Abstract
With the exception of survival, cognitive impairment stemming from the clinical management of cancer is a major factor dictating therapeutic outcome. For many patients afflicted with CNS and non-CNS malignancies, radiotherapy and chemotherapy offer the best options for disease control. These treatments however come at a cost, and nearly all cancer survivors (~11 million in the US alone as of 2006) incur some risk for developing cognitive dysfunction, with the most severe cases found in patients subjected to cranial radiotherapy (~200,000/yr) for the control of primary and metastatic brain tumors1. Particularly problematic are pediatric cases, whose long-term survival plagued with marked cognitive decrements results in significant socioeconomic burdens2. To date, there are still no satisfactory solutions to this significant clinical problem.
We have addressed this serious health concern using transplanted stem cells to combat radiation-induced cognitive decline in athymic rats subjected to cranial irradiation3. Details of the stereotaxic irradiation and the in vitro culturing and transplantation of human neural stem cells (hNSCs) can be found in our companion paper (Acharya et al., JoVE reference). Following irradiation and transplantation surgery, rats are then assessed for changes in cognition, grafted cell survival and expression of differentiation-specific markers 1 and 4-months after irradiation. To critically evaluate the success or failure of any potential intervention designed to ameliorate radiation-induced cognitive sequelae, a rigorous series of quantitative cognitive tasks must be performed. To accomplish this, we subject our animals to a suite of cognitive testing paradigms including novel place recognition, water maze, elevated plus maze and fear conditioning, in order to quantify hippocampal and non-hippocampal learning and memory. We have demonstrated the utility of these tests for quantifying specific types of cognitive decrements in irradiated animals, and used them to show that animals engrafted with hNSCs exhibit significant improvements in cognitive function3.
The cognitive benefits derived from engrafted human stem cells suggest that similar strategies may one day provide much needed clinical recourse to cancer survivors suffering from impaired cognition. Accordingly, we have provided written and visual documentation of the critical steps used in our cognitive testing paradigms to facilitate the translation of our promising results into the clinic.
Keywords: Medicine, Issue 56, neuroscience, radiotherapy, cognitive dysfunction, hippocampus, novel place recognition, elevated plus maze, fear conditioning, water maze
Protocol
Our cognitive testing paradigms are schematically diagrammed in Figure 1. Cognitive testing is initiated one month after irradiation and surgical procedures are performed.
Conducting the Novel Place Recognition (NPR) Task
The Novel Place Recognition (NPR) task is based on procedures described by Mumby and colleagues (2002), and is a spatial recognition memory task that relies on the hippocampus4.
Rats are first familiarized with the testing context by placing them in the testing arenas (custom-made, white, acrylic, open top boxes, 70cm X 70cm X 40cm high) for 20 minutes once per day for three days. Objects that will not be used for later testing are placed in the arenas during these habituation sessions.
For the habituation phase, manual observations of exploratory behavior should be noted, while recording and tracking of behavior are not necessary.
For all phases of the NPR task, the arenas are cleaned thoroughly between animals using 70% ethanol in order to minimize confounding odor cues. For all trials, experimenters place animals in the center of the testing arenas in a consistent orientation to minimize the chance that animals will simply explore the block that they happen to be placed next to.
The familiarization phase and 5-minute test phase are administered on the fourth day.
For the familiarization phase, two identical plastic blocks (8cm X 3cm X 10cm high) that have been thoroughly cleaned with 70% ethanol are placed at specific positions within the testing arenas. One animal is placed in each arena for 5 minutes and allowed to explore freely.
Exploration of the two equally-familiar spatial positions is scored using the automated tracking system. For all phases, exploration is defined as an animal's nose point being within a 4cm radius of and directed toward the blocks.
Following the familiarization phase, the animals are placed in a small holding cage for a 5-minute delay interval. During this time, the testing arenas are cleaned with 70% ethanol, and identical copies of the blocks are added; one in an identical spatial position as during the familiarization phase, and one in a novel spatial position. If a rat remembers the spatial arrangement of the blocks from the familiarization phase, it will spend more time exploring the block located in the novel spatial location.
After the 5-minute delay interval, the animals are returned to the testing arenas for the 5-minute test phase. They explore freely for 3 minutes.
24 hours after the end of the familiarization phase, animals are returned to the testing arenas again for the 24-hour test phase. For this phase, one block remains in the same position as during the familiarization and 5-minute test phases, and the other block is placed in a new, novel spatial position. Again, if a rat remembers the spatial arrangement of the blocks from the familiarization phase the day before, he will spend more time exploring the block located in the novel spatial location.
Now, the amount of time spent exploring the blocks in the various spatial positions is collected using the automated tracking program, and can be analyzed statistically for differences between experimental groups.
For the familiarization phase, total time spent exploring both spatial locations across the entire trial is calculated. There should be no difference in time spent exploring the two spatial locations during this phase as they are equally familiar/unfamiliar to the animals (Fig. 2A). Further, significant differences in total exploration between the experimental groups may indicate group variations in motivation to explore or anxiety levels and should be considered when interpreting spatial recognition memory results from the two test phases.
For both the 5-minute and 24-hour test phases, an exploration ratio is calculated as follows: time spent exploring novel position - time spent exploring familiar position / total time spent exploring (Fig. 2B). In this way, individual variability in motivation to explore the objects is taken into account. Normal animals spend significantly more time exploring the novel spatial position than expected by chance (indicated by the dashed line) following the 5-minute and 24-hour delay intervals (Fig. 2B).
Exploration ratio is typically calculated for the first 60 seconds of the 5-minute and 24-hour tests4. This is because, as the test trials progress, the novel spatial position becomes more familiar to the animals. Thus, the first minute of the test trials provides the most accurate assessment of spatial recognition memory.
2. Conducting the Elevated Plus Maze (EPM) Task
The Elevated Plus Maze (EPM) task is based on procedures from Walf and Frye, 20075.
The EPM task is used to assess anxiety in rodents. The EPM apparatus is a 4-arm maze (45" X 4") raised 45" from floor level, with the arms arranged in a plus shape. Two arms are enclosed and two are open. The apparatus shown is manufactured by Med Associates Inc.
Behavior during this task reflects a conflict between the rat's natural motivation to explore novel environments (time spent in open arms) and preference for remaining in protected areas (closed arms).
The experimental room is brightly lit with spotlights pointed at the maze to ensure adequate motivation to prefer the closed, more dimly-lit arms.
The maze is cleaned thoroughly before the first animal is tested, and between each trial.
The animal is placed in the apparatus by holding it under the forelimbs and placing it in the center portion of the maze, facing the open arm that is away from the experimenter. Each trial lasts for 5 minutes.
The path of the animal is tracked using the automated tracking software so that number of entries and amount of time spent in the open and closed arms can be calculated. Additional behaviors such as rearing, grooming and head dips over the side of the apparatus can be monitored. An entry is counted when the center point of the animal crosses into an arm.
If a rat falls off the maze, it is quickly placed back on the open arm from which it fell, and the event is recorded in the observation notebook. If a rat freezes for an extended period of time, the behavior, along with any possible causes (e.g. sudden, loud noise outside test room) are noted, and testing continues so that all experimental animals have similar exposure times.
Time spent in the open versus closed arms is used to calculate the ratio: time in open arms / total time in arms. Similarly, the ratio of entries to open arms versus total arm entries is calculated. Using the automated tracking software, total distance traveled and velocity can also be measured in order to assess any differences in motor function between experimental groups.
Decreased anxiety is associated with a greater proportion of time spent in the open arms, while increased anxiety and fear are associated with more time spent in the closed arms. Normal animals spend significantly less time in the open arms than expected by chance (indicated by the dashed line, Fig. 3). More anxious animals will spend proportionately less time in the open arms, while less anxious animals will spend more time in the open arms (for example, after multiple exposures to the maze or following anxiolytic drug treatment).
3. Conducting the Contextual and Cued Fear Conditioning (FC) Task
Contextual and Cued Fear Conditioning (FC) task descriptions are based on procedures from Schafe et al., 1999, Winocur et al., 2006 and MacLeod et al., 20076-8.
The Fear Conditioning (FC) task is a behavioral paradigm in which animals learn to fear a previously neutral stimulus (a context, a light or a tone; the conditioned stimulus) by pairing it with noxious stimulus (usually a mild footshock; the unconditioned stimulus). Learning is rapid, robust and long-lasting, and thus the FC task is used often to study the neurobiological circuitry of learning and memory in rodents.
The FC apparatus is a 30cm X 30cm X 40cm high enclosed chamber (Phenotyper, Model 3000, Noldus Information Technology). The floor of the chamber consists of a stainless steel grid which is used to deliver the shock stimulus. The Phenotyper is equipped with a speaker that emits a 2300Hz, 90dB tone on demand, acting as the conditioned stimulus.
The shock is delivered via a shock generator (Med Associates Inc.). The shock is a momentary discomfort which produces no lasting pain or tissue damage. For both training and test trials, delivery of the shock and tone stimuli is timed and controlled automatically using the Ethovision XT add-on Trial and Hardware Control Module (version 7.0; Noldus Information Technology).
On the first day, FC training is conducted. The Phenotyper is cleaned thoroughly with 70% ethanol, and a single animal is placed in the chamber. The training trial consists of three phases; (1) a baseline phase, when no shock or tone is administered, (2) a training phase, when tone-shock pairings are presented, and (3) a post-training phase, when no shock or tone is administered. Each phase lasts approximately 5 minutes for a total training trial time of 15 minutes per animal.
During the training phase, the tone is administered for 30 seconds and coincident with the end of the tone, a 1mA shock is delivered to the grid for 1 second. This tone-shock pairing is repeated a total of 5 times, with a variable 10 to 60 second interval spacing between each pairing.
From the training phase, the percentage of time each animal spends motionless, or freezing, is measured from the final 60 seconds of the initial baseline phase (to establish spontaneous freezing before the tone-shock pairings) and from the final 60 seconds of the post-training phase (to establish freezing behavior in response to the tone-shock pairings). Normal animals display very low levels of freezing during the baseline phase and very high (~95%) levels of freezing during the post-training phase (Fig. 4).
24 hours later, the animal is returned to the identical context and observed for the 5 minute contextual FC test. No shock or tone is delivered during this phase, and percentage of time spent freezing is calculated for the entire trial. Note the importance of keeping the context constant from training to context test. In fact, having the same experimenter handle and test the animals on both days is necessary. Normal animals spend approximately 70% of the context trial engaged in freezing behavior (Fig. 4).
The cued FC test is administered one hour later. The context of the chamber is changed by removing the grid floor, adding panels to the outside of the chamber, and cleaning the floor with a scented household cleaner. The animal's freezing behavior is observed for the first minute, and freezing levels are very low if the context has been sufficiently altered (Fig. 4). The tone is then played for 3 minutes, followed by a final minute of silence, and percentage time spent freezing is calculated. Normal animals show high levels of freezing during this time (Fig. 4).
In total, 5 discrete measures of freezing behavior are collected; (1) baseline; (2) post-training; (3) context test; (4) pre-cue test; (5) cue and post-cue test.
Absence of freezing during the baseline phase establishes normal anxiety levels, while substantial freezing during the post-training phase suggests intact sensory-motor ability. Freezing during the context test establishes 24-hour memory for the training context, which relies on intact hippocampal and amygdala function9. A lack of pre-cue test freezing suggests sufficient alteration to the initial training context. Cue and post-cue freezing establishes 24-hour memory for the tone stimulus, which relies on intact amygdala function9.
4. Conducting the Morris Water Maze (MWM) Task
Morris Water Maze task procedures are based on previous descriptions10,11.
The Morris water maze (MWM) task is a test of spatial learning for rodents that requires the use of distal cues to locate a submerged escape platform in a large pool of opaque water. Large, distinct visual cues are placed around the pool in specific, constant locations. Animals learn to use these cues to navigate to the submerged platform. Animals are monitored and removed from the water if they are unable to swim.
Spatial learning is assessed across repeated trials and reference memory is determined by assessing preference for the platform area when the platform is absent. Reversal trials enhance the detection of spatial impairments, and may tax additional brain regions such as the frontal cortex10.
The MWM task is administered over 8 days and consists of 4 phases; a cued test, an acquisition phase, a 24hr probe test and a reversal learning test.
The pool is a custom-made white, polyethylene tank, 200cm in diameter and 60cm high. It is filled with water to approximately 30cm deep and made opaque with white, non-toxic paint. Water temperature is maintained between 20 and 22°C (68 and 72°F). The escape platform is a custom-made, clear plastic stand with a circular top measuring 10cm in diameter. It sits approximately 1.5cm below the surface of the water.
The cued test consists of 4 trials administered on day 1. For these trials, a distinct black and white flag is placed in the middle of the platform so that the escape location is obvious to the animals. To minimize the use of spatial information during this phase, wall cues are removed and the platform and start locations are varied from trial to trial. This phase helps rule out any sensory-motor-related differences between experimental groups before the acquisition phase starts.
For all phases of the MWM task, animals are held under the forelimbs and gently placed in the water at the designated start position, facing the wall of the pool. Animals are tested in cohorts of 8 such that there is an 8 to 10 minute inter-trial interval between each of the 4 daily trials. During the inter-trial intervals, animals are kept in warming cages to avoid hypothermia.
For the cued test, a maximum swim time of 30 seconds is allowed for the animal to climb onto the platform. If the maximum time is reached before the animal escapes, the experimenter guides it to the platform by hand and places it on the platform. The animal remains on the platform for a rest period of 20 seconds before being placed in a warming cage.
For each cued test trial, the animal is started from a different, pre-determined start location at the perimeter of the pool, and the platform is also moved to each of the 4 different quadrant positions.
The acquisition phase begins the following day and consists of 4 trials per day for 5 consecutive days. During this phase, the hidden platform remains in the same location and animals learn to navigate to it using the distal room cues.
Again, for each of the 4 trials, the animal is placed in the pool at a different, pre-determined start point. Each day, two of the start points are to the left and two of the start points are to the right of the platform, in random order, to avoid the development of egocentric spatial strategies to solve the task.
The maximum swim time for each acquisition trial is 60 seconds, after which the animal is guided to and placed on the platform by the experimenter. The animal remains on the platform for 20 seconds following escape.
On day 7, 24hr after the final training trial, a 60-second probe trial is administered in which the platform is removed. This phase determines the animal's preference for the quadrant that used to contain the platform and is used as a measure of spatial reference memory10. Following the probe trial, the platform is returned to the same quadrant and 3 regular acquisition trials are administered.
For the reversal learning test on day 8, the platform is moved to a new location in order to assess reversal learning ability. Measuring the amount of time animals spend searching for the platform in its previous location versus its new location provides an assessment of perseveration, or the inability to suppress responding to the previous location once the platform has been moved10. As for the acquisition and probe phases, 4 60-second trials are administered.
For the cued test, latency to find the platform and velocity are used as measures of visual and motor function, respectively. Differences between the experimental groups during this phase must be taken into account in interpretation of acquisition and memory results as differences in sensory-motor function between groups may exist.
For the acquisition phase, mean latency to find the platform and/or path length are used as measures of spatial learning. In normal animals, escape latency gradually decreases over the five acquisition days such that mean escape time is around 15 to 20 seconds on day 5 (Fig. 5A).
For the probe phase, mean distance to platform (Fig. 5B) and/or latency to first reach the platform's previous location (Fig. 5C) are used as measures of spatial reference memory.
For the reversal phase, percent time spent in old versus new quadrant containing the platform is used as a measure of perseveration. Over the four trials, preference for the old platform location should decrease as preference for the new platform location is established (Fig. 5D).
5. Representative results:
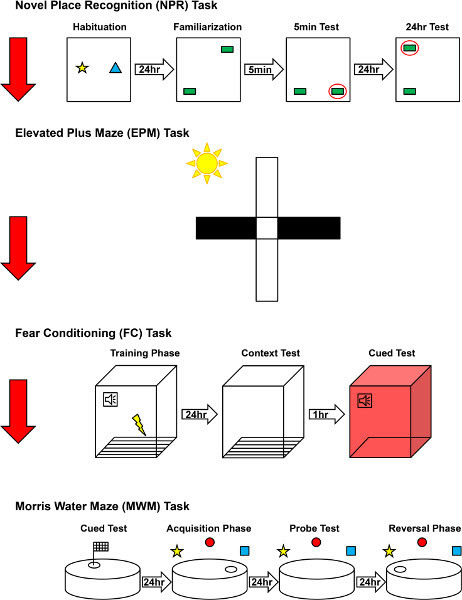 Figure 1. Schematic representation of our cognitive testing paradigms.
Figure 1. Schematic representation of our cognitive testing paradigms.
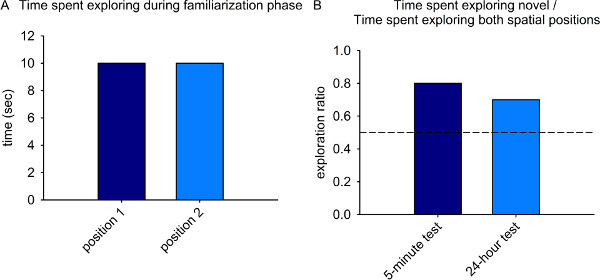 Figure 2. Representative results from control animals on the novel place recognition task. A) Total time spent exploring the two spatial positions should be approximately equal during the familiarization phase. B) Exploration ratio (time spent exploring novel position / time spent exploring both positions) shows a clear preference for the novel spatial position following the 5-minute and 24-hour delay intervals. The dashed line indicates chance performance.
Figure 2. Representative results from control animals on the novel place recognition task. A) Total time spent exploring the two spatial positions should be approximately equal during the familiarization phase. B) Exploration ratio (time spent exploring novel position / time spent exploring both positions) shows a clear preference for the novel spatial position following the 5-minute and 24-hour delay intervals. The dashed line indicates chance performance.
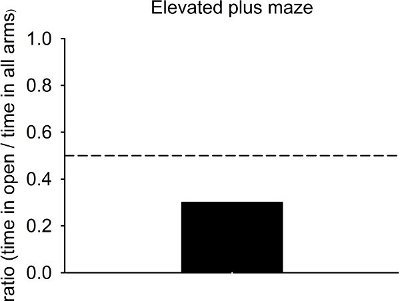 Figure 3. Representative results from control animals on the elevated plus maze. Normal animals spend less time than expected by chance in the open arms (indicated by the dashed line). Anxious animals will spend proportionately less time in the open arms compared to controls, whereas multiple exposures to the maze or treatment with anxiolytic agents will increase time spent in the open arms.
Figure 3. Representative results from control animals on the elevated plus maze. Normal animals spend less time than expected by chance in the open arms (indicated by the dashed line). Anxious animals will spend proportionately less time in the open arms compared to controls, whereas multiple exposures to the maze or treatment with anxiolytic agents will increase time spent in the open arms.
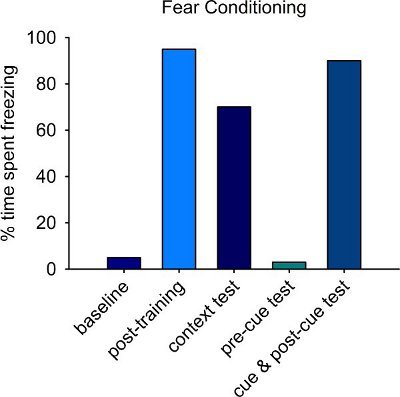 Figure 4. Representative results from control animals on the fear conditioning task. Low levels of freezing are observed during initial exposure to the chamber (baseline), and when the context is altered prior to the cue test (pre-cue test). By contrast, normal animals spend most of the post-training period engaged in freezing behavior. If animals remember the context in which training occurred following a 24hr delay, they will engage in freezing behavior for approximately 70% of the 5-minute context trial. Similarly, if animals remember the tone, they show high levels of freezing during the cue and post-cue test.
Figure 4. Representative results from control animals on the fear conditioning task. Low levels of freezing are observed during initial exposure to the chamber (baseline), and when the context is altered prior to the cue test (pre-cue test). By contrast, normal animals spend most of the post-training period engaged in freezing behavior. If animals remember the context in which training occurred following a 24hr delay, they will engage in freezing behavior for approximately 70% of the 5-minute context trial. Similarly, if animals remember the tone, they show high levels of freezing during the cue and post-cue test.
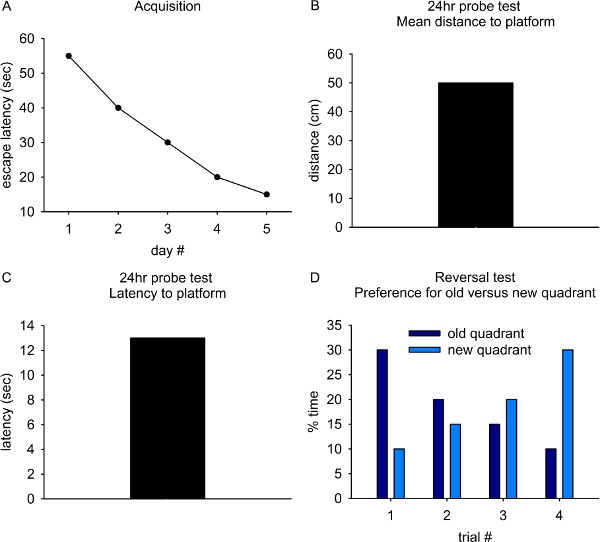 Figure 5. Representative results from control animals on the Morris Water Maze task. A) Escape latency decreases with progressive training during the acquisition phase. B) and C) Mean distance from and latency to first cross the previous platform location during the 24hr probe test are used as measures of spatial reference memory. D) Reversal learning is assessed by changing the location of the platform on the last day of testing. Over the 4 trials, normal animals gradually stop searching the old quadrant and learn that the platform is in a new location.
Figure 5. Representative results from control animals on the Morris Water Maze task. A) Escape latency decreases with progressive training during the acquisition phase. B) and C) Mean distance from and latency to first cross the previous platform location during the 24hr probe test are used as measures of spatial reference memory. D) Reversal learning is assessed by changing the location of the platform on the last day of testing. Over the 4 trials, normal animals gradually stop searching the old quadrant and learn that the platform is in a new location.
Discussion
There is a pressing need for long-term strategies designed to forestall the development of cognitive dysfunction that is becoming increasingly common in cancer survivors12,13. Stem cell therapies may provide the means for ameliorating a range of normal tissue sequelae in the CNS, as they provide the opportunity for replacing cells lost or damaged from cytotoxic cancer treatments14. To hasten the realization of these efforts, we have undertaken a series of proof of principal experiments that have confirmed the capability of cranially engrafted hNSCs to functionally restore cognition within the irradiated tissue bed of the CNS3. Critical to this endeavor was the capability to reliably conduct a series of carefully controlled cognitive tests in a reproducible manner. We have detailed these critical procedures in efforts to expedite the translational potential of stem cell therapies in the clinic. Further considerations that are likely to have a significant impact of the quality of data are highlighted below.
Cognitive testing demands careful attention to detail and adhering to certain standard practices can help minimize artifacts that complicate the interpretation of results. The use of dedicated behavioral testing rooms separate from animal holding rooms and experimenters is preferable. It is also prudent to use "white noise" that blocks external noises in the animal facility that may distract animals from the task at hand. To minimize stress to the animals destined for cognitive testing, it is important for them to be familiar with the experimenters that will perform behavioral testing prior to task administration. In general, if testing females and males, it is recommended to test females first as they tend to be more anxious during testing if male odor cues remain in the testing arenas. When administering a serial battery of tasks, it is important to expose animals to least stressful tasks first (like EPM, NPR) and most stressful tasks last (like FC, MWM).
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by NIH NINDS grant R01 NS074388 581 (C.L. Limoli), California Institute for Regenerative Medicine (CIRM) Grant RS1-00413 (C.L.L.) and a CIRM Grant to JOVE in support of the video documentation. We would also like to thank Rudy Limburg and Edward Lau of University of California, Irvine’s Biological Sciences Machine Shop for their advice, support and superb technical skills in preparation of all custom-made materials used for behavioral testing.
References
- Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta. Oncol. 1996;35:659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Godber T, Smibert E, Weiskop S, Ekert H. Cognitive and academic outcome following cranial irradiation and chemotherapy in children: a longitudinal study. Br. J. Cancer. 2000;82:255–262. doi: 10.1054/bjoc.1999.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19150–19155. doi: 10.1073/pnas.0909293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod JE. Cancer chemotherapy impairs contextual but not cue-specific fear memory. Behav Brain Res. 2007;181:168–172. doi: 10.1016/j.bbr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn. Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain. Res. Brain. Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J. Clin. Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum. Brain. Mapp. 2010. [DOI] [PMC free article] [PubMed]
- Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert. Rev. Neurother. 2008;8:1193–1201. doi: 10.1586/14737175.8.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]


