obtained precipitate was washed with ethanol and water for three times by centrifugation, dried at 60 °C. The obtained white powder was the as-prepared NaYF4:Yb,Er nanoparticles.
2.4 Aminated modification of NaYF4:Yb,Er UCNPs
The modification of as-prepared NPs was carried out according to the approach previously reported by our group.32 First, 20 mg of nanoparticles were dissolved in 70 mL isopropanol, and sonicated for 40 min to form a uniform colloid solution, to which 20 mL water and 2.5 mL ammonia were added. After stirred at 35 °C for 10 min, 25 μL TEOS and 200 μL APTES was dropwise added. After the reaction continued for 5 h, the final colloid solution was centrifuged at 9000 rpm for 10 min. The precipitates were collected, washed with ethanol twice, and dried at 60 °C. The obtained powder was amino-modified NaYF4:Yb,Er nanoparticles.
2.5 Cell labeling and imaging
The modified nanoparticles were first coupled with CEA-8 antibody. Typically, 300 μL of 2.0 mg/mL NaYF4:Yb,Er colloid solution was mixed with 200 μL of 0.1 mg/mL CEA-8 antibody solution. Then 50 μL of 0.2 mg/mL NHS and 100 μL of 0.2 mg/mL EDC were added, leading to the covalent binding reaction between the UCNPs and the antibody. After incubation at 37 °C for 2 h, the antibody coupled nanoparticles were centrifuged, washed with PBS twice, and stored at 4 °C for further use.
The HeLa cells were cultured on glass chamber slides in RPMI 1640 media containing 10% fetal bovine serum and 1% penicillin/streptomycin overnight in a culture box. Next, the cells were washed with PBS for three times and blocked in PBS containing 1% BSA for 20 min at 4 °C. The cells were finally incubated with CEA-8 antibody coupled NaYF4:Yb,Er UCNPs at 37 °C for 2.0 h. In addition, HeLa cells incubated with NPs without linking with antibody were as the control experiment. Before imaging, the cells were washed with PBS for three times, and then placed on an inverted fluorescent microscope (Leica DMIL) equipped with a Nikon digital camera. A 980 nm laser was used for excitation during imaging.
3. Results and Discussion
3.1 Synthesis and Characterization of NaYF4:Yb,Er UCNPs
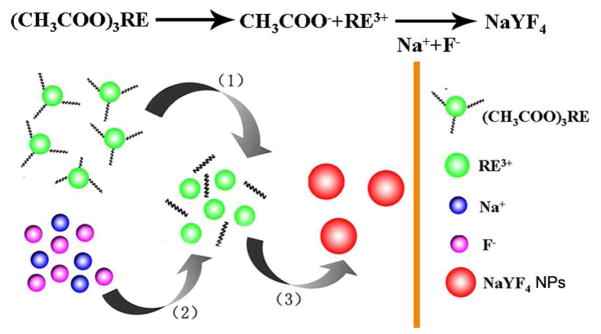
In this work, rare earth acetate was firstly introduced as the precursor for the synthesis of NaYF4:Yb,Er nanoparticles, via a microwave-assisted solvothermal method. Mechanism for the reaction was shown in Scheme 1. The whole process can be divided into three steps: (i) rare earth acetate, NH4F and NaCl were mixed together in ethylene glycol. When the temperature was elevated, the precursor decomposed slowly, so rare earth ions were released from the precursor; (ii) the free rare earth ions encountered and reacted with Na and F ions to form tiny particles; (iii) the tiny particles gradually grew up with the increase of reaction time. Herein, during the synthesis process, the precursor controlled the release of rare earth ions and showed important effect on the nanoparticle formation, so rare earth acetate can lower reaction speed and plays an important role for the particle-size control. Among most method for the synthesis of UCNPs, the NPs were usually prepared at high temperature (300 °C) or using very long time (more than 12 hours for hydrothermal and more than 20 hours for solvothermal method), the synthsis was condition stringent and time consumed. In this study, with the assist of microwave heating, the synthetic process was carried out at a relative lower temperture (160 °C) and a significantly shortened reaction time (only 1 h), the efficiency of the reaction was also improved greatly.
Scheme 1.
Mechanism for the synthesis of NaYF4 UCNPs by microwave-assited solvothermal method.
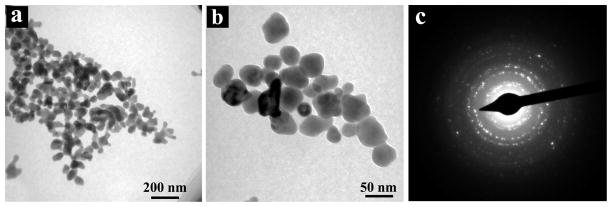
Figure 1 shows the TEM images and selected area electron diffraction (SAED) pattern of NaYF4:Yb,Er UCNPs synthesized at 160 °C for 1 h. Among them, Figure 1(a) and (b) are TEM images of NaYF4:Yb,Er UCNPs with different magnifications. As can be seen from the images, the NaYF4 UCNPs are nearly ellipsoid or spherical, well dispersed, and with the particle size of about 35–45 nm. Because the size of synthesized nanoparticles is comparable to that of biological macromolecules such as proteins, it can be used as biological markers to label the biological samples. Figure 1(c) is the SAED pattern for NaYF4:Yb,Er nanoparticles. It can be seen that there is a series of clear electron diffraction rings, suggesting that the as-prepared nanoparticles are well crystallized and possess a good polycrystalline structure.
Figure 1.
TEM images (a and b) and SAED pattern (c) of NaYF4:Yb,Er NPs.
The sample was next examined by XRD for observation of their crystal structures (Figure 2). Compared with the standard pattern, it can be seen from the figure that diffraction peaks of the prepared NaYF4:Yb,Er UCNPs can be indexed by considering the presence of both hexagonal β-phase (JCPDs No. 00-028-1192) and cubic α-phase NaYF4 (JCPDs No. 01-077-2042) listed in Figure 2(b), indicating that the products are a mixture of hexagonal and cubic crystals. The size of as-synthesized NaYF4:Yb,Er UCNPs could be calculated approximately from Debye-Scherrer equation (D = Kλ/βcosθ), where λ is the wavelength of X-ray radiation (Cu Kα, 1.5406 Ǻ), θ is the Bragg diffraction angle, K is normally equal to 0.89 and β is FWHM of diffraction peak. By this calculation, the average size of NaYF4:Yb,Er UCNPs was about 40.9 nm, in accordance with the TEM result in Figure 2.
Figure 2.
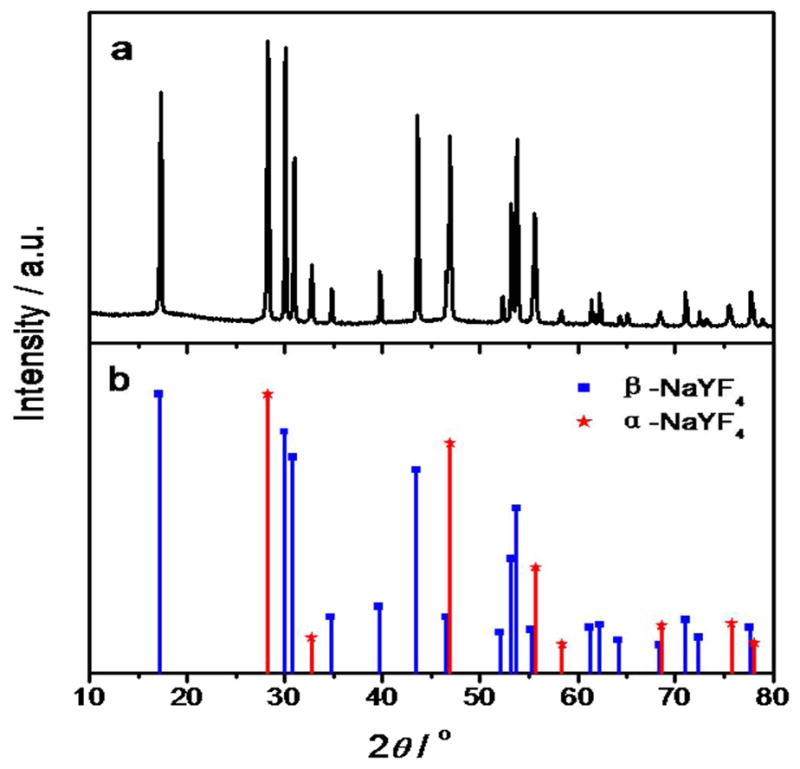
XRD pattern of as-prepared NaYF4:Yb,Er NPs (a) and standard patterns (b).
The NaYF4:Yb,Er nanoparticles (2 mg) were dispersed in water under sonication to form a homogenous solution, then their upconversion fluorescent spectra were characterized on LS-55 fluorescence spectrometer and the photos were taken by a digital camera under 980 nm excitation (Figure 3). The fluorescent spectrum shows that there are three distinct peaks located at 523, 543 and 653 nm separately. The three peaks are typical upconversion emission of Er3+, corresponding to 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transition, respectively,33 which can be attributed to the two-photon processes.34 Among them, the peak located at 543 nm is the strongest, so the nanoparticles exhibit bright green fluorescence (Figure 3, inset).
Figure 3.
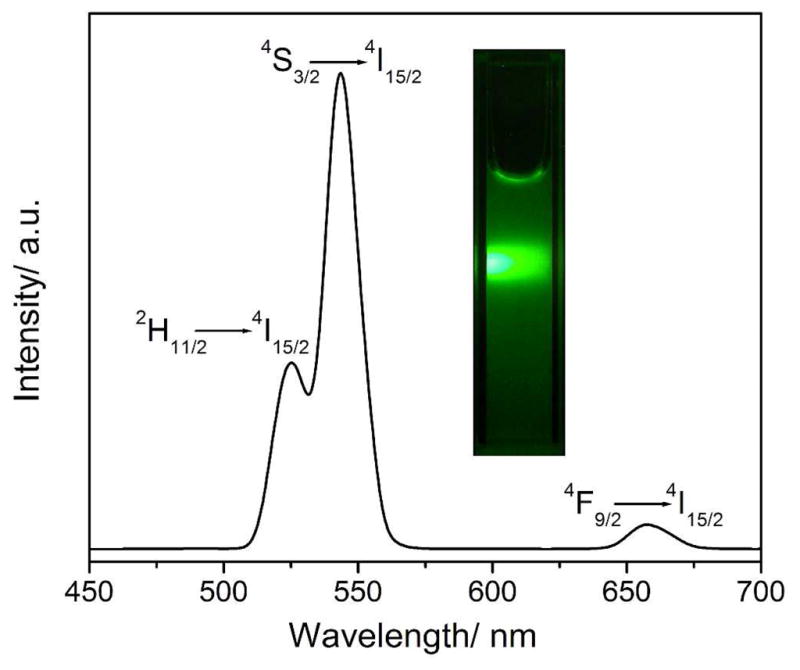
The fluorescence spectrum and digital photo of NaYF4:Yb,Er NPs excited at 980 nm.
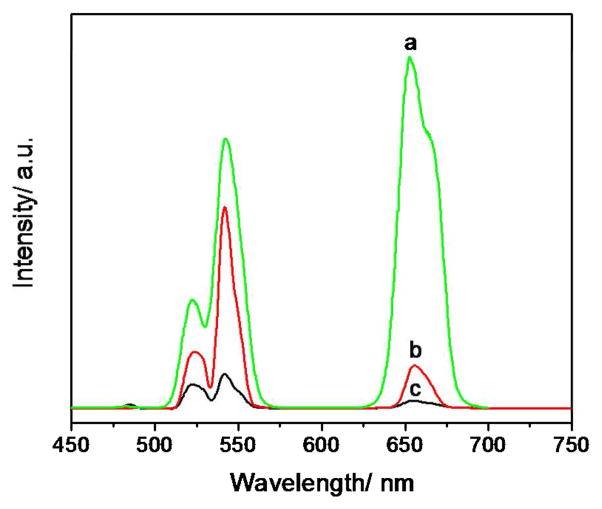
During the synthesis, rare earth ions were first released from the precursor, then reacted with Na and F ions and finally the NaYF4:Yb,Er nanoparticles were formed. The precursor is important in that it can affect the reaction rates and have obvious influence on the UC fluorescence properties of nanoparticles. Here, rare earth acetate, stearate and thioglycollate were used, respectively, as different choices of the rare earth precursor. Luminescence spectra of nanoparticles synthesized with these three precursors were presented in Figure 4. It is obvious that the luminescence intensity of nanoparticles synthesized with rare earth acetate was the strongest which was nearly 10 times more than that synthesized with rare earth stearate.
Figure 4.
The fluorescence spectra of NaYF4:Yb,Er nanoparticles synthesized with (a) rare earth acetate, (b) rare earth stearate and (c) rare earth thioglycollate as a rare earth precursor.
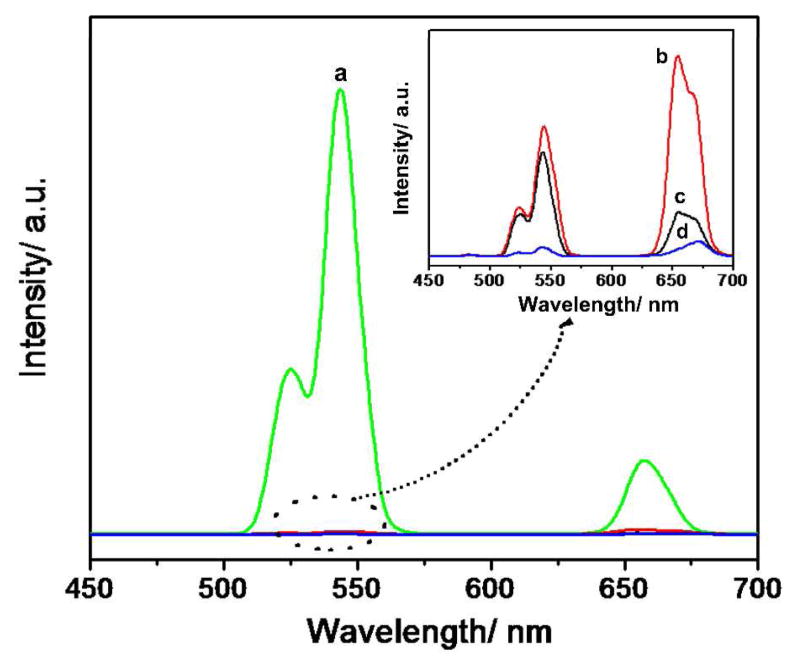
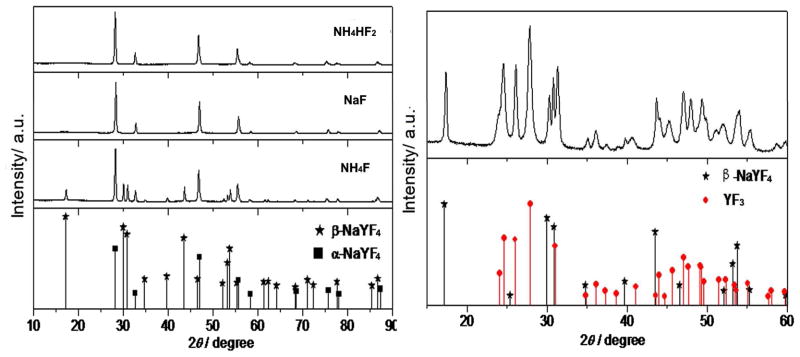
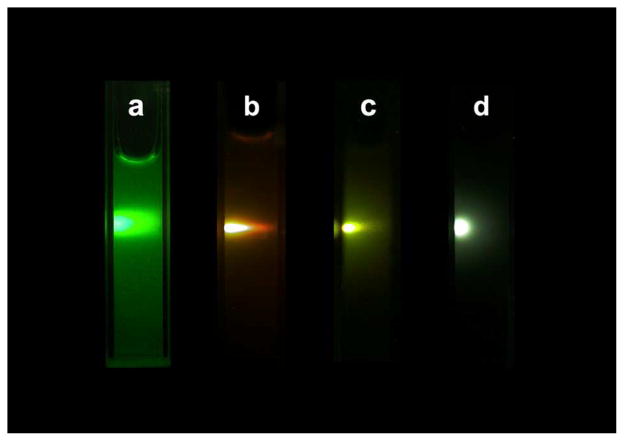
The effect of fluorine source on the nanoparticles was investigated by using NH4F, NH4HF2, NaF, and 1-butyl-3-methyl tetrafluoroborate (BmimBF4) as a fluorine source, respectively. The nanoparticles were synthesized at 160 °C for 1 h, with one of the different fluorine sources mentioned above. UC fluorescence spectra of the corresponding products are shown in Figure 5. It can be seen that the fluorescence intensity of nanoparticles obtained with NH4F was nearly 100 times more than that synthesized with NaF. It can be concluded that F sources exhibited obvious effects on upconversion properties of the synthesized NPs, this phenomenon can be illustrated by the change of crystal structure. As shown in Figure 6, it can be seen that the NPs synthesized with NH4F possessed a mix structure of β-NaYF4 (hexagonal, JCPDS 00-016-0334) and α-NaYF4 (cubic, JCPDS 01-077-2042). The NPs synthesized with NaF and NH4HF2 were pure α-NaYF4 nanocrystals. While, the structure of NPs synthesized with BmimBF4 (Figure 6, right) was mainly YF3 (Orthorhombic, JCPDS 01-074-0911) nanocrystals with very little β-NaYF4. Among them, β-NaYF4 is the most efficient host materials, so fluorescence intensity of nanoparticles obtained with NH4F was the strongest. Figure 7 represents the digital photos of nanoparticles synthesized with NH4F, NaF, BmimBF4 and NH4HF2, respectively. As can be seen from the figure, under the same 980 nm excitation, the nanoparticles synthesized with NH4F as the fluorine source exhibit very bright green fluorescence. When NaF and NH4HF2 were used as fluoride source, the synthesized nanoparticles emit orange and yellow fluorescence, respectively, but the light emission is not so strong. In addition, the fluorescent intensity of nanoparticles synthesized with BmimBF4 is very weak.
Figure 5.
The fluorescence spectra of NPs synthesized with (a) NH4F, (b) NaF, (c) NH4HF2 and (d) BmimBF4 as F source.
Figure 6.
The XRD pattern of NPs synthesized with different F source.
Figure 7.
The digital photo of NPs synthesized with (a) NH4F, (b) NaF, (c) NH4HF2 and (d) BmimBF4 as F source.
3.2 Surface modification of NaYF4:Yb,Er UCNPs
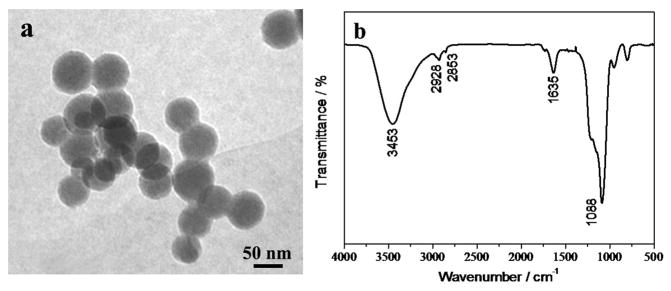
The as-synthesized nanoparticles are bare in that there are no active groups existing on the surface. Hence, they need to be functionalized for the next application, and the typical stöber method can be used for the surface modification.35 The nanoparticles were first coated with a layer of SiO2, and then aminated by basic hydrolysis of an amino-terminated silane coupling agent. The modified nanoparticles were characterized by TEM and FT-IR spectrometer. Figure 8(a) is the TEM image of the functionalized NaYF4:Yb,Er nanoparticles, it clearly shows that the nanoparticles have a core-shell structure. There is a uniform silica layer capped on the surface, with the thickness of approximately 7–10 nm. Figure 8(b) is the FT-IR spectrum of the modified nanoparticles. According to the spectrum, it is evident that after modification, a significant absorption peak can be found at 1088 cm−1 corresponding to Si-O stretching vibration, which proves the existence of SiO2 shell on the surface of nanoparticles. Also, the strong absorption peak at 3441 cm−1 can be attributed to N-H stretching vibration, and the peak at 1635 cm−1 is bending vibration absorption for the N-H bond. These results confirm the successful coating of silica and amino functionalization of NaYF4:Yb,Er UCNPs.
Figure 8.
TEM (a) and FT-IR spectra (b) of NaYF4:Yb,Er NPs after surface modification.
3.3 Applications of UCNPs in HeLa cells imaging
As described above, the synthesized nanoparticles were modified through stöber method, and there are amino groups on their surface, which can be used to conjugate with biomolecules. To verify their upconversion optical properties, the nanoparticles were conjugated with CEA-8 antibody under the activation of NHS and EDC, according to our previous report,36 and used as the immuno-probe for the labeling of HeLa cells. When incubated with HeLa cells, the CEA-8 antibody can specifically recognize the CEA-8 antigen on the cell surface, leading to the labeling of the cell surface by the nanoparticles linked with antibody which can emit bright upconversion fluorescence at 980 nm excitation.
Three groups of HeLa cells were used in this experiment, and one group of cells were used as blank, the other two were incubated with CEA-8 antibody coupled nanoparticles and bare nanoparticles, respectively. After incubation, the cells were first washed three times with PBS buffer solution to remove those unbound nanoparticles, and then placed on an inverted fluorescence microscope for observation. The fluorescence images were taken with an external 980 nm laser as the excitation source. The results for fluorescence imaging were shown in Figure 9. From Figure 9(a), we can see that HeLa cells’ surface showed a bright green fluorescence in dark field. Cells incubated with nanoparticles unlinked with the antibody did not show any fluorescence as shown in Figure 9(b). The results indicated that there was nearly no non-specific adsorption phenomenon, suggesting that our method can achieve specific labeling of HeLa cells. More importantly, the single HeLa cells in Figure 9(c) did not emit any fluorescence under 980 nm laser, indicating that the infrared light can effectively avoid autofluorescence from biological samples, which is superior to other imaging probes in bio-applications.
Figure 9.
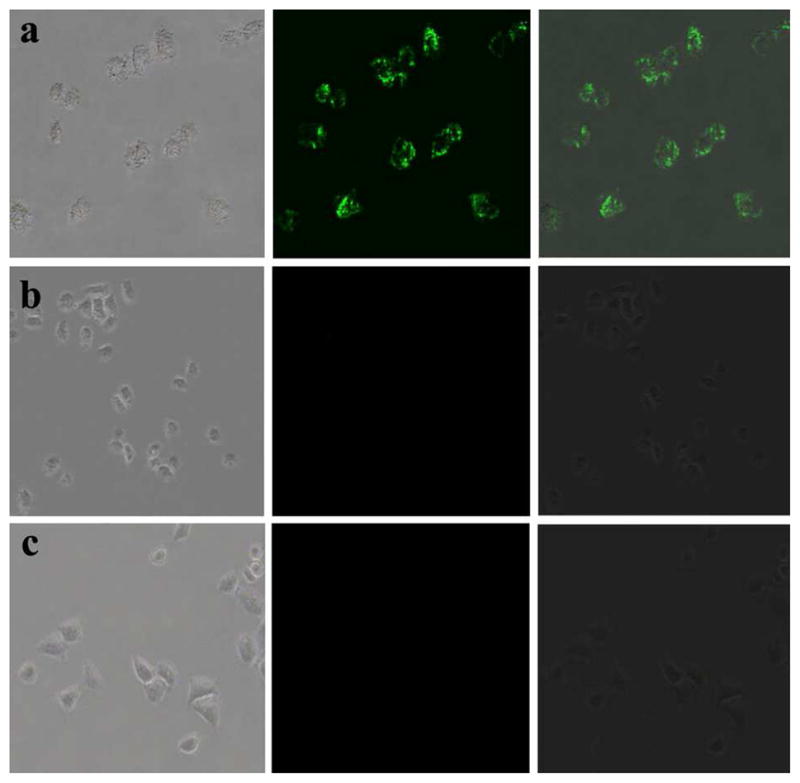
Fluorescence imaging of HeLa cells after incubated with antibody coupled UCNPs (a), amino-modified UCNPs without linking any antibody (b), and blank cells (c). Left rows are images in bright field; the middle rows are fluorescent images in dark field; and right rows are overlays of the left and middle rows.
4. Conclusions
A novel method for the synthesis of NaYF4:Yb,Er upconversion nanoparticles was firstly established with rare earth acetate as the precursor, via a microwave-assisted solvothermal approach using much shorter time (only 1 hour) than conventional method. The obtained nanoparticles were nearly ellipsoidal or spherical, with an average size of about 42 nm. They possessed a mixed structure of hexagonal and cubic, and exhibited excellent UC fluorescent characteristics under 980 nm excitation. After the surface was modified with amino groups through the typical stöber method, the nanoparticles were coupled with CEA-8 antibody to form an immuno bio-probe for the labeling and imaging of HeLa cells. The experimental results proved that the as-prepared nanoparticles satisfy the needs for bio-applications, and the microwave-assisted solvothermal approach is an effective method for the synthesis of UCNPs, which can be expanded for the preparation of other nanomaterials.
Acknowledgments
We are grateful for the support from the National Science Foundation of China (Grant No. 20875011), and the support from Northeastern University on PhD students. CBM would like to thank the financial support from the US National Science Foundation (DMR-0847758, CBET-0854414, CBET-0854465), National Institutes of Health (R21EB009909-01A1, R03AR056848-01, R01HL092526-01A2), and Oklahoma Center for the Advancement of Science and Technology (HR11-006#7823).
References
- 1.Auzel F. Chem Rev. 2004;104:139. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 2.Shan JN, Uddi M, Wei R, Yao N, Ju YG. J Phys Chem C. 2010;114:2452. [Google Scholar]
- 3.Shan GB, Assaaoudi H, Demopoulos GP. ACS Appl Mater Inter. 2011 doi: 10.1021/am200537e. dx.doi.org/10.1021/am200537e. [DOI] [PubMed] [Google Scholar]
- 4.Eliseeva SV, Bunzli JCG. Chem Soc Rev. 2010;39:189. doi: 10.1039/b905604c. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Zhao DY. ACS Nano. 2009;3:159. doi: 10.1021/nn800533v. [DOI] [PubMed] [Google Scholar]
- 6.Jalil RA, Zhang Y. Biomaterials. 2008;29:4122. doi: 10.1016/j.biomaterials.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Nyk M, Kumar R, Ohulchanskyy TY, Bergey EJ, Prasad PN. Nano Lett. 2008;8:3834. doi: 10.1021/nl802223f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Banerjee D, Liu YS, Chen XY, Liu XG. Analyst. 2010;135:1839. doi: 10.1039/c0an00144a. [DOI] [PubMed] [Google Scholar]
- 9.Yang JP, Deng YH, Wu QL, Zhou J, Bao HF, Li Q, Zhang F, Li FY, Tu B, Zhao DY. Langmuir. 2010;26:8850. doi: 10.1021/la904596x. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee DK, Rufaihah AJ, Zhang Y. Biomaterials. 2008;29:937. doi: 10.1016/j.biomaterials.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 11.Lim SF, Riehn R, Ryu WS, Khanarian N, Tung CK, Tank D, Austin RH. Nano Lett. 2006;6:169. doi: 10.1021/nl0519175. [DOI] [PubMed] [Google Scholar]
- 12.Zhang QQ, Qian J, Liang HJ, Somesfalean G, Wang D, He SL, Zhang ZG, Andersson-Engels S. ACS Nano. 2011;5:3744. doi: 10.1021/nn200110j. [DOI] [PubMed] [Google Scholar]
- 13.Jiang S, Gnanasammandhan MK, Zhang Y. J R Soc Interface. 2010;7:3. doi: 10.1098/rsif.2009.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer JC, Manseau MP, Murray JI, van Veggel FCJM. Langmuir. 2010;26:1157. doi: 10.1021/la902260j. [DOI] [PubMed] [Google Scholar]
- 15.Hu H, Yu MX, Li FY, Chen ZG, Gao X, Xiong LQ, Huang CH. Chem Mater. 2008;20:7003. [Google Scholar]
- 16.Zhou J, Sun Y, Du XX, Xiong LQ, Hu H, Li FY. Biomaterials. 2010;31:3287. doi: 10.1016/j.biomaterials.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Park YI, Kim JH, Lee KT, Jeon KS, Na HB, Yu JH, Kim HM, Lee N, Choi SH, Baik SI, Kim H, Park SP, Park BJ, Kim YW, Lee SH, Yoon SY, Song IC, Moon WK, Suh YD, Hyeon T. Adv Mater. 2009;21:4467. [Google Scholar]
- 18.Zhang P, Steelant W, Kumar M, Scholfield M. J Am Chem Soc. 2007;129:4526. doi: 10.1021/ja0700707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian HS, Guo HC, Ho PCL, Mahendran R, Zhang Y. Small. 2009;5:2285. doi: 10.1002/smll.200900692. [DOI] [PubMed] [Google Scholar]
- 20.Gai SL, Yang PP, Li CX, Wang WX, Dai YL, Niu N, Lin J. Adv Funct Mater. 2010;20:1166. [Google Scholar]
- 21.Wang F, Han Y, Lim CS, Lu YH, Wang J, Xu J, Chen HY, Zhang C, Hong MH, Liu XG. Nature. 2010;463:1061. doi: 10.1038/nature08777. [DOI] [PubMed] [Google Scholar]
- 22.Li CX, Lin J. J Mater Chem. 2010;20:6831. [Google Scholar]
- 23.Mader HS, Kele P, Saleh SM, Wolfbeis OS. Curr Opin Chem Biol. 2010;14:582.16. doi: 10.1016/j.cbpa.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Huang YJ, You HP, Jia G, Song YH, Zheng YH, Yang M, Liu K, Guo N. J Phys Chem C. 2010;114:18051. [Google Scholar]
- 25.Zhang F, Wan Y, Yu T, Zhang FQ, Shi YF, Xie SH, Li YG, Xu L, Tu B, Zhao DY. Angew Chem Int Ed. 2007;119:8122. doi: 10.1002/anie.200702519. [DOI] [PubMed] [Google Scholar]
- 26.Buhler G, Feldmann C. Angew Chem Int Ed. 2006;118:4982. doi: 10.1002/anie.200600244. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Wang WJ, Chen XY, Bi JH, Wu L, Li ZH, Fu XZ. Mater Lett. 2009;63:1023. [Google Scholar]
- 28.Wang HQ, Nann T. Nanoscale Res Lett. 2011;6:267. doi: 10.1186/1556-276X-6-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HQ, Nann T. ACS Nano. 2009;3:3804. doi: 10.1021/nn9012093. [DOI] [PubMed] [Google Scholar]
- 30.Chen GY, Ohulchanskyy TY, Kumar R, Agren H, Prasad PN. ACS Nano. 2010;4:3163. doi: 10.1021/nn100457j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang S, Zhang Y. Langmuir. 2010;26:6689. doi: 10.1021/la904011q. [DOI] [PubMed] [Google Scholar]
- 32.Mi CC, Zhang JP, Gao HY, Wu XL, Wang M, Wu YF, Di YQ, Xu ZR, Mao CB, Xu SK. Nanoscale. 2010;2:1141. doi: 10.1039/c0nr00102c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyer JC, Cuccia LA, Capobianco JA. Nano Lett. 2007;7:847. doi: 10.1021/nl070235+. [DOI] [PubMed] [Google Scholar]
- 34.Pichaandi J, Veggel FJM, Raudsepp M. ACS Appl Mater Inter. 2010;2:157. [Google Scholar]
- 35.Wang L, Zhao WJ, Tan WH. Nano Res. 2008;1:99. [Google Scholar]
- 36.Wang M, Mi CC, Wang WX, Liu CH, Wu YF, Xu ZR, Mao CB, Xu SK. ACS Nano. 2009;3:1580–1586. doi: 10.1021/nn900491j. [DOI] [PubMed] [Google Scholar]