Abstract
The activation of vertebrate development at fertilization relies on IP3-dependent Ca2+ release, a pathway that is sensitized during oocyte maturation. This sensitization has been shown to correlate with the remodeling of the endoplasmic reticulum into large ER patches, however the mechanisms involved are not clear. Here we show that IP3 receptors within ER patches have a higher sensitivity to IP3 than those in the neighboring reticular ER. The lateral diffusion rate of IP3 receptors in both ER domains is similar, and ER patches dynamically fuse with reticular ER, arguing that IP3 receptors exchange freely between the two ER compartments. These results suggest that increasing the density of IP3 receptors through ER remodeling is sufficient to sensitize IP3-dependent Ca2+ release. Mathematical modeling supports this concept of ‘geometric sensitization’ of IP3 receptors as a population, and argues that it depends on enhanced Ca2+-dependent cooperativity at sub-threshold IP3 concentrations. This represents a novel mechanism of tuning the sensitivity of IP3 receptors through ER remodeling during meiosis.
Introduction
The egg-to-embryo transition marks the initiation of multi-cellular organismal development and is instigated by a series of cellular events following fertilization collectively referred to as egg activation [1]. These events are encoded in a sequential fashion by the fertilization-induced Ca2+ transient, which possesses specialized spatial and temporal dynamics that are necessary and sufficient for egg activation [2]. The egg acquires the competency to produce the fertilization-specific Ca2+ signal during oocyte maturation, a complex developmental pathway that prepares the egg for fertilization. Oocyte maturation is driven by multiple kinase cascades induced downstream of the hormonal signal that releases meiotic arrest [3]. The dynamic remodeling of Ca2+ signaling is fundamental to the developmental competence of the egg [2]. It further elegantly illustrates the versatility of Ca2+ signals given the broad bandwidth inherent in their temporal and spatial features. This results in an information rich signal that instructs multiple cellular events at egg activation, including the block to polyspermy and completion of meiosis [2].
At steady state IP3 receptors in different cell types cluster in a hierarchical fashion leading to Ca2+ release events of varying sizes, referred to as blips for the smallest events, presumably due to the opening of a single IP3 receptor, or puffs for larger events [4]–[6]. Such elementary events coalesce to produce global Ca2+ release waves [7]–[9]. In addition, studies have shown that sustained IP3 or Ca2+ levels lead to a slow aggregation of IP3 receptors into large clusters independently of ER structure, presumably through protein-protein interactions [10]–[12]. Functionally IP3 receptor clustering allows for a continuum of the IP3-dependent Ca2+ release events that serve specific cellular needs.
An important aspect of Ca2+ signaling differentiation during oocyte meiosis that is conserved among different species, is the increased sensitivity of IP3-dependent Ca2+ release [13]–[15]. Ca2+ release through IP3 receptors is a major contributor to the fertilization-induced Ca2+ signal in vertebrates [16]. The enhanced sensitivity of IP3-dependent Ca2+ release is often described as a larger Ca2+ transient in eggs following store mobilization [14], [17], [18]. However this does not necessarily translate into sensitization of IP3 receptors per se, since additional factors could contribute to the duration and amplitude of the Ca2+ release signal, including Ca2+ extrusion and influx, cytosolic buffering capacity, and Ca2+ reuptake into stores. A more accurate description of IP3 receptor sensitization is reflected as Ca2+ release in the egg at lower threshold IP3 concentrations that induce minimal or no release in the immature oocyte [13], [15]. Also noteworthy is the fact that additional Ca2+ signaling pathways are modulated during meiosis, including inhibition of Ca2+ influx, internalization of the plasma membrane Ca2+-ATPase, and Ca2+ recycling between the cytoplasm and ER lumen due to prolonged opening of IP3 receptors [19]–[23]. Collectively modulation of these different Ca2+ signaling modules defines the dynamics of the fertilization-induced Ca2+ transient.
Enhanced Ca2+ release in the egg correlates with IP3 receptor phosphorylation, and is clearly dependent on the activation of the oocyte maturation kinase cascades [15], [24], [25]. Although this correlation postulates a direct role of IP3 receptor phosphorylation in the sensitization of Ca2+ release [24]–[26], this remains to be shown. A potential mechanism that could contribute to IP3 receptor sensitization is increased affinity of the IP3 receptor [27]. However, this has not been proven experimentally, highlighting the fact that the mechanistic regulation of IP3-dependent Ca2+ release sensitization during meiosis remains largely undefined. In addition, the number of functional IP3 receptors increases during Xenopus oocyte maturation following the disruption of annulate lamellae, which are specialized membranous structures where the activity of a sub-population of IP3 receptors is inhibited [28]. This inhibition is due to nuclear pore complexes, which are enriched in annulate lamellae as a molecular stockpile for embryonic divisions [29].
Furthermore, alterations to IP3-dependent Ca2+ release have been shown to correlate with ER remodeling in oocytes from several species [30]. In Xenopus ER patches nucleate Ca2+ waves and patch appearance coincides with IP3-dependent Ca2+ release sensitization [17], [28]. Similarly in mouse eggs the Ca2+ wave pacemaker localizes spatially with ER patches, and ER remodeling correlates with modulation of IP3-dependent Ca2+ release [31], [32]. This has led to the suggestion that the physical clustering of the ER contributes to the greater sensitivity to IP3 in the mature egg [30], however the mechanisms underlying such a role for ER remodeling are not known. Data presented herein provide a mechanistic explanation for the correlation between ER remodeling and IP3-dependent Ca2+ release sensitization. IP3 receptors enriched in ER patches respond to sub-threshold IP3 concentrations that do not produce Ca2+ release in the neighboring reticular ER, showing that IP3-dependent Ca2+ release is sensitized in ER patches. We present evidence that this sensitization in ER patches is due to enhanced Ca2+-dependent cooperativity between receptors due to the increased density of IP3 receptors within an ER patch.
Results
ER remodels during meiosis
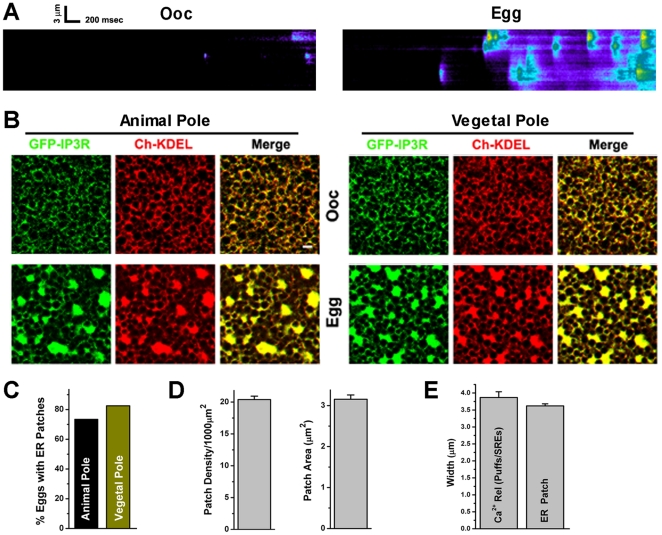
We have previously shown that elementary IP3-dependent Ca2+ release events (Ca2+ puffs) cluster dramatically during oocyte maturation, and that this clustering is important for the mode and speed of Ca2+ wave propagation in the egg [15]. Whereas Ca2+ puffs in the oocyte are separate, they coalesce in the egg following maturation (Fig. 1A). Ca2+ transients were imaged in line scan mode on the animal hemisphere while continuously uncaging IP3 using the near UV 405 nm laser at low intensity (Fig. 1A). This allows for gradual build-up of IP3 concentration thus inducing Ca2+ puffs (Fig. 1A). Note the spatially overlapping Ca2+ puffs in the egg (Fig. 1A). Because ER remodeling was reported to be limited to the vegetal hemisphere of the egg [17], we initially interpreted the clustering of Ca2+ puffs on the animal hemisphere as being due to lateral diffusion of IP3 receptors in the plane of the ER membrane to form overlapping Ca2+ release sites [15]. Attempts to visualize such IP3 receptor clusters in eggs expressing GFP-tagged IP3 receptor were unsuccessful. Rather we observed large three dimensional aggregations of IP3 receptors that were reminiscent of ER patches observed during maturation (Fig. 1B) [17]. Indeed these IP3 receptor aggregations co-localize with the ER marker, mCherry-KDEL (Fig. 1B). In the immature oocyte both the IP3 receptor and ER have a reticular distribution, that remodels into a combination of reticular and large ER ‘patches’ during oocyte maturation (Fig. 1B, Egg). Contrary to what has been previously reported [17], ER reorganization is observed on both the animal and vegetal poles of the egg (Fig. 1B). We assessed ER remodeling following oocyte maturation in 166 eggs from 14 donor females using various markers for the ER, including the IP3 receptor, STIM1, GFP-KDEL and mCherry-KDEL. In all cases we observed ER remodeling on the animal hemisphere. Although ER patches are observed at a slightly lower frequency on the animal pole, with pronounced cell to cell variability, they are present in most eggs (73.5%) (Fig. 1C). ER patch density was ∼20/1000 µm2 with an average area of 3.1+0.087 µm2 (Fig. 1D), consistent with a previous report [17], but not others [28], [29]. Nonetheless, this only provides a snapshot of ER remodeling, since ER patches in the egg are dynamic motile structures that are continuously restructured (Figure 2A). Furthermore, the steady state distribution of ER patches shows a more cortical residence in the vegetal compared to the animal hemisphere (Fig. S1A), and their formation depends on the microtubule network, since 75% of oocytes (n = 36) treated with nocodazole did not form ER patches during maturation (Fig. S1B).
Figure 1. ER remodeling and IP3 receptor clustering during meiosis.
A. Functional clustering of elementary Ca2+ release events during oocyte maturation. Xenopus oocytes were injected with 10 µM caged IP3 and 40 µM Oregon-green. Oocyte maturation was induced with progesterone and both immature oocytes and fully mature eggs were imaged in linescan mode at 488 nm with the 405 nm laser at low intensity (0.2%) to continuously uncage cIP3. The same region in the cell was scanned continuously in linescan mode with the x-axis representing time and the y-axis space. The single isolated Ca2+ puffs observed in the oocyte coalesce into larger release events referred to as single release events (SRE). B. The ER remodels during oocyte maturation to form large patches in the egg. Oocytes were injected with GFP-IP3 receptor (IP3R) (50 ng/cell) and mCherry-KDEL (10 ng/cell). Images show the formation of ER patches in both animal and vegetal hemisphere to which IP3 receptors localize (Scale bar, 2 µm). C. Frequency of ER patches on the animal and vegetable poles (n = 166; 14 frogs). D. ER patch density and area (n = 38). E. Width of elementary Ca2+ release events in the egg as compared to the width of ER patches.
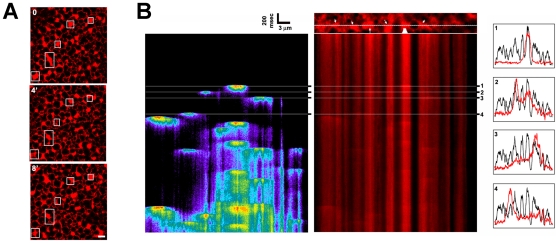
Figure 2. A. ER patches remodel continuously.
Examples of images from a time series from an egg expressing mCherry-KDEL (Scale bar, 2 µm). B. Elementary Ca2+ release events in the egg localize to ER patches. mCherry-KDEL expressing eggs injected with cIP3 and Oregon-green were line-scanned with the 405 nm laser on (0.2%). Left image show Ca2+ release events and the right ER distribution. The xy image with the linescan area is shown on top of the ER linescan. Histograms of Ca2+ release (red trace) and ER distribution (black trace) are shown for four selected areas as indicated by the numbers.
ER remodeling into large patches sensitizes IP3-dependent Ca2+ release
The fact that ER remodeling was observed on both the animal and vegetal hemispheres argues that Ca2+ puffs clustering during meiosis is not due to lateral diffusion of IP3 receptors, but rather to ER remodeling. This is supported by the similar width of ER patches and that of elementary Ca2+ release events (SREs) in eggs (Fig. 1E). In addition, if ER remodeling underlies Ca2+ puff redistribution, then Ca2+ release activity should coincide spatially with ER patches. This is indeed the case as shown in Figure 2B. Gradual uncaging of IP3 results, after a time lag, in Ca2+ release SREs that coalesce into a Ca2+ wave (Fig. 2B). Cross sectional profiles of both ER distribution (black) and Ca2+ release activity (red) demonstrate co-localization of individual SREs to ER patches (Fig. 2B). This co-localization was observed consistently in at least 15 cells tested and shows that elementary Ca2+ release events in the egg localize to ER patches.
IP3 receptors localize to both the reticular ER and ER patches in the egg (Fig. 1B), yet initial Ca2+ release events are observed preferentially within ER patches (Fig. 2B) [28]. This argues for a differential sensitivity of IP3 receptors based on the ER sub-domain they localize to. To test whether this is the case we undertook of series of functional experiments to directly assess Ca2+ release sensitivity in the different ER spatial domains.
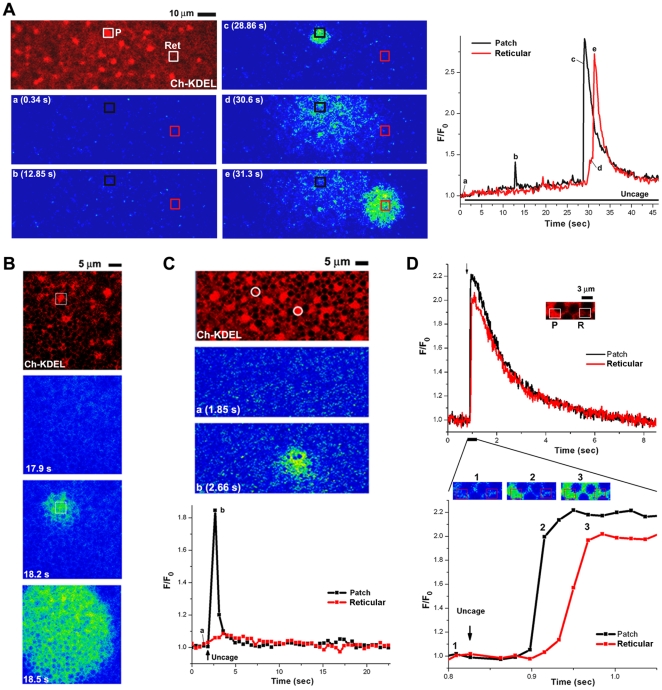
In the first set of experiments we defined two regions of interest, one over an ER patch (P) and the other over the reticular ER (Ret), and imaged Ca2+ dynamics while continuously uncaging cIP3 in these ROIs using the 405 nm laser at low intensity to gradually buildup IP3 concentrations (Fig. 3A). An initial Ca2+ release spike is observed from the ER patch (Fig. 3A, marked b on the images & trace), followed by a larger Ca2+ release transient from the same patch (Fig. 3A, marked c at 28.86 sec). This larger Ca2+ release transient from the ER patch ROI results in a Ca2+ rise over a broad area that eventually diffuses to the reticular ER ROI (Ret). This results in a local Ca2+ rise over the reticular ER ROI (Fig. 3A, observed as a shoulder in the trace marked as d), which triggers Ca2+ release (Fig. 3A, panel e & trace).
Figure 3. Sensitization of Ca2+ release in ER patches.
Eggs expressing mCherry-KDEL were injected with cIP3, and Oregon-green and imaged ∼1 hr after GVBD. A–B. IP3 receptors in ER patches respond to IP3 concentrations not detected by receptors in the reticular ER. ER distribution and Ca2+ release were imaged over time, while continuously uncaging cIP3 only within two ROIs as indicated by the boxes using the 405 nm laser. One ROI was placed over an ER patch (P) and the other over a reticular ER domain (Ret). Ca2+ release events over the entire time course within the two ROIs are shown on the right. B. Same experimental conditions as in A, except that uncaging with the 405 nm laser was performed over the entire field continuously. The white box shows the localization of the first release event to an ER patch. C–D. A single sub-threshold uncaging pulse results in preferential Ca2+ release from ER patches as compared to the reticular ER. In this case a single uncaging pulse was applied (405 nm laser 50% power) only within the circular ROIs (white circles) as indicated by ‘Uncage’ on the traces in the lower panel. One ROI is over an ER patch and the other over the reticular ER. D. Same imaging conditions over a small area (inset, 9.15×3 µm) where a single uncaging pulse was applied over the entire imaged area. Ca2+ release was then analyzed from two region as indicated by the boxes representing an ER patch and the neighboring reticular ER. The lower traces show an expanded time scale of Ca2+ release.
Under this experimental paradigm IP3 is uncaged at the same rate in both the patch and reticular ER ROIs, arguing that IP3 receptors in both ROIs are subject to similar IP3 concentrations over a prolonged period of time (10–20 sec). Nonetheless, only IP3 receptors in the patch ER ROI respond by releasing Ca2+. IP3 receptors in the reticular ER ROI respond only when a local Ca2+ rise occurs due to the diffusion of Ca2+ released from the ER patch ROI. This fits with Ca2+ acting as a co-agonist of the IP3 receptor, where at a constant IP3 concentration a Ca2+ rise below a certain threshold increases the probability of opening of IP3 receptors, since IP3 receptor gating exhibits a bell-shaped dependence on Ca2+ [33], [34]. These results suggest that IP3 receptors in ER patches are more sensitive when compared to those in the reticular ER. Note that the amount of Ca2+ released from the reticular ER ROI is comparable to that observed over the ER patch ROI (Figure 3A, trace), arguing that local luminal Ca2+ content is not limiting.
We next replicated the conditions in the line scan protocol (Fig. 2B) by imaging Ca2+ transients while continuously uncaging IP3 at low amplitude across the entire field (Fig. 3B). This results in a propagative wave that is invariably initiated over an ER patch (Fig. 3B, white box) in all 12 cells tested. Therefore, as IP3 levels build up, Ca2+ release is preferentially triggered from ER patches as compared to the reticular ER, favoring the argument that IP3 receptors in ER patches are sensitized to IP3 compared to receptors in the reticular ER. In these experiments IP3 receptors in the two ER domains are exposed to similar IP3 concentration for several seconds. This prolonged exposure to IP3 shows that IP3 receptors in ER patches respond to sub-threshold IP3 concentrations that are incapable of inducing Ca2+ release from the reticular ER.
In the previous experimental approaches IP3 was gradually increased over time through continuous uncaging of cIP3. Given that IP3 can result in Ca2+-independent IP3 receptor inactivation [35], [36], the gradual slow increase in IP3 could potentially result in receptor inactivation, which may dampen Ca2+ release from the reticular ER. With this concern in mind, we designed experiments to define the sensitivity of IP3-dependent Ca2+ release in different ER domains following a single uncaging pulse within two ROIs (circles), one over an ER patch and the other over a reticular ER region (Fig. 3C). The uncaging pulse induced Ca2+ release from the ER patch but not the reticular ER ROI (Fig. 3C). This experiment was repeated on 11 cells with 8/11 showing Ca2+ release only from the ER patch domain (Fig. 3C), and 3/11 releasing Ca2+ from both the ER patch and reticular domain, albeit with a lower amplitude from the reticular domain. In a complementary approach a small region was scanned continuously and the entire imaged field stimulated by a single uncaging pulse (Fig. 3D). The expanded time scale and corresponding images show that Ca2+ release is initiated from the ER patch domain and spreads to the rest of the imaged field (Fig. 3D). This was observed in 14/17 cells, while 3/17 showed simultaneous release from both ER domains. Collectively these different approaches show that IP3 receptors within ER patches can sense and respond to IP3 concentrations not detectable by IP3 receptors in the neighboring reticular ER. This suggests that increasing IP3 receptor density due to ER remodeling sensitizes IP3-dependent Ca2+ release.
Role of Ca2+-dependent cooperativity within ER patches in sensitizing IP3-dependent Ca2+ release
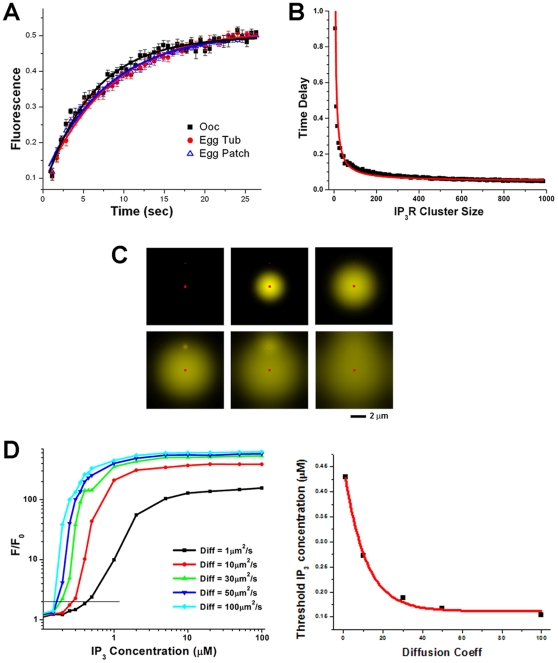
How can the sensitivity of IP3 receptors in distinct ER sub-domains be modulated differentially? One possibility is that there exists a different population of IP3 receptors in ER patches versus the reticular ER with differential posttranslational modifications and/or protein-protein interactions. To test whether this is the case we measured the mobility of GFP-tagged IP3 receptors in the two ER sub-domains using fluorescence recovery after photo-bleaching (FRAP) (Fig. 4A). The rate of recovery from photo-bleaching was indistinguishable in ER patches and the reticular ER, and was comparable to that of IP3 receptors in oocytes before ER remodeling (Fig. 4A). This argues that IP3 receptors' lateral mobility is equivalent between the different ER subdomains. However, the recovery from photobleaching was not complete (Fig. 4A) showing that a sub-population of IP3 receptors in immobile. The immobile fraction of IP3 receptors in oocytes (57.14±0.86), egg reticular ER (56.5+0.86) and egg ER patches (57.61+0.57) is also similar (n = 14–21). Given the percent immobile fraction it is possible that a sub-population of IP3 receptors localize differentially to ER compartments. This is unlikely though, because ER patches are continuously remodeling and fusing with reticular ER domains (Fig. 2A), arguing that the same population of IP3 receptors exists in ER patches and reticular ER domains.
Figure 4. A. IP3 receptors lateral mobility in ER patches and reticular ER.
Fluorescence recovery after photobleaching (FRAP) in oocytes and eggs expressing GFP-IP3 receptor (n = 7–8; mean±SE). The bleaching ROIs in eggs were positioned over ER patches or reticular ER. Recovery kinetics were fitted with a monoexponential decay function. Recovery kinetics show that the lateral mobility of IP3 receptors is comparable within ER patches and the reticular ER. B–D. Mathematical modeling. Modeling the time delay in Ca2+ release as a function of IP3 receptor cluster size. As IP3 receptor cluster size increases the delay in response to an IP3 pulse decreases. C. Modeling Ca2+ release spatial and amplitude distribution from two IP3 receptor clusters of varying size (20 and 1000) in response to a sub-threshold IP3 pulse. D. Simulation of the dependence of Ca2+ release within an IP3 receptor cluster as a function of IP3 concentration for varying Ca2+ diffusion coefficients. Lowering the Ca2+ diffusion coefficient results in less Ca2+-dependent cooperativity between neighboring receptors. Decreasing Ca2+ diffusion coefficient leads to decreased sensitivity of IP3 receptors as illustrated by the threshold IP3 concentration required to initiate Ca2+ release.
We then considered the possibility that the density of IP3 receptors in the different ER sub-domains modulates their sensitivity as a population. The probability of opening (Po) of IP3 receptors exhibits a bell-shaped dependence on Ca2+ [33], [34], which plays a role in the cooperativity of gating of IP3 receptors within a cluster [4], [37], [38]. At the ultrastructural level ER patches in the egg are formed by a complex three dimensional ER rearrangement (Fig. S2) [17], which brings IP3 receptors in close physical proximity. Therefore, increased density of IP3 receptors within an ER patch could cooperatively increase their Po, leading to functional sensitization. To determine whether this is the case we modeled the time delay in Ca2+ release as a function of IP3 receptor cluster size, which shows an inverse relationship (Fig. 4B). Therefore, the higher the density of IP3 receptors (large cluster) the shorter the time delay in responding to IP3 to gate the receptor open (Fig. 4B). This is consistent with IP3 receptor sensitization due to increased density. We then modeled the response of two IP3 receptor clusters of different sizes (20 or 1000 receptors) (Fig. 4C). Simulating an IP3 rise across the entire field leads to Ca2+ release from the large IP3 receptor cluster, which diffuses to the smaller cluster leading to Ca2+ release (Fig. 4C), replicating the experimental observation in Figure 3A, and showing that IP3 receptors within the large cluster are more sensitive to IP3. Details of the mathematical model are discussed in Appendix S1.
We then tested whether Ca2+-dependent cooperativity within a large cluster of IP3 receptors modulates their sensitization. To alter Ca2+-dependent cooperativity we varied Ca2+ diffusion coefficient, reasoning that higher Ca2+ diffusion coefficients increase IP3 receptors cooperativity by enhancing cross-talk (Ca2+-dependent gating) between channels within a cluster. Changing the Ca2+ diffusion coefficient will not affect IP3 receptor affinity to its ligand IP3. A dose response relationship of IP3-dependent Ca2+ release as a function of IP3 concentrations shows a shift to the right as Ca2+ diffusion coefficient decreases (Fig. 4D). From this plot one can estimate the sensitivity of IP3-dependent Ca2+ release as the threshold IP3 concentration required to produce Ca2+ release (F/F0), in this case set as double the resting Ca2+ (Fig. 4D, left panel). The threshold IP3 concentration decays exponentially as a function of the Ca2+ diffusion coefficient (Fig. 4D, right panel). This supports a model, where clustering of IP3 receptors within the three dimensional space of an ER patch enhances channel cooperativity leading to their sensitization. The sensitization of IP3 receptors in this case is a population property that is independent from the IP3 affinity of each individual IP3 receptor. These results show that ER remodeling tunes the sensitivity of IP3 receptors by increasing their density and as such Ca2+-dependent cooperativity. When IP3 receptors are concentrated in a three-dimensional volume as in the context of an ER patch, stochastic opening of individual receptors would lead to Ca2+-dependent sensitization of neighboring receptors resulting in Ca2+ release.
ER remodeling modulate Ca2+ wave propagation
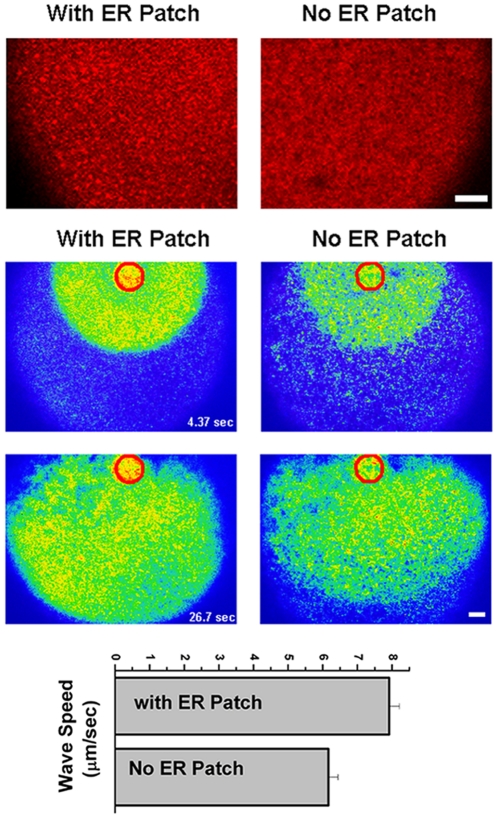
To test the role of ER patches in Ca2+ wave propagation we measured the speed of propagation of the Ca2+ wave in eggs with or without ER patches on the animal hemisphere. As shown in Figure 1C a small percentage of eggs exhibit no ER patches. We tried to increase this percent by treating cells with nocodazole, however found that when cells were injected with Oregon-green, nocodazole was no longer effective at preventing patch formation. The reasons for this observation are unclear but could be due to a Ca2+-dependence of nocodazole action on microtubules. Nonetheless, the natural diversity of patch occurrence in individual oocytes allowed us to test whether the presence of ER patches has any effect on the speed of propagation of the Ca2+ wave. An uncaging pulse on the animal hemisphere leads to a global Ca2+ wave in both cells with or without ER patches (Fig. 5). However, the speed of propagation was significantly slower is cells with no ER patches (p = 1.2×10−4) (Fig. 5), arguing that ER patches are important in modulating Ca2+ wave speed.
Figure 5. Role of ER remodeling in Ca2+ wave propagation.
Cells expressing mCherry-KDEL to visualize ER distribution were injected with caged-IP3 and Oregon-Green to image Ca2+ dynamics. Top panel shows an enlarged region from two cells, one with ER patches and one without. Ca2+ waves were induced by uncaging IP3 within an ROI (red circle) and wave propagation speed was measured. Example images of wave propagation are shown. These experiments were performed on the animal hemisphere (n = 12–13).
Role of the oocyte maturation kinase cascade in ER remodeling and IP3 receptor sensitization
In both Xenopus and mouse oocytes the kinase cascade driving maturation plays an important role in the sensitization of IP3-dependent Ca2+ release [24]–[26]. In Xenopus, sensitization of Ca2+ release correlates with the activation of MPF and hyper-activation of either the MAPK cascade or MPF is sufficient to sensitize Ca2+ release [24]. The IP3 receptor is phosphorylated at consensus MPF/MAPK sites during oocyte maturation [24]. Furthermore, modeling studies suggest that increased IP3 receptor affinity is a determinant of the changes observed in both elementary and global Ca2+ signaling during oocyte maturation [27]. This raises questions regarding the relative contribution of the oocyte maturation kinase cascade, ER remodeling and IP3 receptor phosphorylation to IP3 receptor sensitization. To address this issue, we correlated Ca2+ release sensitivity with the activation state of the kinase cascades and the formation of ER patches. To assess the sensitivity of IP3-dependent Ca2+ release a threshold uncaging pulse was empirically defined in oocytes and applied to eggs (Fig. 6). Normalization of the data to the Ca2+ release levels in oocytes provides a measure of the sensitization of Ca2+ release in response to IP3 [24].
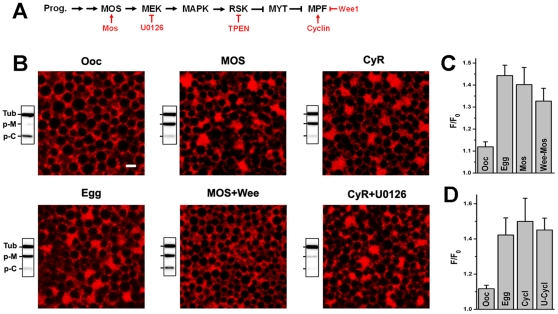
Figure 6. MPF is required for ER remodeling during meiosis.
A. Simplified kinase cascade activated during oocyte maturation. Indicated in red is the action of different molecular and pharmacological modulators. The arrow indicates activation and the bar inhibition. B. Confocal fields from individual cells expressing mCherry-KDEL and treated as indicated showing ER structure. After imaging cells were lysed and subjected to Western blotting analysis for phosphor-MAPK (p-M), phosphor-Cdc2 (p-C) and tubullin (Tub) as the loading control (Scale bar, 2 µm). Mos indicates cells injected with Mos RNA, CyR: Cyclin B1 RNA; MOS+Wee: inject Wee1 RNA and incubate cells overnight before Mos RNA injection; CyR+U0126: pre-treat with U0126 (50 µM) for 1 h before Cyclin B1 RNA injection. The effectiveness of these treatments is illustrated in Western blots performed on individual oocytes after confocal imaging. The top band (Tub) represents the tubulin loading control, the middle band phosphorylated MAPK (p-M) and the lower band phosphorylated cdc2 at Tyr-15 (p-C). MAPK phosphorylation indicates its activation, whereas the phosphorylation of Cdc2, the kinase subunit of MPF, indicates its inhibition. C–D. Sensitivity of IP3-dependent Ca2+ release measured as indicated in the text.
Modulation of the kinase cascade by different molecular or pharmacological regulators allows differential activation of kinases along the cascade as summarized in Figure 6A. In contrast to oocytes, in eggs MAPK and MPF are active, ER patches are apparent and IP3-dependent Ca2+ release is sensitized (Fig. 6B–D, Egg). Expressing Mos or Cyclin B (CyR) activates both MAPK and MPF, and results in ER patch formation and sensitization of Ca2+ release (Fig. 6B–D). Activation of MPF independently of MAPK using the Cyclin B-U0126 approach (Fig. 6A), results in ER patch formation and sensitization of Ca2+ release (Fig. 6B & D), showing that MPF activation is sufficient to remodel the ER during meiosis. This is confirmed by activation of the MAPK cascade independently of MPF, using Wee1 followed by Mos expression, which does not result in ER reorganization into patches (Fig. 6B). However, in this case Ca2+ release is still sensitized (Fig. 6C), as previously shown [24]. These data argue that ER remodeling during meiosis requires MPF, the master kinase that drives the cell division phase. However, the sensitization of IP3-dependent Ca2+ release still occurs in the absence of ER remodeling when the MAPK cascade is hyper-activated, suggesting that both ER remodeling and phosphorylation of either the IP3 receptor, or other effectors that modulate IP3 receptor activity, contribute to sensitization of IP3-dependent Ca2+ release during meiosis. As such they may represent redundant mechanisms that ensure IP3 receptor sensitization, and egg activation at fertilization.
Discussion
The IP3 receptor transduces signals downstream of PLC-coupled receptors into Ca2+ transients, and acts as a central signal integrator through its large cytoplasmic domain, which interacts with multiple modulators [39]. Ca2+ released through IP3 receptors plays critical cellular functions, including the regulation of gene expression, cell growth and secretion. IP3 receptors coalesce into clusters of several IP3 receptors, which underlie elementary Ca2+ release events. The size of these elementary events depends on the number of active receptors, which is regulated by cross talk between IP3 receptors within a cluster through Ca2+-dependent potentiation or potentially direct protein-protein interaction [4]–[6]. Whether the size and function of these clusters is modulated by the ligand IP3 is a matter of debate [40], [41]. In addition to this steady-state physiological clustering of IP3 receptors, significantly larger clusters detectable by fluorescence microscopy (0.6–1 µm), are induced following sustained stimulation of Ca2+ signaling [10]–[12], however the physiological significance of this clustering is unclear. These large IP3 receptor aggregations appear to be due to lateral diffusion of IP3 receptors and protein-protein interactions, and do not involve changes in ER structure [10]–[12]. In contrast, the clustering of IP3 receptors observed during oocyte meiosis is due to restructuring of the ER. We show that ER remodeling during Xenopus oocyte meiosis requires MPF activation, consistent with results in mouse [31] and nemertean eggs [42]. Furthermore, MPF activation has been shown in several species to be important for Ca2+ signaling differentiation during oocyte maturation [2], [22], [24], [43]–[45].
In the context of oocyte maturation in preparation for fertilization, IP3-dependent Ca2+ release is sensitized and contributes to the generation of the specialized Ca2+ transient, which is essential for egg activation [2]. Remodeling of the ER during oocyte meiosis is well documented in different species, and ER patches are enriched in the egg's cortex [17], [32], [46]–[49]. The formation of such ER patches correlates with the sensitization of IP3-dependent Ca2+ release [17], [28], [32], and as such they have been postulated as initiation sites for repetitive Ca2+ waves in mouse, and as important for wave propagation in Xenopus [17], [30]. Furthermore, the tight correlation between ER reorganization and Ca2+ signaling remodeling during oocyte maturation suggests a has (reviewed in [30]. However, the mechanisms underlying a potential role for ER restructuring during meiosis in modulating Ca2+ signaling are unknown. Here we show that the remodeling of the ER underlies the clustering of elementary Ca2+ release events observed during meiosis [15], since the large elementary Ca2+ release events in the egg (SREs) localize to ER patches (Fig. 2). Furthermore, ER remodeling also affects global Ca2+ dynamics in the egg by modulating the propagation speed of the Ca2+ wave in the egg (Fig. 5), which mediates egg activation events such as the block to polyspermy and completion of meiosis [2]. Hence ER remodeling modulates both elementary and global Ca2+ dynamics and as such plays a central role in endowing the egg with the competency to activate at fertilization.
An important aspect of Ca2+ signaling remodeling during oocyte maturation is the sensitization of IP3-dependent Ca2+ release, the main pathway underlying the Ca2+ transient at fertilization [2]. We show that ER remodeling contributes to sensitization of IP3-dependent Ca2+ release through a simple and elegant mechanism that we refer to as ‘geometric sensitization’. In the context of ER patches formed during meiosis, IP3 receptors are sensitized as a population (Fig. 3) apparently due to their increased density in the three-dimensional space of the ER patch (Fig. 4). The packing of membranes in ER patches brings IP3 receptors into close proximity (40–50 nm) (Fig. S2). This is predicted to enhance Ca2+-dependent cooperativity between neighboring receptors. Given the bell-shaped dependence of IP3 receptor gating on Ca2+ and the fact that Ca2+ acts as a co-agonist for the IP3 receptor, increased Ca2+-dependent cooperativity between IP3 receptors within an ER patch could sensitize them to respond to sub-threshold IP3 concentration as observed in mature eggs. Mathematical modeling supports this conclusion. Simply increasing Ca2+-dependent cooperativity between IP3 receptors in a cluster sensitize them to gate open at lower IP3 concentration (Fig. 4). We altered Ca2+-dependent cooperativity between IP3 receptors by modulating the Ca2+ diffusion coefficient in a cluster of IP3 receptors to represent the context of an ER patch (Fig. 4). This is consistent with what is observed experimentally, where IP3 receptors within an ER patch can sense and gate open in response to IP3 concentrations that are undetectable by IP3 receptors in the reticular ER (Fig. 3).
Therefore, ‘geometric sensitization’ of IP3 receptors due to ER remodeling represents a physiological modulator of IP3 receptor function during meiosis. This mechanism could be applicable to other physiological situations as during mitosis where the ER restructures with the preponderance of the evidence arguing for a transition from a tubular to a cisternae-like structures in mitosis [50]–[52].
In summary we show that the physical structure of the ER has a profound effect on IP3 receptor function. During oocyte meiosis ER remodeling regulates both elementary and global aspects of Ca2+ signaling and is involved in sensitizing IP3 receptors. We argue that ER patch formation during meiosis increases the density of IP3 receptors thus enhancing Ca2+-dependent cooperativity. This leads to sensitization of IP3 receptors allowing them to gate open at sub-threshold IP3 concentrations. IP3 receptor sensitization is central to producing the fertilization specific Ca2+ transient, and as such for egg activation. Therefore, ER remodeling through geometric sensitization of IP3 receptors plays a central role in oocyte maturation.
Materials and Methods
Ethics Statement
All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and in accordance with Qatari regulations regarding the use of animal subjects in research as outlined by the Supreme Council of Health. Studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Weill Cornell Medical College protocol #0806-759A.
Molecular Biology
pSp64s-GFP-KDEL was a gift from Mark Terasaki [17]. pGEM-HE-NOT-GFP-rIP3R1 was generous gift from Patricia Camacho. pSGEM-mCherry-KDEL and pSGEM-mCherry-STIM1 plasmids were previously described [23], [53]. All constructs were verified by DNA sequencing and by analytical endonuclease restriction enzyme digestion. For in vitro transcription, after linearization with NheI or NotI, capped RNAs were transcribed using T7 RNA polymerase with T7 mMESSAGE mMACHINE kit (Ambion). Xenopus oocytes were obtained as described previously [22]. Oocytes injected with the different RNAs were incubated either at room temperature or 18°C for 1–3 days. Oocytes injected with GFP-rIP3R1 RNA required significantly longer time to express the GFP-tagged IP3 receptor and were kept at 18°C for 7–9 days depending on the batch of oocytes before analysis. For experiments aimed at modulating the kinase cascade differentially in the oocyte (Figure 6), cells were incubated for a few hours after Mos or cyclin B RNA injection until they resulted in GVBD as indicated by the appearance of a white spot on the animal pole. Wee1 RNA was injected 12–16 hours before Mos injection [22]. The plasmid used as templates to produce Mos, Wee and Cyclin B RNA were as previously described [22]. For the U0126-cyclib B treatment cells were preincubated with U0126 (50 µM) for 1 hour before injecting Cyclin B RNA.
Imaging
Live cell imaging was performed on a Zeiss LSM710 confocal using a Plan Apo 63×/1.4 oil DIC II objective. The image size was typically set at 744×744 pixel (67.38×67.38 µm) with pinhole set at 1 airy unit (0.9 µm) unless otherwise specified. GFP and Oregon-Green signals were excited with 488 nm laser and emissions collected through a bandwidth of 492–558 nm, mCherry was excited at 561 nm and emission collected through a bandwidth of 572–699 nm. Cells were imaged in OR2 solution (in mM: 82.5 NaCl, 2.5 KCl, 1 CaCl2, 1 Na2HPO4, 5 HEPES, pH 7.5). Images were analyzed using ZEN 2008 and MetaMorph and figures compiled using Adobe Photoshop.
For FRAP analyses pre-bleach and recovery images were scanned (pixel dwell time: 0.84 µs) at 561 nm with 1% laser power. A 25×25 pixels area was bleached at 60% laser power at reduced scanning speed (pixel dwell time: 5.31 µs). FRAP recovery curves were derived by comparison with reference unbleached area, subtracting the background, and fitting with a monoexponential decay function.
Ca2+ waves were analyzed in eggs expressing mCherry-KDEL and injected with caged-IP3 (NPE-caged inositol 1,4,5-trisphosphate (Invitrogen I23580) and 40 µM Oregon Green 488 BAPTA-1 hexapotassium salt (Invitrogen O6806). Cells were scanned in Ca2+-free OR2 solution using Plan-Neofluar 40×/1.3 oil DIC objective with 488 and 561 nm laser set at 2% intensity. The image size is 256×256 pixel (352×352 µm) with pinhole set at 4 µm. After 5 initial scans, an circular ROI (diameter 30 µm) was bleached 50 times with the 405 nm laser set at 20% power resulting in propagating Ca2+ wave that crossed the entire cell.
Immunoblot Analysis
Phospho-MAPK and phospho-Cdc2 Westerns were performed as described previously [54], and α-tubulin was used as the loading control.
Mathematical Modeling
The details of the model used are included in Appendix S1.
Supporting Information
ER patch spatial distribution. A. ER patches form in GFP-KDEL expressing eggs. Planes at which confocal images were taken along z-stack are indicated by the matching number in the orthogonal z-section. B. Images from eggs expressing mCherry-KDEL at GVBD which were either untreated (Egg Con) or treated with Nocodazole (25 µg/ml) for 1 hr.
(TIF)
Low (A) and high (B) magnification transmission EM images of ER patches in a Xenopus egg. Patches in the low magnification image are highlight by the white contour.
(TIF)
Computational Modeling.
(DOCX)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by grants from the Qatar National Research Fund (QNRF) NPRP08-395-3-088 and NPRP08-138-3-050. Additional support for the authors comes from Biomedical Research Program funds at Weill Cornell Medical College, a program funded by Qatar Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Horner VL, Wolfner MF. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev Dyn. 2008;237:527–544. doi: 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- 2.Machaca K. Ca2+ signaling differentiation during oocyte maturation. Journal of Cellular Physiology. 2007;213:331–340. doi: 10.1002/jcp.21194. [DOI] [PubMed] [Google Scholar]
- 3.Nebreda AR, Ferby I. Regulation of the meiotic cell cycle in oocytes. Curr Opin Cell Biol. 2000;12:666–675. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 4.Ionescu L, Cheung KH, Vais H, Mak DO, White C, et al. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal [corrected] Ca2+release. J Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol 482 (Pt. 1995;3):533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bootman M, Niggli E, Berridge M, Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J Physiol. 1997;499(Pt 2):307–314. doi: 10.1113/jphysiol.1997.sp021928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callamaras N, Marchant JS, Sun XP, Parker I. Activation and co-ordination of InsP3-mediated elementary Ca2+ events during global Ca2+ signals in Xenopus oocytes. J Physiol. 1998;509(Pt 1):81–91. doi: 10.1111/j.1469-7793.1998.081bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1048. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- 9.Lipp P, Niggli E. A hierarchical concept of cellular and subcellular Ca2+-signalling. Prog Biophys Molec Biol. 1996;65:265–296. doi: 10.1016/s0079-6107(96)00014-4. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers M, Schell MJ, Thorn P. Agonist-evoked inositol trisphosphate receptor (IP3R) clustering is not dependent on changes in the structure of the endoplasmic reticulum. Biochem J. 2006;394:57–66. doi: 10.1042/BJ20051130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tateishi Y, Hattori M, Nakayama T, Iwai M, Bannai H, et al. Cluster formation of inositol 1,4,5-trisphosphate receptor requires its transition to open state. J Biol Chem. 2005;280:6816–6822. doi: 10.1074/jbc.M405469200. [DOI] [PubMed] [Google Scholar]
- 12.Wilson BS, Pfeiffer JR, Smith AJ, Oliver JM, Oberdorf JA, et al. Calcium-dependent clustering of inositol 1,4,5-trisphosphate receptors. Mol Biol Cell. 1998;9:1465–1478. doi: 10.1091/mbc.9.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiba K, Kado RT, Jaffe LA. Development of calcium release mechanisms during starfish oocyte maturation. Dev Biol. 1990;140:300–306. doi: 10.1016/0012-1606(90)90080-3. [DOI] [PubMed] [Google Scholar]
- 14.Mehlmann L, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod. 1994;51:1088–1098. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- 15.Machaca K. Increased sensitivity and clustering of elementary Ca2+ release events during oocyte maturation. Dev Biol. 2004;275:170–182. doi: 10.1016/j.ydbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 17.Terasaki M, Runft LL, Hand AR. Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation. Mol Biol Cell. 2001;12:1103–1116. doi: 10.1091/mbc.12.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem. 1994;270:6671–6677. doi: 10.1074/jbc.270.12.6671. [DOI] [PubMed] [Google Scholar]
- 19.El Jouni W, Jang B, Haun S, Machaca K. Calcium signaling differentiation during Xenopus oocyte maturation. Dev Biol. 2005;288:514–525. doi: 10.1016/j.ydbio.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 20.El Jouni W, Haun S, Machaca K. Internalization of plasma membrane Ca2+-ATPase during Xenopus oocyte maturation. Dev Biol. 2008;324:99–107. doi: 10.1016/j.ydbio.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machaca K, Haun S. Store-operated Calcium Entry Inactivates at the Germinal Vesicle Breakdown Stage of Xenopus Meiosis. J Biol Chem. 2000;275:38710–38715. doi: 10.1074/jbc.M007887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machaca K, Haun S. Induction of maturation-promoting factor during Xenopus oocyte maturation uncouples Ca2+ store depletion from store-operated Ca2+ entry. J Cell Biol. 2002;156:75–85. doi: 10.1083/jcb.200110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci U S A. 2009;106:17401–17406. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Haun S, Jones RC, Edmondson RD, Machaca K. Kinase-dependent regulation of IP3-dependent Ca2+ release during oocyte maturation. J Biol Chem. 2009;284:20184–20196. doi: 10.1074/jbc.M109.004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, et al. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006;133:4355–4365. doi: 10.1242/dev.02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito J, Yoon SY, Lee B, Vanderheyden V, Vermassen E, et al. Inositol 1,4,5-trisphosphate receptor 1, a widespread Ca2+ channel, is a novel substrate of polo-like kinase 1 in eggs. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.05.548. In Press: doi 10.1016/j.ydbio.2008.05.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullah G, Jung P, Machaca K. Modeling Ca(2+) signaling differentiation during oocyte maturation. Cell Calcium. 2007;42:556–564. doi: 10.1016/j.ceca.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Boulware MJ, Marchant JS. IP3 receptor activity is differentially regulated in endoplasmic reticulum subdomains during oocyte maturation. Curr Biol. 2005;15:765–770. doi: 10.1016/j.cub.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 29.Boulware MJ, Marchant JS. Nuclear pore disassembly from endoplasmic reticulum membranes promotes Ca2+ signalling competency. J Physiol. 2008;586:2873–2888. doi: 10.1113/jphysiol.2008.153379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kline D. Attributes and dynamics of the endoplasmic reticulum in mammalian eggs. Curr Top Dev Biol. 2000;50:125–154. doi: 10.1016/s0070-2153(00)50007-6. [DOI] [PubMed] [Google Scholar]
- 31.FitzHarris G, Marangos P, Carroll J. Cell Cycle-dependent Regulation of Structure of Endoplasmic Reticulum and Inositol 1,4,5-Trisphosphate-induced Ca(2+) Release in Mouse Oocytes and Embryos. Mol Biol Cell. 2003;14:288–301. doi: 10.1091/mbc.E02-07-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kline D, Mehlmann L, Fox C, Terasaki M. The cortical endoplasmic reticulum (ER) of the mouse egg: localization of ER clusters in relation to the generation of repetitive calcium waves. Dev Biol. 1999;215:431–442. doi: 10.1006/dbio.1999.9445. [DOI] [PubMed] [Google Scholar]
- 33.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 34.Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol trisphosphate [correction of tris-phosphate] receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci U S A. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sneyd J, Dufour JF. A dynamic model of the type-2 inositol trisphosphate receptor. Proc Natl Acad Sci U S A. 2002;99:2398–2403. doi: 10.1073/pnas.032281999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchant JS, Taylor CW. Rapid activation and partial inactivation of inositol trisphosphate receptors by inositol trisphosphate. Biochem. 1998;37:11524–11533. doi: 10.1021/bi980808k. [DOI] [PubMed] [Google Scholar]
- 37.Parker I, Choi J, Yao Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 1996;20:105–121. doi: 10.1016/s0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- 38.Vais H, Foskett JK, Daniel Mak DO. Unitary Ca(2+) current through recombinant type 3 InsP(3) receptor channels under physiological ionic conditions. J Gen Physiol. 2010;136:687–700. doi: 10.1085/jgp.201010513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 40.Smith IF, Wiltgen SM, Shuai J, Parker I. Ca2+ Puffs Originate from Preestablished Stable Clusters of Inositol Trisphosphate Receptors. Science Signaling. 2009;2:ra77, 1-ra77, 7. doi: 10.1126/scisignal.2000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman T-U, Skupin A, Falcke M, Taylor CW. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+. Nature. 2009;458:655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stricker SA, Smythe TL. Endoplasmic reticulum reorganizations and Ca2+ signaling in maturing and fertilized oocytes of marine protostome worms: the roles of MAPKs and MPF. Development. 2003;130:2867–2879. doi: 10.1242/dev.00508. [DOI] [PubMed] [Google Scholar]
- 43.Levasseur M, McDougall A. Sperm-induced calcium oscillations at fertilisation in ascidians are controlled by cyclin B1-dependent kinase activity. Development. 2000;127:631–641. doi: 10.1242/dev.127.3.631. [DOI] [PubMed] [Google Scholar]
- 44.Deng MQ, Shen SS. A specific inhibitor pf p34(cdc2)/cyclin B suppresses fertilization-induced calcium oscillations in mouse eggs. Biol Reprod. 2000;62:873–878. doi: 10.1095/biolreprod62.4.873. [DOI] [PubMed] [Google Scholar]
- 45.Gordo AC, Kurokawa M, Wu H, Fissore RA. Modifications of the Ca2+ release mechanisms of mouse oocytes by fertilization and by sperm factor. Molecular Human Reproduction. 2002;8:619–629. doi: 10.1093/molehr/8.7.619. [DOI] [PubMed] [Google Scholar]
- 46.Campanella C, Andreuccetti P, Taddei C, Talevi R. The modifications of cortical endoplasmic reticulum during in vitro maturation of Xenopus laevis oocytes and its involvement in cortical granule exocytosis. J Exp Zool. 1984;229:283–293. doi: 10.1002/jez.1402290214. [DOI] [PubMed] [Google Scholar]
- 47.Mehlmann L, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphoaphate receptors after meiotic maturation of the mouse oocyte. Dev Biol. 1996;180:489–498. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- 48.Jaffe LA, Terasaki M. Structural changes in the endoplasmic reticulum of starfish oocytes during meiotic maturation and fertilization. Dev Biol. 1994;164:579–587. doi: 10.1006/dbio.1994.1225. [DOI] [PubMed] [Google Scholar]
- 49.Shiraishi K, Okada A, Shirakawa H, Nakanishi S, Mikoshiba K, et al. Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5-trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes. Dev Biol. 1995;170:594–606. doi: 10.1006/dbio.1995.1239. [DOI] [PubMed] [Google Scholar]
- 50.Lu L, Ladinsky MS, Kirchhausen T. Cisternal organization of the endoplasmic reticulum during mitosis. Mol Biol Cell. 2009;20:3471–3480. doi: 10.1091/mbc.E09-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCullough S, Lucocq J. Endoplasmic reticulum positioning and partitioning in mitotic HeLa cells. J Anat. 2005;206:415–425. doi: 10.1111/j.1469-7580.2005.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puhka M, Vihinen H, Joensuu M, Jokitalo E. Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells. J Cell Biol. 2007;179:895–909. doi: 10.1083/jcb.200705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu F, Sun L, Machaca K. Constitutive recycling of the store-operated Ca2+ channel Orai1 and its internalization during meiosis. J Cell Biol. 2010;191:523–535. doi: 10.1083/jcb.201006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun L, Machaca K. Ca2+cyt negatively regulates the initiation of oocyte maturation. J Cell Biol. 2004;165:63–75. doi: 10.1083/jcb.200309138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ER patch spatial distribution. A. ER patches form in GFP-KDEL expressing eggs. Planes at which confocal images were taken along z-stack are indicated by the matching number in the orthogonal z-section. B. Images from eggs expressing mCherry-KDEL at GVBD which were either untreated (Egg Con) or treated with Nocodazole (25 µg/ml) for 1 hr.
(TIF)
Low (A) and high (B) magnification transmission EM images of ER patches in a Xenopus egg. Patches in the low magnification image are highlight by the white contour.
(TIF)
Computational Modeling.
(DOCX)