Abstract
Background
Adverse childhood experiences (ACEs) increase the risk for adult depression and substance dependence, possibly mediated by the corticotropin-releasing hormone type 1 receptor (CRHR1). In some studies, a three-SNP “T-A-T” haplotype in CRHR1, which encodes CRHR1, exerted a protective moderating effect on risk of depression in adults with ACEs. Other studies have shown a main or moderating effect of SNPs in CRHR1 on alcohol consumption.
Methods
We tested the moderating effects of the three-SNP haplotype on lifetime risk of a major depressive episode (MDE) and alcohol dependence (AD) in 1,211 European Americans (EAs) and 1,869 African Americans (AAs), most of whom had a lifetime substance use disorder.
Results
There were no significant main or interaction effects of the TAT haplotype on AD. There was a significant interaction of ACE by TAT on risk of depression only in AA women (p=0.005); each copy of the TAT haplotype reduced the odds of MDE by almost 40% (OR = 0.63). In AA women without an ACE and two TAT haplotypes, the risk of MDE was increased (OR=1.51).
Conclusion
Our findings in relation to the TAT haplotype of CRHR1 extend those obtained in other populations to a largely substance-dependent one. The complex structure of CRHR1 may help to explain why some variants in the gene moderate the effects of an ACE only on depression risk while others moderate the effect of an ACE only on AD risk.
Keywords: Childhood maltreatment, Association analysis, Genetic risk, Gene by environment interaction, Depression, Alcoholism
INTRODUCTION
There is a strong association between negative childhood experiences, such as sexual and physical abuse, and adult psychiatric illness, particularly depression and post-traumatic stress disorder [Chapman et al., 2004; Molnar et al. 2001; Penza et al. 2003; Widom et al. 2007, Xie et al. 2009]. Early childhood abuse is also an important risk factor for alcohol use disorders in adults [Dube et al., 2003; Kendler et al., 2000; Nelson et al., 2002]. Such adverse childhood experiences (ACEs) appear to modify the stress response [Carpenter et al. 2007, 2009, 2011; Tryka et al. 2009], imparting a greater susceptibility to psychiatric and substance use disorders in adulthood [Nemeroff 2004].
Corticotropin releasing hormone (CRH) stimulates the stress response by activation of the corticotropin-releasing hormone type 1 receptor (CRHR1) [Bale and Vale 2004]. CRHR1 is a G-protein coupled receptor that binds neuropeptides of the CRH family, which are major regulators of the hypothalamic-pituitary-adrenal (HPA) axis. CRH also has neural effects mediated by CRHR1 that are independent of the HPA axis. These effects appear relevant both to alcohol dependence (AD) [Heilig et al. 2010] and to depression [Kling et al. 2009], particularly that which is elicited by chronic stress [Wang et al. 2010].
CRHR1 is encoded by CRHR1, which maps to 17q21.31. The gene is approximately 51.55 kb in length (http://www.genecards.org/cgi-bin/carddisp.pl?gene=CRHR1&search=crhr1; accessed Aug. 8, 2011) and is one of several genes contained within a 900-kb inversion polymorphism (Stefansson et al. 2005) resulting in H1 or H2 haplotypes. These represent two distinct genetic lineages that appear to have diverged some 3 million years ago. Due to the nature of the large inversion, markers in the region do not recombine across the H1 and H2 haplotypes. Markers in this inversion region have been associated with several neuropsychiatric disorders including Parkinson disease [Oliveira et al. 2004] and Alzheimer disease [Laws et al 2007]. Although individuals of African ancestry are largely homozygous for the non-inversion H1 haplotype, the frequency of the H2 haplotype is approximately 20% in individuals of European ancestry [Stefansson et al. 2005].
Bradley et al. [2008] genotyped 15 single-nucleotide polymorphisms (SNPs) spanning 57 kb of CRHR1. In a predominantly African-American (AA) and largely female adult sample, these investigators found that specific SNPs moderated the effects of child abuse on risk of depressive symptoms. The most robust effect was exerted by a protective three-SNP haplotype (comprised of alleles of rs7209436, rs110402, and rs242924). The SNPs comprising the haplotype are in high linkage disequilibrium (LD), and thus they must be contained within a single, high-LD block. Notably, these SNPs are monomorphic in the H2 haplotype in which the high-LD block is absent. Bradley et al. [2008] validated the moderating effect of the TAT haplotype on risk of depressive symptoms associated with ACEs in an independent sample that was predominantly European American (EA) and all women. Heim et al. [2009] re-analyzed the predominantly AA sample from the Bradley et al. study after more than doubling it in size, to examine the sex-specificity of the moderation of phenotype. These investigators examined only one CRHR1 SNP (rs110402), which “tagged” the haplotype. They found a moderating effect of the SNP only among men, with the A allele being protective against depressive symptoms among individuals who had experienced childhood trauma. The weight of the interaction effect was carried by the physical abuse subtype of childhood trauma. In an independent sample, Heim et al. [2009] found that male carriers of the A allele at rs110402 who had experienced childhood trauma showed a significantly lower cortisol response to the dexamethasone/CRH test than men without exposure to childhood trauma or women (irrespective of their trauma exposure); this suggested a biological mechanism for the observed effect on diagnosis phenotype.
Polanczyk et al. [2009] sought to replicate the findings of Bradley et al. [2008] in two prospective longitudinal cohort samples by examining the moderating effect of the TAT haplotype. In only one of the samples, the TAT haplotype protected against the development of major depressive disorder among adults exposed to childhood maltreatment. The authors attributed the inconsistent findings to the different methods used to assess childhood trauma.
Grabe et al. [2010] examined 28 SNPs in CRHR1 in a large German population sample. Included among the SNPs were the three representing the high LD block of interest in the studies by Bradley et al. [2008] and Polanczyk et al. [2009], though the majority of SNPs examined had minor alleles that tagged the H2 haplotype. Grabe et al. [2010] observed a significant moderator effect of the TAT haplotype on the risk of adult depression symptoms. However, rather than being protective, the haplotype was associated with greater depressive symptoms only among individuals who experienced moderate-to-severe physical neglect. Another SNP (rs17689882), which tags the H2 inversion haplotype and had not been examined specifically in previous studies, was the most robust moderator of the effects of physical neglect on depressive symptoms. The minor (A) allele of this SNP was protective against depressive symptoms in the overall sample, with significant effects only among men when the analysis was stratified by sex.
Treutlein et al. [2006] examined the association of two haplotype tagging (ht)SNPs (rs242938 and rs1876831) in CRHR1 on drinking behavior in a sample of adolescents from a longitudinal outcome study and a sample of adults with DSM-IV AD. In the adolescent sample, genotype frequencies differed significantly in relation to ever having drunk alcohol, ever having drunk heavily, and ever having been intoxicated. Among alcohol-dependent patients there was an association of CRHR1 with very heavy drinking. Using the same adolescent study sample, Blomeyer et al. [2008] examined the same two CRHR1 htSNPs as moderators of the effects of recent (i.e., during the preceding three years) stressful life events on drinking behavior. They found that SNPs rs1876831 moderated the association of stressful events with a greater amount of alcohol consumed per occasion and a higher lifetime rate of heavy drinking. Dahl et al. [2005] examined the association of five tightly linked CRHR1 SNPs (hCV449763, rs7209436, rs242924, rs1396862, and rs878887) that were spaced in 10–20 kb intervals across the gene in a small sample (i.e., 120 alcohol-dependent individuals and 180 screened control subjects). They found no association to phenotype in this sample.
Nelson et al. [2010] examined 13 SNPs, including those studied by Treutlein et al. [2006], Blomeyer et al. [2008], and Bradley et al. [2008] and nearby SNPs identified from dbSNP, in a sample of 1,128 Australians from 476 families. They found that individuals with a history of childhood sexual abuse reported a significantly higher quantity of alcohol consumed and greater risk for AD. Further, the H2 haplotype had a protective moderating effect on the impact of childhood sexual abuse on both alcohol consumption and AD. They also examined the association of several additional SNPs that are monomorphic in H2 [including rs7209436, rs242924, and rs110402] and found no evidence of significant main or interaction effects on the alcohol-related phenotypes.
In summary, variation in CRHR1 has been shown to moderate the effects of stressful events both on risk of depression and on alcohol-related behaviors. However, the variants associated with the different phenotypes do not appear to overlap, consistent with the structure of the gene. Further, some studies have failed to show a moderating effect of different variants, on one or the other phenotype.
In the present study, we tested the effects of ACEs and moderation of those effects by the 3-SNP, LD haplotype examined by Bradley et al. [2008], Polanczyk et al. [2009], and Grabe et al. [2010], comprised of rs7209436, rs110402, and rs242924, on the risk of 1) a lifetime major depressive episode (MDE) and 2) a lifetime diagnosis of AD in well-characterized participants in studies of the genetics of substance dependence.
METHODS
Subjects
The study sample consisted of 3,080 subjects [1,211 European Americans (EAs) and 1,869 African Americans (AAs)] selected from among participants in family-based linkage or association studies of the genetics of substance dependence [Gelernter et al., 2005, 2006a,b]. The family-based study sub-sample was ascertained through two or more siblings affected with cocaine dependence (CD) and/or opioid dependence (OD). The association study sub-sample was comprised of unrelated individuals with AD, CD, or OD and healthy controls. Subjects were recruited at four sites: 1,282 from the University of Connecticut Health Center (Farmington, CT), 1,238 from Yale University (New Haven, CT), 233 from the Medical University of South Carolina (Charleston, SC), 308 from University of Pennsylvania (Philadelphia, PA), and 19 from McLean Hospital (Belmont, MA). The institutional review board at each participating institution approved the study protocol and informed consent forms. Subjects provided written informed consent after receiving a complete description of the study and were paid to participate.
We included subjects for whom there were complete genotype phenotype data [including the presence or absence of a lifetime diagnosis of a major depressive episode (MDE) and AD, as well as whether there was a childhood history of having witnessed or experienced violent crime or been subjected to physical or sexual abuse (i.e., adverse childhood experiences)]. Although multiple members of a family were recruited in the family-based sub-sample, generally only the proband from each family was included in the analysis. When complete information was not available for a proband, a family member of the proband with full information was selected for study inclusion. Because a substantial minority of subjects had no lifetime substance dependence diagnosis, we conducted a secondary analysis in which we excluded these non-substance-dependent individuals.
Measures
All participants were interviewed using the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA) to obtain information on demographics, the childhood home environment, and Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) diagnoses of substance dependence and major depressive episodes [American Psychiatric Association 1994]. The instrument and its administration methods and reliability are described in detail elsewhere [Feinn et al., 2009; Pierucci-Lagha et al., 2005; Pierucci-Lagha et al., 2007].
The primary dependent variables in the analyses were dichotomous indicators of whether a subject had experienced an MDE or AD. We focused on the presence of an “independent MDE” to avoid the confounding effects of AD on ascertainment of a major depression diagnosis [Schuckit et al. 1997, 2007; Niciu et al. 2009]. In an independent MDE, the depressive symptoms are reported by respondents to not be associated with substance use, a general medical condition, or bereavement. As a secondary analysis to determine whether similar findings obtained using a more inclusive depression diagnosis, we also used “any lifetime MDE” (which included substance-induced depression and depression due to bereavement or a medical disorder) as a dependent measure in the analyses.
The SSADDA-derived diagnoses relevant to the present study showed good-to-excellent inter-rater and test-retest reliabilities [Pierucci-Lagha et al. 2005]. For independent lifetime MDE, values of κ were 0.68 (95% confidence interval = 0.55, 0.81) and 0.76 (0.62, 0.89), respectively. The respective inter-rater and test-retest reliabilities for lifetime AD were κ = 0.66 (0.54, 0.78) and 0.87 (0.77, 0.97) [Pierucci-Lagha et al. 2005].
Information on the childhood home environment included three items that reflected ACEs: 1) exposure to a violent crime (“Did you witness or experience a violent crime, like a shooting or a rape, by age 13?”), 2) sexual abuse (“By the time you were age 13, were you ever sexually abused?”), and 3) physical abuse (“By the time you were age 13, were you ever beaten by an adult so badly that you needed medical care, or had marks on your body that lasted for more than 30 days?”). A positive response to any of the items was coded as positive for the dichotomous variable ACE. The inter-rater and test-retest reliability for this variable was κ = 0.88 (0.78, 0.97) and 0.92 (0.80, 1.00), respectively.
Genotyping/Haplotyping Procedures
DNA was extracted from whole blood or immortalized cell lines. We used the Taqman genotyping technique [Shi et al. 1999] to genotype the 3 SNPs (rs7209436, rs110402, and rs242924). The PCR reaction used 1X Universal PCR Master Mix, 0.2X BSA, 0.5X 40X Assay, and 2 ng of genomic DNA. PCR amplification was carried out under the following conditions: 95° C for 10 min, followed by 15 s at 92° C, and then 60 s at 60° C for 65 cycles. Signals were analyzed with an ABI Prism 7900HT sequence detector using software version 2.1 from Applied Biosystems (Foster City, CA).
We estimated the haplotype frequencies using PHASEv2 software [Stephens, Smith, Donnelly 2001], which employs a Bayesian method for haplotype reconstruction [Stephens & Donnelly 2003]. Each subject has a TAT haplotype value of 0–2.
Analyses
Because allele frequencies differed by population and the prevalence of MDE and AD differed by sex, all analyses were conducted separately by population and sex. We examined the main and interaction effects of ACE (present/absent) and TAT haplotype (0, 1, or 2 copies) on the likelihood of a lifetime diagnosis of MDE (present/absent) using logistic regression and SPSS PASW v17 software [Norusis 2008]. We used a similar analytic approach to examine the main and interaction effects of ACE and TAT haplotype on risk of a lifetime diagnosis of AD (coded as present or absent).
Additional logistic analyses were conducted using the bootstrap re-sampling method [Efron & Tibshirani, 1993] to determine the robustness of the results. The bootstrap involved generating 1000 samples for each outcome and was conducted in R v2.10 [R Development Core Team, 2008].
Because the groups differed socioeconomically, we repeated the logistic regression analyses with age, education level, and income level included as covariates. To examine the association of the number of TAT haplotypes with the presence or absence of an ACE, we conducted chi-square analysis of these variables for each of the four groups (AA women, EA women, AA men, and EA men) separately.
RESULTS
Sample Characteristics
Demographics
As shown in Table 1, the AA sample consisted of 1,050 men and 819 women with a mean age of nearly 42 years. Most AA participants had completed high school, with about one-third having attended college. A majority of AAs were unemployed, with an annual income below $10,000. The EA sample was comprised of 708 men and 503 women, with a mean age of nearly 38 years. Over 40% of the sample had attended college. As was true of the AA sample, most EAs were unemployed. However, a larger percentage of EAs than AAs had an annual income above $30,000.
Table 1.
Demographic and Clinical Features and Haplotype Distribution of the Study Sample
| African-American | European-American | |||
|---|---|---|---|---|
| Women (N = 819) |
Men (N = 1,050) |
Women (N = 503) |
Men (N = 708) |
|
| Age [Mean yr (± SD)] | 40.3 ± 9.3 | 43.1 ± 9.1 | 37.3 ± 11.3 | 38.0 ± 11.7 |
| Education | ||||
| < High school | 306 (37%) | 413 (39%) | 141 (28%) | 210 (30%) |
| High School | 233 (28%) | 316 (30%) | 130 (26%) | 211 (30%) |
| Some College | 221 (27%) | 272 (26%) | 153 (30%) | 193 (27%) |
| College Degree | 58 (7%) | 49 (5%) | 79 (16%) | 92 (13%) |
| Employment Status | ||||
| Full-time | 158 (19%) | 192 (18%) | 90 (18%) | 189 (27%) |
| Part-time | 122 (15%) | 154 (15%) | 87 (17%) | 82 (12%) |
| Not working | 538 (66%) | 700 (67%) | 324 (65%) | 432 (61%) |
| Income Level | ||||
| ≤ $10,000 | 472 (58%) | 560 (53%) | 224 (45%) | 285 (40%) |
| $10,000 – $29, 999 | 206 (25%) | 334 (32%) | 150 (30%) | 204 (29%) |
| ≥ $30,000 | 132 (16%) | 147(14%) | 121 (24%) | 211 (30%) |
| Substance Dependence1 | ||||
| None | 185 (23%) | 93 (9%) | 117 (23%) | 106 (15%) |
| Alcohol | 397 (49%) | 641 (61%) | 232 (46%) | 408 (58%) |
| Cocaine | 560 (68%) | 827 (79%) | 300 (60%) | 451 (64%) |
| Opioid | 126 (15%) | 251 (24%) | 248 (49%) | 366 (52%) |
| Adverse Childhood Experiences2 | ||||
| None | 547 (67%) | 688 (66%) | 329 (65%) | 552 (78%) |
| Violent Crime | 174 (21%) | 275 (26%) | 78 (16%) | 81 (11%) |
| Sexual Abuse | 188 (23%) | 98 (9%) | 134 (27%) | 75 (11%) |
| Physical Abuse | 79 (10%) | 92 (9%) | 68 (14%) | 64 (9%) |
| Major Depressive Episode | 130 (16%) | 81 (8%) | 134 (27%) | 83 (12%) |
| No. of TAT Haplotypes | ||||
| 0 | 431 (53%) | 543 (52%) | 189 (38%) | 237 (34%) |
| 1 | 320 (39%) | 418 (40%) | 225 (45%) | 341 (48%) |
| 2 | 68 (8%) | 89 (9%) | 89 (18%) | 130 (18%) |
Some subjects had multiple substance dependence diagnoses
Some subjects had multiple adverse childhood events
Adverse Childhood Experiences
The distribution of the three types of ACEs is shown in Table 1. The mean number of ACEs among AAs was 0.48 (SD=0.8); the most common ACE was having witnessed or experienced a violent crime (24%). Although among AAs there was no sex difference in the frequency of physical abuse, a significantly higher proportion of men experienced violent crime (χ2(1)=6.15, p=0.013), while more women experienced sexual abuse (χ2(1)=66.23, p<0.001). Among EAs, the mean number of ACEs was 0.41 (SD=0.77) and the most common ACE was sexual abuse (17%). EA women were more likely to have witnessed or experienced violent crime (χ2(1)=4.23, p=0.039) and to have experienced sexual abuse (χ2(1)=53.12, p<0.001) and physical abuse (χ2(1)=6.05, p=0.014) than EA men. Overall, AAs and EAs reported comparable rates of sexual and physical abuse. However, AAs were more likely to have witnessed or experienced violent crime than EAs [24% versus 13%; χ2(1)=55.26, p<0.001], an effect that differed by sex (p=0.002). As shown in Table 1, in AAs, exposure to violent crime was more common among men, but in EAs, it was more common among women.
The number of TAT haplotypes (on a scale of 0–2) was significantly associated with the presence or absence of an ACE only among AA women (χ2(2)=10.83, p=0.004): 54.8% of AA women who reported no ACE had no TAT haplotypes, 35.5% had one TAT haplotype, and 9.7% had two TAT haplotypes. Among AA women who had experienced an ACE, the respective numbers were 48.2%, 46.3%, and 5.5%. However, the percentage of TAT haplotypes did not differ significantly in the two groups (i.e., 27.4% vs. 28.7%, respectively) (χ2(1)=0.28, p=0.60).
Psychiatric Diagnoses
The lifetime prevalence of MDE was significantly higher among EAs than AAs [18% vs. 11%; χ2(1)=26.99, p<0.001]. As shown in Table 1, in both populations, women were at least twice as likely as men to meet criteria for the disorder [AA: χ2(1)=30.58, p<0.001, EA: χ2(1)=44.49, p<0.001]. A comparable majority of subjects in both populations (85% of AAs and 82% of EAs) met criteria for at least one substance dependence diagnosis, reflecting the ascertainment scheme (i.e., which was aimed predominantly at substance-dependent individuals, with a smaller sample of control subjects).
Haplotype Frequencies
In AAs, the three SNPs were in tight LD (r2>0.8 for all comparisons). In this population group, the most common haplotype was CGG (67.3%) and the estimated frequency of the TAT haplotype was 27.9%. Six other haplotypes accounted for the remaining 4.8% of haplotypes observed. The three SNPs were also in tight LD (r2>0.9 for all comparisons) in EAs. The CGG haplotype was also the most common (54.3%) in EAs, followed by the TAT haplotype (41.0%). Six other haplotypes accounted for the remaining 4.7% of haplotypes in EAs.
Main and Interaction Effects of ACE and TAT Haplotype on MDE Risk
Table 2 shows the logistic regression results for MDE as a function of ACE and haplotype separately by population and sex. ACE was a significant predictor of MDE among AA women (p < 0.001), AA men (p = 0.005) and EA women (p < 0.001), but not EA men (p = 0.11). TAT had a significant main effect on risk of MDE only in AA women (p = 0.035), in whom there was also a significant interaction of TAT and ACE (B = −0.88, p = 0.005). Neither the main effect of TAT nor the interaction of ACE by TAT on risk of MDE was significant for the other three groups (p’s all > 0.30). As shown in Figure 1, in AA women who experienced at least one ACE, each copy of the TAT haplotype reduced the odds of MDE by 37% (OR = 0.63); in women without an ACE but with two TAT haplotypes, the odds of having a lifetime MDE was approximately 50% greater (OR=1.51).
Table 2.
Logistic Regression Results for Risk of Major Depressive Episode
| African-American | European-American | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | P | Coefficient | SE | P | |
| Women | ||||||
| ACE | 1.62 | .27 | <.001 | 1.10 | .31 | <.001 |
| Number of TAT Haplotypes | .414 | .20 | .035 | −.021 | .19 | .91 |
| Interaction of TAT × ACE | −.875 | .31 | .005 | −.141 | .29 | .63 |
| Men | ||||||
| ACE | 0.82 | .29 | .005 | 0.67 | .42 | .11 |
| Number of TAT Haplotypes | −.278 | .27 | .30 | .196 | .21 | .35 |
| Interaction of TAT × ACE | −.317 | .40 | .43 | .289 | .35 | .41 |
ACE, adverse childhood event
Figure 1.
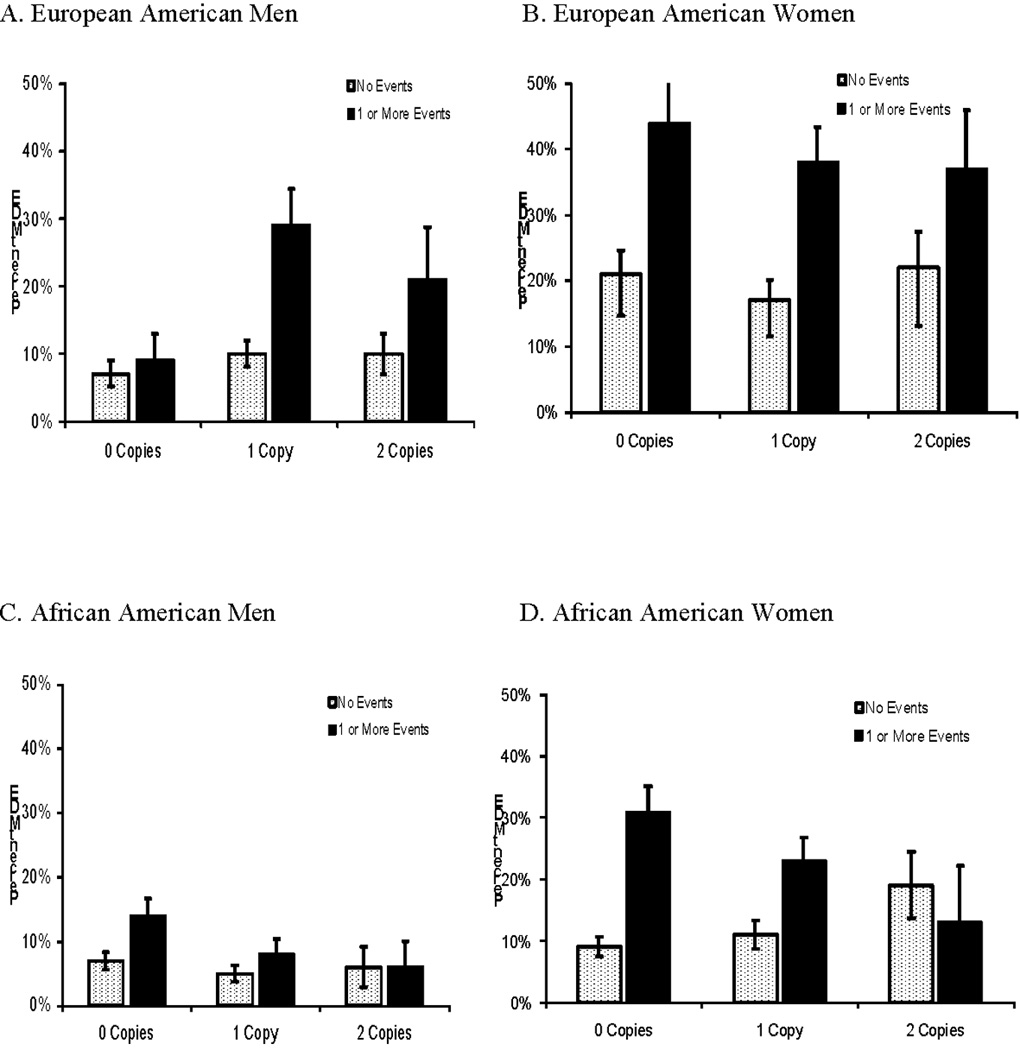
MDE Risk by the Number of TAT Haplotypes and Presence or Absence of ACE
Follow-up analyses using the three components of ACE (violent crime, sexual abuse, and physical abuse) individually in place of a summary measure in the models showed no significant TAT by adverse event interaction among EAs or AA men. Among AA women, the interaction of TAT haplotype was significant for exposure to violent crime (p=0.011) and approached significance when using sexual abuse (p=0.098) and physical abuse (p=0.060).
Main and Interaction Effects of ACE and TAT Haplotype on AD Risk
As can be seen in Table 3, there was a significant main effect of ACE on risk of AD only among EA women (p < 0.001). There was neither a main effect of TAT haplotype nor an interaction effect of ACE by TAT on AD risk in any of the four subgroups (p’s all > 0.10).
Table 3.
Logistic Regression Results for Risk of Alcohol Dependence
| African-American | European-American | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | Sig | Coefficient | SE | Sig | |
| Women | ||||||
| ACE | .276 | .20 | .17 | 1.00 | .29 | <.001 |
| Number of TAT Haplotypes | −.031 | .13 | .81 | .187 | .16 | .23 |
| Interaction of TAT × ACE | .339 | .25 | .17 | −.237 | .27 | .38 |
| Men | ||||||
| ACE | .141 | .18 | .43 | .091 | .29 | .75 |
| Number of TAT Haplotypes | .031 | .12 | .80 | .043 | .12 | .72 |
| Interaction of TAT × ACE | .163 | .21 | .61 | .414 | .27 | .13 |
Bootstrap Procedure
All logistic models described above were re-analyzed using the bootstrap re-sampling method. The results were consistent with what was reported above: namely, there was a significant ACE by TAT interaction on MDE risk for AA women [B = −0.88 (95%CI = −1.48 to −0.27)]. The interaction term in all other models was not significant.
Both the analyses restricted to individuals with substance dependence (n=2,579) and those using the more inclusive diagnosis of “any lifetime MDE” yielded the same findings as the main analyses, i.e., there was a significant moderator effect only in AA women.
DISCUSSION
Our finding that the TAT haplotype in CRHR1 reduced the risk of major depression among AA women who experienced one or more ACEs extends findings from some prior studies in non-substance-dependent populations to a predominantly substance-dependent one. Our findings are consistent with those originally reported by Bradley et al. [2008] and may be limited to AAs by virtue of the different haplotype structure among AAs and EAs. Notably, not all studies have replicated the effects reported by Bradley et al. [2008], possibly due to differences in the study populations. The populations studied have been of African or European ancestry, in varying proportions. Heim et al. [2009], extended the AA sample initially studied by Bradley et al. and found a moderating effect of a “tag SNP” for the TAT haplotype (rs110402) only among men. In an initial sample of women of European ancestry, Polancyzk et al. [2009] found a significant moderating effect of the TAT haplotype, but failed to replicate the finding in a second sample of European ancestry comprised of both sexes. Grabe et al. [2010], in a German population sample, found that the presence of the TAT haplotype increased the risk of depression. After correction for multiple comparisons, only one SNP (rs17689882) of the 16 examined by Grabe et al. [2010] significantly moderated the effect of physical neglect on depressive symptoms. This SNP, as well as the haplotype block in which it was located (i.e., Block 3), were found to be protective. Of note, these Block 3 SNPs all effectively tag the H2 haplotype and would be expected to be in complete LD. It is thus surprising that the level of significance reported for rs17689882 exceeded those found for other H2-tagging SNPs by an order of magnitude. The findings reported by Grabe et al. [2010], which run counter to those of most other reports, may reflect in part the confounding effects of different haplotype blocks in CRHR1. Although the Block 3 (H2) haplotype and the TAT haplotype (located in Block 1 and not present in H2) showed modest linkage disequilibrium (R2=0.21), moderator analyses did not take the relative contributions of these two haplotypes into account.
Studies in this area have also used different instruments to measure ACEs and have examined different kinds of ACEs. The Childhood Trauma Questionnaire [Bernstein et al. 1994], a validated measure of ACEs, was used by Bradley et al. [2008], Grabe et al. [2010], and in one of the two samples studied by Polanczyk et al. [2009]. We used a measure of self-reported ACEs for which reliability data are available [Douglas et al. 2010], but the validity is not established. An important limitation of this measure of ACEs is that it does not assess differences in the timing and severity of the events, which could have important effects on the risk associated with them. Although Grabe et al. [2008] found that genetic moderation was limited to physical neglect, our most robust finding was for exposure to (i.e., having witnessed or experienced) violent crime. We obtained similar effects for both physical and sexual abuse, although, perhaps due to the fact that these ACEs were not endorsed as frequently as violent crime, the effects did not reach statistical significance. Although the four subgroups examined by us differed in the proportion of individuals endorsing the different kinds of ACEs, these differences do not appear to explain why we observed genetic moderation of the risk of MDE only among AA women.
Another source of variability in the findings reported in the literature on the moderating effect of variation in CRHR1 on depression risk is the specific measure of depression used. We used a dichotomous lifetime MDE as the dependent measure in our analyses, which is most similar to the measure used by Polanczyk et al. [2009], who defined depression in terms of a DSM-IV major depressive disorder, assessed over the preceding year. These dichotomous diagnoses contrast with the studies by Bradley et al. [2008] (and the extension of that study by Heim et al. [2009] and Ressler et al. [2010]) and Grabe et al. [2010], which used the Beck Depression Inventory, a dimensional approach, to measure current depressive symptoms.
Differences in socioeconomic status among the four groups in the present report do not appear to explain the observed effects on risk of depression, since the inclusion of age, education level, and income level as covariates in the logistic regression analyses did not substantially alter the findings. However, because only among AA women was there a correlation between having experienced an ACE and the number of TAT haplotypes, it is possible that the observed interaction in this group between the number of TAT haplotypes and ACE on risk of depression could reflect a statistical, rather than a biological effect.
Findings reported by Tyrka et al. [2009] support a biological basis for the observed moderating effect of CRHR1 variation on risk of MDE. These investigators found a moderating effect of the CRHR1 SNPs rs110402 and rs242924, which together with rs7209436 constitute the 3-SNP Block 1 haplotype, on a history of childhood maltreatment, using the dexamethasone/corticotropin-releasing hormone (DEX/CRH) test. In this study, both SNPs (which were in near-complete linkage disequilibrium) moderated the effect of maltreatment on cortisol response to the DEX/CRH test. The authors suggested that excessive HPA axis activation could represent a mechanism of interaction of risk genes with stress in the development of mood disorders. Heim et al. (2009) found that the moderating effect of rs110402 on the cortisol response to the DEX/CRH test was present only in men, consistent with their findings on the risk of depressive symptoms. Recent evidence points to multiple brain regions being involved in the effects of stress, including not only HPA-mediated effects, but also CRH neurotransmission in, for example, the amygdala [Gallagher et al. 2008, Wang et al. 2010]. Further research on the specific mechanisms by which stress and variation in CRHR1 interact to produce depression could help to refine the pathophysiology of the disorder.
The findings reported here may also have implications for the treatment of depression. Liu et al. [2007] examined the moderating effect of three CRHR1 SNPs (rs1876828, rs242939 and rs242941) on the response to 6 weeks of treatment with fluoxetine in 127 Han Chinese patients with MDD. Individuals who were homozygous for the G allele at rs242941 and those with two copies of the GAG haplotype comprised of these three SNPs showed a more robust fluoxetine therapeutic response, an effect that was limited to individuals with high levels of anxiety. If replicated, these findings may help to identify individuals for whom SSRI therapy may be most efficacious.
In summary, we found evidence that the TAT haplotype, variants limited to the H1 inversion haplotype moderated the risk of MDE in AA women exposed to ACEs and extend the findings of a protective effect of this haplotype on risk of depression to a largely substance-dependent population. We found no moderating effects of the TAT haplotype on the risk of AD. These findings add to a growing, but complex and inconsistent, literature on the moderating role of this variation in depression and are consistent with prior negative findings in relation to AD [Treutlein et al. 2006, Blomeyer et al. 2008, Nelson et al. 2010]. Differences in the measurement of adverse childhood experiences and depression, as well population differences could contribute to the variable results. Nevertheless, taken together, these findings suggest a differential impact of variation in the two large non-recombinant haplotypes containing CRHR1 among individuals who have experienced ACEs: namely, that variation moderating the risk of depression may be localized to the H1 haplotype and the one moderating risk of AD may be localized to the H2 haplotype. A model of differential vulnerability associated with the H1 and H2 haplotypes is supported by the lack of recombination across the two haplotypes (Stefansson et al. 2005). Further, other differential effects have been seen with the two haplotypes, including ongoing positive selection of the H2 haplotype (Stefansson et al. 2005) and a propensity for microdeletions, which have been associated with a risk of developmental delay and learning disability (Shaw-Smith et al. 2006). Further research to test the hypothesis that the H1 and H2 haplotypes are differentially associated with risk of depression and AD is warranted.
Acknowledgment
Supported by NIH grants R01 DA12690, R01 DA12849, R01 DA018432, R01 AA11330, R01AA017535, K24 AA013736, P60 AA03510, and M01 RR06192.
REFERENCES
- American Psychiatric Association. Diagnostic and Statisical Manual of Mental Disorders. Fourth Edition. Washington: American Psychiatric Press; 1994. [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63(2):146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2011;214(1):367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66(1):69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82(2):217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Dahl JP, Doyle GA, Oslin DW, Buono RJ, Ferraro TN, Lohoff FW, Berrettini WH. Lack of association between single nucleotide polymorphisms in the corticotropin releasing hormone receptor 1 (CRHR1) gene and alcohol dependence. J Psychiatr Res. 2005;39(5):475–479. doi: 10.1016/j.jpsychires.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Douglas KR, Chan G, Gelernter J, Arias AJ, Anton RF, Weiss RD, Brady K, Poling J, Farrer L, Kranzler HR. Adverse childhood events as risk factors for substance dependence: partial mediation by mood and anxiety disorders. Addict Behav. 2010;35(1):7–13. doi: 10.1016/j.addbeh.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111(3):564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirana RJ. An Introduction to the Bootstrap. London: Chapman & Hall; 1993. [Google Scholar]
- Feinn R, Gelernter J, Cubells JF, Farrer L, Kranzler HR. Sources of unreliability in the diagnosis of substance dependence. J Stud Alcohol Drugs. 2009;70(3):475–481. doi: 10.15288/jsad.2009.70.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583(2–3):215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Hesselbrock V, Rounsaville B, Poling J, Wilcox M, Farrer L, Kranzler HR. Genomewide linkage scan for cocaine dependence and related traits: significant linkages for a cocaine-related trait and cocaine-induced paranoia. Am J Med Genet B Neuropsychiatr Genet. 2005;136B(1):45–52. doi: 10.1002/ajmg.b.30189. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Wilcox M, Hesselbrock V, Rounsaville B, Poling J, Weiss R, Sonne S, Zhao H, Farrer L, Kranzler HR. Genomewide linkage scan for opioid dependence and related traits. Am J Hum Genet. 2006a;78(5):759–769. doi: 10.1086/503631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ, Kranzler HR, Farrer L. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006b;15(24):3498–3507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Schwahn C, Appel K, Mahler J, Schulz A, Spitzer C, Fenske K, Barnow S, Lucht M, Freyberger HJ, John U, Teumer A, Wallaschofski H, Nauck M, Volzke H. Childhood maltreatment, the corticotropin-releasing hormone receptor gene and adult depression in the general population. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1483–1493. doi: 10.1002/ajmg.b.31131. [DOI] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, Hommer D, Barr CS. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev. 2010;35(2):334–344. doi: 10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Ressler KJ, Binder EB. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front Behav Neurosci. 2009;3(41):1–10. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kling MA, Coleman VH, Schulkin J. Glucocorticoid inhibition in the treatment of depression: can we think outside the endocrine hypothalamus? Depress Anxiety. 2009;26(7):641–649. doi: 10.1002/da.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws SM, Friedrich P, Diehl-Schmid J, Muller J, Eisele T, Bauml J, Forstl H, Kurz A, Riemenschneider M. Fine mapping of the MAPT locus using quantitative trait analysis identifies possible causal variants in Alzheimer's disease. Mol Psychiatry. 2007;12(5):510–517. doi: 10.1038/sj.mp.4001935. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Tang J, Liu W, Wang H, Liu H, Wang X, Wu Y, Cao Z, Li W. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett. 2007;414(2):155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. Am J Public Health. 2001;91(5):753–760. doi: 10.2105/ajph.91.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Wang JC, Whitfield JB, Saccone FS, Kern J, Grant JD, Schrage AJ, Rice JP, Montgomery GW, Heath AC, Goate AM, Martin NG, Madden PA. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict Biol. 2010;15(1):1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Madden PA, Cooper ML, Dinwiddie SH, Bucholz KK, Glowinski A, McLaughlin T, Dunne MP, Statham DJ, Martin NG. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry. 2002;59(2):139–145. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65 Suppl 1:18–28. [PubMed] [Google Scholar]
- Niciu MJ, Chan G, Gelernter J, Arias AJ, Douglas K, Weiss R, Anton RF, Farrer L, Cubells JF, Kranzler HR. Subtypes of major depression in substance dependence. Addiction. 2009;104(10):1700–1709. doi: 10.1111/j.1360-0443.2009.02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norusis M. SPSS 17.0 Guide to Data Analysis. Upper Saddle River: Prentice Hall; 2008. [Google Scholar]
- Oliveira SA, Scott WK, Zhang F, Stajich JM, Fujiwara K, Hauser M, Scott BL, Pericak-Vance MA, Vance JM, Martin ER. Linkage disequilibrium and haplotype tagging polymorphisms in the Tau H1 haplotype. Neurogenetics. 2004;5(3):147–155. doi: 10.1007/s10048-004-0180-5. [DOI] [PubMed] [Google Scholar]
- Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: implications for the pathophysiology of depression and anxiety. Arch Womens Ment Health. 2003;6(1):15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, Farrer L, Kranzler HR. Reliability of DSM-IV diagnostic criteria using the semi-structured assessment for drug dependence and alcoholism (SSADDA) Drug Alcohol Depend. 2007;91(1):85–90. doi: 10.1016/j.drugalcdep.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80(3):303–312. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66(9):978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statisical Computing; 2008. [Google Scholar]
- Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, Gillespie CF, Nemeroff CB, Cubells JF, Binder EB. Polymorphisms in CRHR1 and the serotonin transporter loci: gene × gene × environment interactions on depressive symptoms. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(3):812–824. doi: 10.1002/ajmg.b.31052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Pierson J, Trim R, Nurnberger JI, Kramer J, Kuperman S, Bierut LJ, Hesselbrock V. A comparison of factors associated with substance-induced versus independent depressions. J Stud Alcohol Drugs. 2007;68(6):805–812. doi: 10.15288/jsad.2007.68.805. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psychiatry. 1997;154(7):948–957. doi: 10.1176/ajp.154.7.948. [DOI] [PubMed] [Google Scholar]
- Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, Curley R, Cumming S, Dunn C, Kalaitzopoulos D, Porter K, Prigmore E, Krepischi-Santos ACV, Varela MC, Koiffmann CP, Lees AJ, Rosenberg C, Firth HV, de Silva R, Carter NP. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nature Genetics. 2006;38(9):1032–1037. doi: 10.1038/ng1858. [DOI] [PubMed] [Google Scholar]
- Shi MM, Myrand SP, Bleavins MR, de la Iglesia FA. High throughput genotyping for the detection of a single nucleotide polymorphism in NAD(P)H quinone oxidoreductase (DT diaphorase) using TaqMan probes. Mol Pathol. 1999;52(5):295–299. doi: 10.1136/mp.52.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J, Baker A, Jonasdottir A, Ingason A, Gudnadottir VG, Desnica N, Hicks A, Gylfason A, Gudbjartsson DF, Jonsdottir GM, Sainz J, Agnarsson K, Birgisdottir B, Ghosh S, Olafsdottir A, Cazier JB, Kristjansson K, Frigge ML, Thorgeirsson TE, Gulcher JR, Kong A, Stefansson K. A common inversion under selection in Europeans. Nat Genet. 2005;37(2):129–137. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73(5):1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11(6):594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66(7):681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Yan XB, Hofman MA, Swaab DF, Zhou JN. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neurosci Bull. 2010;26(4):297–303. doi: 10.1007/s12264-010-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64(1):49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66(11):1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]


