Abstract
Background
Many patients with heart failure (HF) have cognitive deficits, including memory loss.
Objectives
To evaluate the efficacy of a cognitive training intervention on memory (primary outcome), working memory, psychomotor speed, executive function, and performance of cognitive activities and instrumental activities of daily living (IADLs).
Methods
Forty patients with HF were randomly assigned to the computerized plasticity-based cognitive training intervention called Brain Fitness and to the health education active control intervention. Advanced practice nurses made weekly home visits to assess symptoms and monitor intervention adherence. Patients completed demographic and clinical data (baseline), neuropsychological tests (baseline, 8, and 12 weeks), and measures of cognitive and IADLs performance (baseline and 12 weeks)and satisfaction (12 weeks).
Results
Linear mixed models analyses indicated a significant group by time interaction for delayed recall memory (p = .032) and a significant time effect for total (list learning) (p < .001) and delayed (p = .015) recall memory, psychomotor speed (p = .029), and performance of IADLs (p = .006). Intervention adherence and patient satisfaction were high.
Conclusions
To our knowledge, this was thefirst test of Brain Fitness in HF. Although it was a preliminary study with limitations, results support the need for a largerrandomized, controlled trialto determine whether the memory loss of HF is amenable to plasticity-based interventions.
Keywords: heart failure, cognitive deficits, memory loss, cognitive training
Introduction
More than 5.7 million Americans have heart failure (HF) and 670,000 new cases are reported annually in people age 45 years and older.1The 12-month mortality rates for HF remain high, ranging from 15 to 50%, depending upon severity. Heart failure continues to be a leading cause of hospital readmission, contributing substantially to increased health care costs.2In patients with HF, quality of life is diminished by dyspnea, fatigue, cognitive deficits,and depression.3-5
In past studies, 23% to 50% of patients with HF have cognitive deficits, including the devastating problem of memory loss.3,4,6,7In 166 HF patients, left ventricular ejection fraction (LVEF) and cognitive deficits predicted 12-month all-cause mortality; memory loss was the most significant cognitive deficit that predicted mortality.8 Despite the prevalence and importance of memory loss in HF, few interventions have been tested to improve memory and no evidence-based recommendations are currently available for HF patients whose care is complicated by memory loss and other cognitive deficits.9 Novel interventions are needed to address this life-threatening problem.
Therefore, the Nurse Enhanced Memory Intervention in HF Study (MEMOIR), a randomized controlled pilot study, was conducted among patients with chronic HF. The aim was to evaluate the efficacy of the cognitive training intervention on the primary outcome of memory (verbal learning) and the secondary outcomes of working memory, psychomotor speed, executive function, performance of cognitive activities, and performance of instrumental activities of daily living (IADLs) compared with patients in the active control group who receivedthe health education intervention. All patients received weekly nurse visits from advanced practice nurses (APNs) to assess physical health and monitor intervention adherence. Adherence to and patient satisfaction with the interventions wereevaluated.
Theoretical and Scientific Background
The exact etiology of memory loss in HF patients has not been determined, but it is most likely the result of brain injury due to decreased cerebral blood flow (this may be due to elevated vascular resistance, elevated central venous pressure, decreased arterial pressure, or decreased cardiac output)or microembolileading to cerebral hypoperfusion, hypoxia, and disrupted cerebral autoregulation.10-13In a recent study examining cognitive deficits in 249 HF patients, memory loss was the most common type of deficit, found in 23% of patients; psychomotor slowing and decreased executive function were found in 19% of the patients.4The HF patients had significantly more cognitive deficits than comparison groups of 63 healthy participants and 102 participants with major medical conditions other than HF. Increased HF severity was associated with more memory deficits; increased HF severity and older age were associated with more deficits in executive function. In unstructured interviews, the HF patients discussed the challenges of living with memory loss, including the inability to perform complex cognitive activities and IADLs (e.g., managing a checkbook).14
The memory deficits of HF detected on neuropsychological tests are consistent with the injured brain areas in the hippocampus and prefrontal lobes detected in animal models of cerebral hypoxia,15case and autopsy reports,16 and neuroimagingstudies of patients with HF.13,17,18Neuronal loss has been documented in the hippocampus in animals and humans who have experienced cerebral ischemia.11,15,16
In addition to cerebral hypoperfusion and hypoxia, other factors may contribute to memory loss and deficits in other cognitive domains in HF. First, age-related cognitive decline begins as early as the third decade of life,19and many HF patients are elderly. The peripheral sensory organs may deteriorate with age and the input from the visual, auditory, tactile, and proprioceptive systems is degraded. The time required to integrate auditory stimuli and detect signals in speech is increased. As a result, the brain may not be able to accurately interpret details of complex auditory signals. This inability is manifested as slowed psychomotor speed, which occurred in 19% or more of HF patients.4The neurotransmitters (acetylcholine, dopamine, serotonin, norepinephrine) that modulate the synaptic plasticity in the cerebral cortex and hippocampus and that regulate learning and plasticity may degrade with age and further contribute to memory loss.20
Decreased stimulation of the sensory and cognitive systems may occur because of illness-related secondary problems, such as reduced interactions with others and with the environment. Patients may perform fewer cognitively demanding activities because of such problems as well as age-associated environmental issues (e.g., retirement). Together, these factors may decrease sensory and cognitive stimulation and potentially lead to negative changes in neuronal metabolism, learning, and memory. Performing novel or demanding cognitive activities becomes more difficult.20In HF, the physical and cognitive demands of illness become progressively complex and patients perform fewer cognitively challenging activities.14,21
In the current study, a plasticity-based cognitive training program called Brain Fitness was selected for testing. Many computerized cognitive training programs are available, but most have not been scientifically tested.22-37 After evaluating and using Brain Fitness and other cognitive training programs, Brain Fitness was selected because it: 1) was developed based on scientific principles of neuroplasticity and incorporates core elements to improve plasticity;38,39 2) had demonstrated efficacy among healthy elders;38,40 3) focused on improving sensory function and processing speed to improve memory and these functions may underlie some memory deficits in HF; and 4) is customized so that the difficulty of the program progresses based on the individual’s performance as ability improves.40,41The plasticity-based cognitive training intervention used in this study, Brain Fitness, is designed to enhance sensory stimulation in order to improve memory, psychomotor speed, executive function, performance of cognitive activities, and performance of IADLs. The intervention is derived from knowledge about neurogenesis and neuroplasticity.42-44Neurogenesis is the ability to generate new neurons and neuroplasticity is the capacity that neurons have to modify their synaptic structure and form new neural connections. The knowledge that brain neurons are capable of neuroplasticity in the presence of injury can be used as a basis for intervention. Neuroplasticity occurs in brain areas that are associated with learning and memory. Recent evidence indicates that neurogenesis can occur in the hippocampus after injury and in HF, the hippocampus is damaged.11,13,44,45Neurogenesis and neuroplasticity are mechanisms through which the brain may recover from small, silent infarcts andcompensate for periods of oxygen deprivation.42,44,45Data from animal and human studies provide promising, preliminary evidence that intensive training to increase sensory stimulation and performance of cognitively challenging activities promotes neuroplasticity and improves cognitive outcomes.20,40,43,46-47
Cognitive training interventions based on scientific principles of neuroplasticity have been tested among healthy elders, including persons at risk of age-related cognitive decline.Individuals who received plasticity-based interventions had improved memory, psychomotor speed, reasoning, and IADLs.38,48-50Using a non-computerized cognitive training intervention delivered in ten sessions over 5 to 6 weeksamong 2,832 healthy elders, participants who received the specific cognitive training interventions had improvement immediately after the intervention in psychomotor speed (87% improved), reasoning (74%), and memory (26%).48For the memory training group, improved memory scores seen immediately after the intervention were sustained over two and five years.50Self-reported performance of IADLs significantly improved two years after the reasoning intervention and performance of IADLs significantly improved five years after the speed of processing intervention with booster training.50
In a plasticity-based, computerized cognitive training intervention among 182 healthy elders, those who completed the intervention had significant improvement in memory compared with older adults in the attention control and no-contact groups.40In a multisite randomized controlled double-blind trial, the Improvement in Memory with Plasticity-Based Adaptive Cognitive Training (IMPACT) Study using the Brain Fitness computerized training program in 487 community-dwelling older adults without cognitive impairment, improvement in memory and attention was significantly greater (p = .02) in the group who received the plasticity-based cognitive training intervention (3.9 points, 95% confidence interval = 2.7 – 5.1) than in the active control group who received general cognitive stimulation (1.8 points, 95% CI 0.6 – 3.0).38The investigators concluded that the magnitude of the effect sizes is clinically meaningful.
No studies testing plasticity-based interventions, including Brain Fitness,aimed at improving memory or other cognitive abilities in HF were found in an extensive review of the literature.3No evidence-based recommendations are available for patients who have cognitive loss in HF. In the current randomized pilot study, it was hypothesized that the patients who received the plasticity-based computerized Brain Fitness intervention would have improved memory (list learning and recall), working memory, psychomotor speed, executive function, performance of cognitive activities, and performance of IADLs compared with the patients who received the health education attention control intervention.
Methods
Designand Procedures
The MEMOIR Study used a two-group randomized experimental design to evaluate the cognitive training intervention in HF patients. The study was approved by the university institutional review board and all patients gave written informed consent prior to participation. The data were collected from April 2008 to July 2010. Data were collected at baseline, 8 weeks (defined as within 1 week of completion of the 8-week interventions), and 12 weeks (defined as within 4 weeks of completion of the 8-week interventions). The length of time to obtain consent and administer the baseline questionnaires and tests was 90 to 120 minutes. After baseline data collection, patients were randomly assigned to the Brain Fitness and health education interventions using a computer-generated list of random numbers and a 1:1 ratio. During the eight weeks of intervention delivery, APNs who were members of the research team made weekly home visits to assess HF-related physical symptoms, discuss problems or concerns about the interventions, and monitor intervention adherence. All patients were requested to maintain a calendar log of the time they spent performing the interventions.The data were collected by research assistants (RAs) who were graduate nursing students and a licensed psychologist with training in neuropsychological testing.The neuropsychologist co-investigator (BG) selected the test battery and assisted with training for administration and scoring of the neuropsychological tests. All neuropsychological testing was completed in patients’ homes and environmental distractions were controlled. Fifty-one percent of the follow-up data collection was completed by data collectors who were blinded to group assignment.
Sample
The patients were recruited from a multidisciplinary HF clinic affiliated with the university medical center in a Midwestern city. Clinic staff approached potentially eligible patients and invited them to participate; the names and contact information of interested patients were provided to research team members. Eligibility criteria were: 1) adults age ≥ 21 years; 2) able to understand English; 3) have access to a working telephone; 4) able to hear normal conversation; 5) able to see and read a computer screen; 6) have a current diagnosis of chronic systolic HF documented by echocardiography, nuclear imaging, or cardiac catheterization within the past two years and LVEF equal to or less than 40%;517) NYHA class 2, 3, or 4; and 8) optimized medical therapy.9 Patients were excluded from participation if they had a condition likely to cause reduced cognitive function (e.g., neurological disorders) or terminal illness.
Interventions
Brain Fitness Intervention
Patients randomly assigned to the intervention group received the Brain Fitness Program developed by PositScience. This program, based on principles of neuroplasticity, was designed to increase sensory integration of information and strengthen neuromodulatory chemicals necessary for encoding information. A person’s ability to remember information (i.e., encode, recall, and use information) is closely linked to the ability to hear and interpret auditory information accurately. The computerized program is designed to be completed in 40 1-hour sessions over eight weeks. Computers were placed in patients’ homes and usage was demonstrated to patients by the APNs.All computers were a standardized size with a 14 inch monitor screen to provide delivery of high quality visual and auditory components of the intervention. Noise-cancelling headphones that were able to be worn alone or over hearing aids were provided to patients to use while completing the intervention.
The Brain Fitness program incorporates interesting visual and auditory components to deliver exercises, scenes, and stories designed to strengthen the person’s ability to learn and recall information. The program provides intensive and progressive training of the auditory system by asking the person to distinguish between similar speech components.41Stories are provided that have novel events and the person is asked to answer series of questions about the events.Intensive practice is believed to improve precision and accuracy in understanding speech and thereby improve memory recall. Progressive training is provided in the program - the exercises become increasingly complex as the person progresses through the program. Speech used in the program is processed by an algorithm to increase the rate and complexity over the training sessions in order to facilitate the person’s ability to comprehend and encode speech. The computer program monitors the person’s performance and ensures that he or she is training at threshold level (i.e., the uppermost level of current skill). This is a unique element of the program because it individualizes the training to the person’s performance. Information that is associated with stronger emotions is more novel and more easily recalled,15,41and Brain Fitness incorporates surprising andnovel content to strengthen recall. Finally, feedback and rewards are provided by Brain Fitness to strengthen learning.40,41Intervention adherence (time on task) is monitored by the computer program.
Health Education Intervention
The health education intervention was designed to provide an active control intervention. Patients randomly assigned to the health education intervention were required to read information about maintaining cardiovascular health. The reading materials were eight issues of Heart Insight, the quarterly consumer-oriented magazine published by the American Heart Association (AHA) and Lippincott Williams and Wilkins.52This official AHA publication is focused on preventing and managing cardiovascular disease and was developed for patients and their families. Patients were requested to read one issue each week for at least one hour.
Measures
Demographic and clinical variables were obtained at the baseline interviews (age, gender, race, ethnicity, marital status, education, and blood pressure) and from the medical records (LVEF and NYHA classification, devices, and medications) to describe the sampleand evaluate baseline equivalencies of the groups. Perceived physical functional capacity, HF-related physical symptoms, and comorbiditieswere assessed at baseline to describe the sample and evaluate baseline equivalenciesbecause these variables may influence patients’ cognitive function and limit their ability to perform and adhere to interventions.
The Duke Activity Status Index was used to measure perceived physical functional capacity, defined as the maximal capacity that a person believes he/she has to perform particular physical tasks.53The Index consists of twelve items on 4-point response scales. Possible weighted scores range from 0 to 58.2; higher scores indicate greater perceived functional capacity. Internal consistency of the Duke Activity Status Index is satisfactory; Cronbach’s alpha was 0.82 in 249 HF patients and 0.86 in 246 patients with implantable cardioverter defibrillators.4,54Validity was supported by a correlation of 0.80 between the index and peak oxygen uptake by exercise testing.53,55In the current sample, the Cronbach’s alpha was 0.87.
The Minnesota Living with Heart Failure (LHFQ) Physical Subscale was used to measure physical symptom impact.56The LHFQ is a HF-specific quality of life questionnaire that was developed to measure the impact of physical and emotional HF-related symptoms on patients’ ability to live as they wanted in the past month. The Physical Subscale consists of five 6-point response scales. Possible scores on the Physical Subscale range from 0 to 25; higher scores indicate greater impact of physical symptoms and worse quality of life. Internal consistency of the LHFQ Physical Subscale was 0.94 and 0.91 in 211 HF patients and construct validity was supported by factor analysis.57,58In the current sample, the Cronbach’s alpha was 0.85.
The Charlson Comorbidity Index(chart form) was used to measure comorbidity. This Index evaluates patients’ history of having medical problems in 12 areas.59Itprovides a score based on the severity of the existing health problems; higher scores indicate more or more severe comorbid conditions. The Charlson Comorbidity Index has predictive validity among patients with chronic medical conditions.60
The Mini-Mental State Examination (MMSE) was used to measure global cognitive functionat baseline and aid in interpretation of the neuropsychological test data.61The test has 11 items that produce a total possible score ranging from 0 to 30 points; higher scores indicate better mental status. Twenty-four hour test-retest reliability was r = 0.83 with different testers and r = 0.89 with the same tester.61,62Construct validity of the MMSE has been supported in elders and it is sensitive to dementia across different age levels.63The MMSE is a screening questionnaire to identify patients in need of further evaluation; in past studies it was less sensitive than other measures in detecting possible cognitive impairment.64,65
Outcome variables
TheHopkins Verbal Learning Test - Revised total and delayed recall scores were used to measure the primary outcome of memory (specifically, list learning and recall).66,67The Hopkins is a well-established neuropsychological test developed to measure recall and retention of information. In this test, the person is asked to learn and remember a list of 12 words over three trials and again after a 20-minute delay. The test yields a total list learning score (the sum of the three trials) and a delayed recall score. The possible total list learning score ranges from 0 to 36 and the possible delayed recall score ranges from 0 to 12; higher scores indicate better performance. Nine-week test-retest was r = 0.66 (nonsignificant).66The Hopkins discriminated between patients with mild cognitive impairment and cognitively healthy persons with a sensitivity of 0.79 and a specificity of 0.95 among 21 patients with mild cognitive impairment and 98 cognitively healthy control participants64and a sensitivity of 0.96 and specificity 0.80 among 541 healthy participants.68In this study, equivalent forms were counterbalanced and randomized at the three administrations to minimize the likelihood of practice effects.Raw scores were used in the analyses.
The Digit Symbol Subtest of the Wechsler Adult Intelligence Scale-3 was used to measure psychomotor speed (WAIS).69The Digit Symbol is a widely used neuropsychological test in which the person is asked to match a series of numbers with characters over 120 seconds. The number matched is summed and a scaled score is obtained from the standardization sample (Mean = 10, SD = 3). Higher scores indicate better performance. Test-retest and validity have been supported.69In the current study, comparable parallel forms were counterbalanced and randomized at the three administrations to minimize the likelihood of practice effects.The different forms have not been formally equated, but they are considered comparable based on common practice.Scaled scores were used in the analyses.
The Controlled Oral Word Association Test was used to measure executive function (verbal fluency).70This is a widely used neuropsychological test in which the person is presented with a letter and asked to name as many words as possible that begin with the letter over 60 seconds. This is done three times with three different letters. The sum of the words generated is adjusted by age and education and a percentile score is obtained as the final measure; higher scores indicate better performance. Test-retest reliability and validity have been supported.62In this study, equivalent forms were counterbalanced and randomized at the three administrations to minimize the likelihood of practice effects.Scores adjusted by age and education were used in the analyses.
Four tests from the CogState Healthneuropsychological test battery were administered to evaluate working memory, memory, and psychomotor speed.71In addition, the CogState tests were used to compare them with the traditional neuropsychological tests in HF patients for use in future studies. The CogState Health neuropsychological test battery is based on traditional neuropsychological tests but has the advantage of computerized delivery that can be standardized and scored as it is completed. The battery uses playing cards on the computer as the stimulus set. The four tests used in this study were the Detection Taskto measure psychomotor speed, the Identification Task to measure working memory, the One Back Task Speed and Accuracy to measure working memory, and the International Shopping List total (list learning) and delayed recall to measure memory. Reliability and construct validity of the CogState Health battery have been supported. One week test-retest reliability among 60 young volunteers had an intraclass correlation coefficient of 0.69 to 0.90.71TheCogState tests were significantly different among samples of 113 healthy young adults, 10 patients with mild head injury, 15 patients with mild cognitive impairment, 15 patients with schizophrenia, and 15 patients with AIDS dementia complex.64,72-74The transformed scores for the Detection, Identification, and One Back tasks were used in the analysis. The numbers of items learned and remembered after three trials and recalled after a 20-minute delay on the International Shopping List were used in the analysis.
The Florida Cognitive Activities Scalewas used to measure self-reported cognitive activity performance.75This scale was developed to assess the frequency with which people engage in cognitive activities, including challenging cognitive activities (e.g., preparing new recipes, driving to unfamiliar places). The total Scale has twenty-five items on 5-point response scales. Possible scores range from 0 to 100; higher scores indicate more frequent performance of cognitive activities, including complex activities. Internal consistency reliability of the Scale was Cronbach’s alpha from 0.65 to 0.79 in healthy elders and 0.68 among 223 African-American older adults.75,76Construct validity was supported by factor analysis and discriminant validity was supported by comparing scores of healthy elders and persons with neurological impairments.75,76In the current study, the Cronbach’s alpha reliability was 0.77 at baseline and 0.76 at 12 weeks.
The Everyday Problems Test for Cognitively Challenged Elderly was used to measure IADLs.77This test was developed to assess older adults’ ability to perform a set of complex tasks that are part of everyday living. The tasks involve global cognitive functions and higher-order executive functions on seven common domains (managing finances, taking medications, transportation, shopping, doing light household chores, preparing meals, and using the telephone).77,78The test has 16 situations with two questions each for a total of 32 items. The difficulty level of the tasks is within the range of performance of patients in the early stage of dementia and elderly persons who have low education level but not dementia.78Possible scores range from 0 to 32; higher scores indicate better performance on the tasks that represent IADLs. Six-month test-retest reliability was 0.81 among 65 patients with Alzheimer Disease.78Construct validity was supported among the 65 patients with Alzheimer Disease because the Everyday Problem Test for Cognitively Challenged Elders was significantly correlated with other neuropsychological tests and with direct observation of tasks performed.78Change in the Everyday Performance Test for Cognitively Challenged Elders was a significant predictor of mortality.79
The Patient Satisfaction Questionnaire was used to measure patient satisfaction with the Brain Fitness intervention. This Questionnaire, adapted from Northouse and colleagues,80 asked patients to complete five structured items on 5-point response scales (possible score range 5 to 25 with higher scores indicating more satisfaction) and 4 open-ended items. Patients completed the Questionnaire at the 12-week data collection visit. In this sample, the Cronbach’s alpha was 0.80.
Intervention adherence was assessed by the Brain Fitness computer program for patients assigned to the intervention group. Completion of the entire program took 2310 minutes. Adherence with the health education intervention was assessed by patients’ weekly report of time they spent reading health information, including the Heart Insight magazines. Completion of the control intervention took 480 minutes, although some patients read health information for more than an hour per week. Patients were categorized as adherent if they met or exceeded 90% of the required minutes for the interventions and categorized as non-adherent if they completed less than 90% of the required minutes.
statistically analysis
Baseline descriptive statistics were computed for the demographic, clinical, and studyvariables and compared between the patients assigned to the Brain Fitness and health education groups using independent t-tests for continuous variables and Chi-square for categorical variables. Cronbach’s alpha was computed to estimate internal consistency reliability for multi-item scales. Linear mixed models analyses wereconducted for the dependent variables to compare the group, the time, and the group-by-time interaction effects. The dependent variables were the measures for memory(Hopkins Verbal Learning Test), psychomotor speed (Digit Symbol), executive function (Controlled Oral Word Association), performance of cognitive activities (Florida Cognitive Activities Scale), performance of IADLs (Everyday Problem Test) and the CogState Health tests for psychomotor speed (Detection), working memory (Identification and One Back Task Speed and Accuracy), and memory (International Shopping List). The patterns of change were assessed using planned contrasts between baseline and 8 weeks, baseline and 12 weeks, and 8 and 12 weeks. The mixed models analyses were completed using the intent-to-treat approach with all available data, including baseline data from the patients who did not complete the study. Statistical analyses were completed using SPSS. Intervention adherence and patient satisfaction with the Brain Fitness intervention were evaluated using descriptive statistics. The significance level was set at alpha <.05 for the analyses.
Results
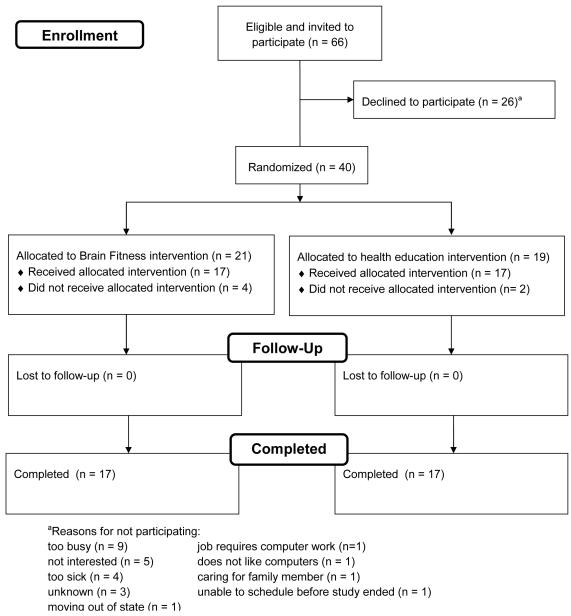
The numbers of patients invited to participate, enrolled, and who completed the study are presented in Figure 1. Forty patients wereenrolled and randomly assigned to the Brain Fitness (n = 21) and health education (n = 19) intervention groups. Sample characteristics of the 40 patients who were randomly assigned in the study are presented in Table 1. No significant differences were found between the groups ondemographic and clinical characteristics, butpatients assigned to the health education group were on average older, more likely to be men, had lower LVEF, were less likely to be receiving ACE-inhibitors, had fewer devices, and had more comorbidities. Compared with patients assigned to Brain Fitness, patients assigned to the health education interventionreported more physical symptoms on the Living with Heart Failure Questionnaire Physical Subscale at baseline (p = .044).
Figure 1.
Numbers of Patients Invited, Enrolled, and Who Completed the Study
Table 1.
Sample Characteristics of Total Group and Patients Who Were Randomly Assigned to the Brain Fitness and Health Education Groups (n = 40)
| Characteristic | Total (n = 40) |
Brain Fitness (n = 21) |
Health Education (n = 19) |
p-value |
|---|---|---|---|---|
| Age, mean ± SD | 57.8 ± 13.1 | 54.7 ± 13.9 | 61.1 ± 11.5 | .124 |
|
| ||||
| Gender, n (%) | ||||
| Men | 28 (70) | 13 (62) | 15 (79) | .240 |
| Women | 12 (30) | 8 (38) | 4 (21) | |
|
| ||||
| Race, n (%) | ||||
| African-American | 7 (17.5) | 5 (24) | 2 (11) | .543 |
| Asian | 2 (5) | 1 (5) | 1 (5) | |
| White | 31 (77.5) | 15 (71) | 16 (84) | |
|
| ||||
| Ethnicity, n (%) | ||||
| Hispanic | 3 (7.5) | 2 (10) | 1 (5) | .609 |
| Non-hispanic | 37 (92.5) | 19 (90) | 18 (95) | |
|
| ||||
| Marital status, n (%) | ||||
| Married | 26 (65) | 13 (62) | 13 (68) | .666 |
| Not married | 14 (35) | 8 (38) | 6 (32) | |
|
| ||||
| Education, years, mean ± SD | 14.0 ± 2.5 | 13.9 ± 2.4 | 14.2 ± 2.6 | .657 |
|
| ||||
| LVEF,a mean ± SD | 26.7 ± 10.3 | 28.5 ± 9.6 | 24.7 ± 10.8 | .252 |
|
| ||||
| NYHAb class, n (%) | ||||
| I | 11 (27.5) | 6 (29) | 5 (26) | |
| II | 20 (50) | 11 (52) | 9 (47) | .860 |
| III | 9 (22.5) | 4 (19) | 5 (26) | |
| IV | 0 (0) | 0 (0) | 0 (0) | |
|
| ||||
| Systolic blood pressure, mean ± SD | 113.3 ± 20.4 | 116.2 ± 20.0 | 110.0 ± 20.9 | .341 |
|
| ||||
| Diastolic blood pressure, mean ± SD | 68.0 ± 13.4 | 69.4 ± 13.8 | 66.4 ± 13.1 | .492 |
|
| ||||
| Implantable Cardioverter Defibrillator, n (%) |
22 (55) | 13 (62) | 9 (47) | .356 |
|
| ||||
| Cardiac Resynchronization Therapy, n (%) |
11 (28) | 5 (24) | 6 (32) | .583 |
|
| ||||
| Dual-Chamber Pacemaker, n (%) | 3 (8) | 1 (5) | 2 (11) | .489 |
|
| ||||
| Left Ventricular Assist Device, n (%) | 1 (3) | 1 (5) | ||
|
| ||||
| Medications, n (%) | ||||
| ACE-inhibitorc | 21 (53) | 12 (57) | 9 (47) | .536 |
| ARBd | 11 (28) | 5 (24) | 6 (32) | .583 |
| Beta-adrenergic blocking agent | 36 (90) | 19 (90) | 17 (89) | .916 |
| Diuretic | 29 (73) | 15 (71) | 14 (74) | .873 |
| Aldosterone antagonist | 19 (48) | 11 (52) | 8 (42) | .516 |
|
| ||||
| Duke Activity Status Index, mean ± SD |
22.9 ± 17.1 | 24.0 ± 15.5 | 21.6 ± 19.1 | .662 |
|
| ||||
| LHFQe Physical Subscale | 8.6 ± 7.0 | 6.4 ± 5.1 | 11.0 ± 8.2 | .044 |
|
| ||||
| Charlson Comorbidity Index, mean ± SD |
2.3 ± 1.1 | 2.1 ± 1.0 | 2.4 ± 1.1 | .336 |
|
| ||||
| MMSE,f mean ± SD | 28.2 ± 1.7 | 28.1 ± 1.8 | 28.2 ± 1.6 | .978 |
Left ventricular ejection fraction
New York Heart Association
Angiotensin-converting enzyme inhibitor
Angiotensin receptor blocking agent
Living with Heart Failure Questionnaire
Mini-mental State Examination
Thirty-four (85%) patients completed the 12-week study (17 patients completed the Brain Fitness intervention and 17 patients completed the health education intervention). Six patients withdrew or were withdrawn by the investigators (four from the Brain Fitness intervention and two from the health education intervention). Reasons for withdrawal were: too ill and too many doctors’ appointments (n = 2); unable to use the computer (n = 1); family obligations (n=1); too busy with work (n = 1); and inadequate incentive fees (n = 1). No significant differences were found between the patients who did and did not complete the study in demographics (age, gender, race, ethnicity, marital status, years of education), clinical variables (LVEF, NYHA, systolic or diastolic blood pressure,dual chamber pacemakers, cardiac resynchronization therapy, or cardiac medications), perceived functional capacity (Duke Activity Status Index), physical symptoms (Living with Heart Failure Questionnaire Physical Subscale),and the MMSE. Patients who completed the intervention were significantly more likely to have an implantable cardioverter-defibrillator (Chi square = 4.2, p = .041) and tended to have fewer comorbidities (CharlsonComorbidity Index mean score, completed, 2.1 ± 1.0; did not complete, 3.0 ± .9, t = 2.0, p = .058). At baseline, all of the mean neuropsychological test scores were lower for the patients who did not complete the study compared with the mean scores of the patients who completed the study. The scores of the patients who did not complete the study were significantly lower than the patients who completed the study on tests of memory (Hopkins Verbal Learning Test total list learning, t = 2.3, p = .025; CogState International Shopping List learning, t = 2.5, p = .018) and working memory (CogState One Back Task Speed, t = 2.4, p = .021) and approached significance on other tests of memory (Hopkins Verbal Learning Test delayed recall, t, = 1.9, p = .070; CogState International Shopping List delayed recall, t = 1.9, p = .064), working memory (CogState Identification Task, t = 1.9, p = .064), psychomotor speed(Digit Symbol scaled score, t = 1.9, p = .063), and executive function (Controlled Oral Word Association, t = 1.8, p = .075). No baseline differences were found between the patients who did and did not complete the study on self-reported performance of cognitive activities (Florida Cognitive Activities Scale, t = 0.4, p = .926) but patients who did not complete the study had a significantly lower mean score on the measure of performance of IADLs (Everyday Problems Test, t = 2.4, p = .019).
The mean scores for patients in both groups are presented in Table 2. Small improvement was found across the groups in memory, working memory, psychomotor speed, executive function, and IADLs. Results for the linear mixed models analyses are presented in Table 2. Memory as measured by the Hopkins Verbal Learning Test total list learning score had a strong and significant time effect (F [2,65] = 9.04, p < .001)and nonsignificant group (F[1,37] = 0.03, p = .866) and group by time interaction (F [2,65] = 0.64, p = .530) effects, indicating that patients in both groups improved over time on total number of words learned and recalled over the test trials. The Hopkins delayed recalldemonstrated significant time (F [2,64] = 4.48, p = .015) and group by time interaction (F[2,64] = 3.65, p = .032) effects(Table 2) and a nonsignificantgroup effect (F[1,37] = 0.49, p = .488), indicating that patients who received the Brain Fitness intervention improved more in delayed recall memory over time than patients who received the health education intervention. The primary hypothesis of the study was supported.
Table 2.
Means, Standard Deviations, and Linear Mixed Models Analysis for Outcome Variables
| Baseline | 8 Weeks | 12 Weeks | Mixed Models Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Brain Fitness (n = 21) Mean ± SD |
Health Education (n = 19) Mean ± SD |
Brain Fitness (n = 17) Mean ± SD |
Health Education (n = 17) Mean ± SD |
Brain Fitness (n = 17) Mean ± SD |
Health Education (n = 17) Mean ± SD |
Group p-value |
Time p-value |
Group x Time p-value |
| Hopkins Verbal | |||||||||
| Learning Test | |||||||||
| List learning | 20.5 ± 4.9 | 21.7 ± 5.4 | 23.7 ± 5.0 | 23.1 ± 5.8 | 25.2 ± 5.4 | 24.1 ± 5.4 | .866 | < .001 | .530 |
| Delayed recall | 6.0 ± 3.2 | 7.8 ± 3.0 | 7.7 ± 3.0 | 7.8 ± 2.9 | 8.5 ± 2.7 | 8.1 ± 3.1a | .488 | .015 | .032 |
|
| |||||||||
| Digit Symbol Scaled |
8.7 ± 1.7 | 9.8 ± 2.6 | 8.8 ± 2.1 | 10.7 ± 2.4a | 9.3 ± 2.0 | 10.5 ± 2.7a | .067 | .479 | .532 |
|
| |||||||||
| Controlled Oral Word Association (Percentile) |
52.5 ± 30.8 | 45.6 ± 30.6 | 57.9 ± 28.3 | 48.5 ± 34.5 | 65.3 ± 26.0 | 51.7 ± 32.2 | .680 | .100 | .561 |
|
| |||||||||
| CogState | |||||||||
| Detection | 2.5 ± 1.0 | 2.5 ± 0.1 | 2.5 ± 0.2 | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.6 ± 0.1 | .313 | .315 | .525 |
| Identification | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | .112 | .159 | .704 |
| One Back Speed |
2.9 ± 0.1 | 3.0 ± 0.1 | 2.9 ± 0.1a | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.1 | .372 | .029 | .108 |
| One Back Accuracy |
1.3 ± 0.2 | 1.3 ± 0.3 | 1.3 ± 0.2a | 1.3 ± 0.3 | 1.3 ± 0.2 | 1.2 ± 0.2 | .718 | .423 | .193 |
|
| |||||||||
| International | |||||||||
| Shopping List | |||||||||
| List learning | 23.6 ± 8.6 | 24.8 ± 6.7 | 27.0 ± 6.9a | 24.2 ± 9.2 | 25.0 ± 9.1 | 24.7 ± 7.7 | .846 | .914 | .557 |
| Delayed recall | 8.3 ± 3.6 | 9.1 ± 3.2 | 8.5 ± 4.2a | 8.5 ± 3.7 | 8.5 ± 3.5 | 8.9 ± 3.6 | .587 | .575 | .926 |
|
| |||||||||
| Florida Cognitive Activities Scale |
45.1 ± 9.8 | 45.6 ± 12.1 | – | – | 47.2 ± 9.7 | 44.7 ± 12.7 | .759 | .779 | .276 |
|
| |||||||||
| Everyday | |||||||||
| Problems Test |
27.0 ± 2.8 | 27.8 ± 2.7 | – | – | 29.3 ± 2.5 | 28.2 ± 4.6 | .793 | .006 | .144 |
n = 16 (data missing or patient unable to complete test at the time of data collection)
On the secondary outcomes, significant results were found in working memory and performance of IADLs (Table 2). Working memory as measured by the CogState One Back Speed Task indicated a significant time effect (F [2,65] = 3.75; p = .029) and nonsignificant group and group by time interaction effects. The performance of IADLs as measured by the Everyday Problems Test indicated a strong significant time effect (F [1,30] = 8.62, p = .006) and nonsignificant group and group by time interaction effects.
The 17 patients who completed the Brain Fitness intervention had a mean adherence time of 1996 (SD = 677)minutes and 13 (77%) patients had 90% and higher adherence rates.The 17 patients who completed the health education intervention had a mean adherence time of 749 (SD = 643)minutes and 14 (82%) patients had 90% and higher adherence rates.
Scores on the patient satisfaction questionnaire for the Brain Fitness intervention were high (mean = 24.3; standard deviation = 1.3). Patients reported that the most helpful aspect of the program was the practice with memory and that the information contained in Brain Fitness did not overlap with any information they had received from their health care providers. Several patients reportedthat the program became tedious at times because of the repetitive training. Patients had positive comments about the APNs who completed the weekly visits.
Discussion
MEMOIR is an important study because, to our knowledge, it is the first test of the Brain Fitness intervention in HF. Although this was a preliminary study with limitations, the results support the urgent need for further testing to determine whether thememory loss of HF may be amenable to plasticity-based interventions. Linear mixed models analyses using an intent-to-treat design indicated that compared with patients who received the health education intervention, patients who received the Brain Fitness intervention had significantly improved performance over time on the test of delayed recall memory. These results are consistent with the IMPACT study in which investigators found that healthy elders improved on the word list delayed recall as measured by the Rey Auditory Verbal Learning test.38
Patients in both groups demonstrated improvement over time in memory (list learning and delayed recall), working memory, psychomotor speed, executive function, and IADLs. There are three plausible explanations for the improvement noted in both groups. First, the health education intervention provided patients with a high quality magazine, Heart Insight, that publishes articles related to cardiovascular health. Second, patients in both groups received the nurse enhanced component of the intervention that included weekly home visits by APNs and this may have contributed, in part, to the improvement. Home visits and care coordination by APNs reduce hospitalizations and improve quality of life, although no studies were found that evaluated the effects of APNs on specific cognitive abilities in HF and the APNs in this study did not provide care coordination.81,82In this first study, it was important to include the nurse enhanced component to validate that patients could complete the intervention. Results are consistent with past studies that reported cognitive training interventions improved memory and psychomotor speed among healthy elders.38,48A larger randomized controlled trial isurgently needed to evaluate the efficacy of the Brain Fitness intervention in HF independent of APN visits. Third, practice effects may have occurred with repeated administrations of the neuropsychological tests. The finding of a significant group by time interaction for delayed recall memory in the Brain Fitness group compared with the health education active control group suggests that practice effects may not have occurred. Furthermore, alternative test forms were used to minimize the likelihood of practice effects, but future studies are needed to fully evaluatethese effects.
The use of an active control intervention that allowed the patients in both groups to receive interventions that might improve cognitive function was an important aspect of the study. To fully evaluate the efficacy of the Brain Fitness intervention, a no-contact control group of HF patients who continue to receive optimized medical therapy but receive no specific intervention that would increase their performance of cognitively-challenging activities is needed. The use of an active control group of patients who perform increased cognitively challenging activities independently on the computeris also necessary to provide a comparison that controls for computer usage.
Another important result of this study was the identification of a subset of patients who were unable or unwilling to complete the interventions. Although the patients reported other reasons for not completing the study, they had lower scores on all neuropsychological tests and on the measure of IADLs. These results support the use of enrollment criteria that pre-specifies a cutoff level for eligibility. Patients who are unable to complete the interventions may be in need of further neuropsychological evaluation and alternative interventions. Although exclusion criteria in the current study included the existence of documented cognitive disorders, it is plausible that the patients who were unable to complete the interventions had early or undiagnosed cognitive disorders.
Adherence with both interventions was high, supporting the feasibility of using these interventions in future studies among HF patients. The high adherence level is particularly important because these patients had advanced HF as evidenced by the low mean LVEF and perceived functional capacity. They were willing and able to complete the interventions when delivered in the home and supported by APNs. Patients had a high level of satisfaction with the program and with the nurse enhanced care. They were interested in maintaining and improving their cognitive health and the content in the Brain Fitness intervention did not duplicate information they had received from their health care providers.
This study had limitations that may have influenced the results. First, the sample size was small; results need to be replicated in a larger randomized controlled trial. With the sample size of 40 patients, the current study had 34% power to detect medium-sized effects and 69% power to detect large effects.83Thesignificant group by time interaction for the primary outcome variable of delayed memory recall suggests a large effect size.Second, patients randomly assigned to the health education group were on average older, more often men, had lower LVEF, received fewer ACE-inhibitor therapies, had fewer devices, had slightly more comorbidities, andreported poorer physical symptoms at baseline than the Brain Fitness group. Although only differences in physical symptoms were statistically significant, the health education group may have been sicker compared with the Brain Fitness group.Third, the possibility of selection bias cannot be excluded because patients were recruited from one academic medical center and received care from a multidisciplinary team of providers. However, the baseline mean scores on the neuropsychological tests in this sample were consistent with the scores reported in a larger sample of 249 HF patients enrolled at seven sites in a different state.4The enrollment of patients receiving optimal medical care at a multidisciplinary specialty clinic eliminated some variables that could be responsible for impaired cognition (e.g., lack of medications recommended by guidelines). Academic medical centers often provide care to those patients with the most advanced HF and the finding that memory can be improved in these patients is promising for application to patients with less severe HF treated in other settings. The mean age of the sample was relatively young and this may be different than a community-based sample. Whether this type of intervention would work in groups of older HF patients is unknown. Fourth, the greater time spent by patients who completed the Brain Fitness intervention compared to the time spent by patients in the active control intervention may have contributed to the improvement found in the Brain Fitness group. Additionally, twopatients in the health education group reported increasing their cognitive activities as a result of the baseline testing which may have contributed to improved performance. Fifth, the funding of the pilot study limited the ability to have all follow-up data collected by data collectors who were blinded to group assignment. Sixth, depressive symptoms and sleep-disordered breathing, both of which are common in HF, may be alternative explanations for the results and need to be evaluated in a larger study.Finally, plasticity was not directly measured in this study. If the intervention is efficacious in a larger study, investigations into the neural mechanismsresponsible for improvement need to be determined.
In conclusion, patients who received the computerized, plasticity-based Brain Fitness cognitivetraining intervention had improved performance in delayed memory at 12 weeks. Although significant improvement was not found in working memory and psychomotor speed, the improvement in overall delayed recall was consistent with the IMPACT study.38Patients had high levels of adherence and satisfaction with the intervention. These preliminary results require testing in a larger randomized controlled trial.
Acknowledgements
Assistance with recruitment, data collection, and data management: Judith Grossi, BS, University of Michigan Health System; Letitia Baxter, RN, MSN, Seung Hee Choi, RN, MSN, and Ya-Ru Tsai, RN, University of Michigan School of Nursing
Brain Fitness software donated by PositScience and Heart Insight magazines donated by the American Heart Association
Funding sources: National Institute of Nursing Research R01NR008147 and P30NR009000
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: There are no other disclosures.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, DeSimone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics – 2009 update: a report from the AHA Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Titler MG, Jensen GA, Dochterman JM, Xie X, Kanak M, Reed D, et al. Cost of hospital care for older adults with heart failure: Medical, pharmaceutical, and nursing costs. Health Serv Res. 2008;43(2):635–655. doi: 10.1111/j.1475-6773.2007.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pressler SJ. Cognitive functioning and chronic heart failure: A review of the literature (2002 -July 2007) J CardiovascNurs. 2008;23:239–249. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- 4.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, et al. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59(2):127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: A meta-analytic review of prevalence, intervention effects, and association with clinical outcomes. JAm CollCardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 6.Sauve MJ, Lewis WR, Blankenbiller M, Rickabaugh B, Pressler SJ. Cognitive impairmentsin chronic heart failure: A case controlled study. J Card Fail. 2009;15:1–10. doi: 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Vogels RLC, Oosterman JM, van Harten B, Scheltens P, van der Flier WM, Schroeder-Tanka JM, et al. Profile of cognitive impairment in heart failure. J Am GeriatrSoc. 2007;55:1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 8.Pressler SJ, Kim J, Riley P, Ronis DL, Gradus-Pizlo I. Memory dysfunction, psychomotor slowing, and decreased executive function predict mortality in patients with heart failure and low ejection fraction. JCardFail. 2010;16(9):750–760. doi: 10.1016/j.cardfail.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, et al. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. JCard Fail. 2010;16:475–539. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm. 2010;7:433–437. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Woo MA, Birrer BVX, Macey PM, Fonarow GC, Hamilton MA, et al. Mammillary bodies and fornix fibers are injured in heart failure. Neurobiol Dis. 2009;33:236–242. doi: 10.1016/j.nbd.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siachos T, Vanbakel A, Feldman D, Uber W, Simpson KN, Pereira NL. Silent strokes in patients with heart failure. J Card Fail. 2005;11(7):485–489. doi: 10.1016/j.cardfail.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic,emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15(3):214–223. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sloan RS, Pressler SJ. Cognitive deficits and chronic heart failure: Re-cognition of vulnerability as a strange new world. J CardiovascNurs. 2009;24:241–248. doi: 10.1097/JCN.0b013e3181a00284. [DOI] [PubMed] [Google Scholar]
- 15.Briones TL, Therrien B, Metzger B. Effects of environment on enhancing functional plasticity following cerebral ischemia. Biol Res Nurs. 2000;1:299–309. doi: 10.1177/109980040000100406. [DOI] [PubMed] [Google Scholar]
- 16.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serber SL, Kumar R, Woo MA, Macey PM, Fonarow GC, Harper RM. Cognitive test performance and brain pathology. Nurs Res. 2008;57(2):75–83. doi: 10.1097/01.NNR.0000313483.41541.10. [DOI] [PubMed] [Google Scholar]
- 18.Vogels RLC, Oosterman JM, van Harten B, Gouw AA, Schroeder-Tanka JM, Scheltens P, et al. Neuroimaging and correlates of cognitive function among patients with heart failure. Dement GeriatrCognDisord. 2007;24:418–423. doi: 10.1159/000109811. [DOI] [PubMed] [Google Scholar]
- 19.Cansino S. Episodic memory decay along the adult lifespan: A review of behavioral and neurophysiological evidence. Int J Psychophysiol. 2009;71:64–69. doi: 10.1016/j.ijpsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: Scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 21.Dickson VV, Tkacs N, Riegel B. Cognitive influences on self-care decision making in persons with heart failure. Am Heart J. 2007;154:424–431. doi: 10.1016/j.ahj.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed May 9, 2011];Brain Train. Website: http://www.braintrain.com/main/cognitive_training_research.htm.
- 23. [Accessed May 9, 2011];Mindfit and Cognifit. http://www.cognifit.com/affiliations/Catalyst/MindFit.htm.
- 24.Rabiner D, Murray D, Skinner A. A randomized trial of two promising computer-based interventions for children with attention difficulties. J Abnorm Child Psych. 2010;38(1):131–142. doi: 10.1007/s10802-009-9353-x. [DOI] [PubMed] [Google Scholar]
- 25.Slate SE, Meyer TL, Burns WJ, Montgomery DD. Computerized cognitive training for severely emotionally disturbed children with ADHD. BehavModif. 1998;22(3):415–437. doi: 10.1177/01454455980223012. [DOI] [PubMed] [Google Scholar]
- 26.Burda PC, Starkey TW, Dominguez F, Vera V. Computer-assisted cognitive rehabilitation of chronic psychiatric inpatients. Compute Hum Behav. 1994;10(3):359–368. [Google Scholar]
- 27.Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD—a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Westerberg H, Klingberg T. Changes in cortical activity after training of working memory—a single-subject analysis. PhysiolBehav. 2007;92:186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson ML, Bartfai A, et al. Computerized working memory training after stroke – a pilot study. Brain Injury. 2007;21(1):21–29. doi: 10.1080/02699050601148726. [DOI] [PubMed] [Google Scholar]
- 30.Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging Ment Health. 2005;9(3):262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- 31.Craik FIM, Winocur G, Palmer H, Binns MA, Edwards M, Bridges K, et al. Cognitive rehabilitation in the elderly: effects on memory. J IntNeuropsycholSoc. 2007;13:132–142. doi: 10.1017/S1355617707070166. [DOI] [PubMed] [Google Scholar]
- 32.Stuss DT, Robertson IA, Craik FIM, Levine B, Alexander MP, Black S, et al. Cognitive rehabilitation in the elderly: A randomized trial to evaluate a new protocol. J IntNeuropsycholSoc. 2007;13:120–131. doi: 10.1017/S1355617707070154. [DOI] [PubMed] [Google Scholar]
- 33.Winocur G, Palmer H, Dawson D, Binns MA, Bridges K, Stuss DT. Cognitive rehabilitation in the elderly: An evaluation of psychosocial factors. J IntNeuropsycholSoc. 2007;13:153–165. doi: 10.1017/S135561770707018X. [DOI] [PubMed] [Google Scholar]
- 34.Winocur G, Craik FIM, Levine B, Robertson IH, Binns MA, Alexander M, et al. Cognitive rehabilitation in the elderly: Overview and future directions. J IntNeuropsycholSoc. 2007;13:166–171. doi: 10.1017/S1355617707070191. [DOI] [PubMed] [Google Scholar]
- 35.Oswald WD, Gunzelmann T, Rupprecht R, Hagen B. Differential effects of single versus combined cognitive and physical training with older adults: The SimA study in a 5-year perspective. Eur J Ageing. 2006;3:179–192. doi: 10.1007/s10433-006-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnett SM, Ceci SJ. When and where do we apply what we learn? A taxonomy for far transfer. Psychol Bull. 2002;128(4):612–637. doi: 10.1037/0033-2909.128.4.612. [DOI] [PubMed] [Google Scholar]
- 37.Zelinski EM. Far transfer in cognitive training of older adults. RestorNeurolNeuros. 2009;27:455–471. doi: 10.3233/RNN-2009-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith GE, Housen P, Yaffe K, Russ R, Kennison RF, Mahncke HW, et al. A cognitive training program based on principles of brain plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) Study. J AmGeriatrSoc. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: Scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 40.Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: A randomized, controlled study. ProcNatlAcadSci USA. 2006;103:12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.PositScience . Companion Guide: Using the Brain Fitness Program and Understanding the Science Behind It. PositScience; San Francisco: 2005-2007. [Google Scholar]
- 42.Castellani RJ, Zhu X, Lee HG, Perry G, Smith MA, Casadesus G. Neurogenesis in human hippocampus: Implications for Alzheimer disease pathogenesis. Neuroembryology& Aging. 2006-07;4:175–182. [Google Scholar]
- 43.Gage FH. Brain, repair yourself. Sci Am. 2003;283(6):46–63. doi: 10.1038/scientificamerican0903-46. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity of newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 45.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature: Neuroscience. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 46.Briones TL, Suh E, Jozsa L, Woos J. Behaviorally induced synaptogenesis and dendritic growth in the hippocampal region following transient global cerebral ischemia are accompanied by improvement in spatial learning. ExpNeurol. 2006;198:530–538. doi: 10.1016/j.expneurol.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 47.Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: An interim report on the effects 6 months later. Schizophr Bull. 2010;36(4):869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. for the ACTIVE Study Group Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, et al. ACTIVE: A cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. for the ACTIVE Study Group Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald’sHeart Disease: A Textbook of Cardiovascular Medicine. 7thed Elsevier Saunders; Philadelphia: 2005. [Google Scholar]
- 52.American Heart Association [Accessed August 27, 2010];Heart Insight. website, 2010. http://journals.lww.com/heartinsight/pages/default.aspx.
- 53.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am JCardiol. 1989;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 54.Dunbar SB, Langberg JJ, Reilly CM, Viswanathan B, McCarty F, Culler SD, et al. Effect of a psychoeducational intervention on depression, anxiety, and health resource use in implantable cardioverter defibrillator patients. PACE. 2009;32:1249–1272. doi: 10.1111/j.1540-8159.2009.02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson CL, Herndon JE, Mark DB, Pryor DB, Califf RM, Hlatky MA. Relation of clinical and angiographic factors to functional capacity as measured by the Duke Activity Status Index. Am J Cardiol. 1991;68:973–975. doi: 10.1016/0002-9149(91)90423-i. [DOI] [PubMed] [Google Scholar]
- 56.Rector TS, Kubo SH, Cohn JN. Patients’ self-assessment of their congestive heart failure, Part 2: Content, reliability and validity of a new measure, the Minnesota Living with Heart Failure Questionnaire. Heart Failure. 1987;3:198–209. [Google Scholar]
- 57.Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, et al. Discriminant properties of commonlyusedquality of life measures in heartfailure. Qual Life Res. 2002;11:349–359. doi: 10.1023/a:1015547713061. [DOI] [PubMed] [Google Scholar]
- 58.Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, et al. Comparison of quality of life measures in heartfailure. NursRes. 2003;52:207–216. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 60.Perkins AJ, Kroenke K, Unutzer J, Katon W, Williams JW, Jr, Hope C, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J ClinEpidemiol. 2004;57:1040–1048. doi: 10.1016/j.jclinepi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 62.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4thed Oxford; New York: 2004. [Google Scholar]
- 63.Giordani B, Boivin MJ, Hall AL, Foster NL, Lehtinen SJ, Bluemlein LA, et al. The utility and generality of Mini-Mental State Examination scores in Alzheimer’s disease. Neurology. 1990;40:1894–1896. doi: 10.1212/wnl.40.12.1894. [DOI] [PubMed] [Google Scholar]
- 64.DeJager CA, Schrijnemaekers AMC, Honey TEM, Budge MM. Detection of MCI in the clinic: Evaluation of the sensitivity and specificity of a computerised test battery, the Hopkins Verbal Learning Test and the MMSE. Age Ageing. 2009;38:455–460. doi: 10.1093/ageing/afp068. [DOI] [PubMed] [Google Scholar]
- 65.Riegel B, Bennett JA, Davis A, Carlson B, Montague J, Howard R, Glaser D. Cognitive impairment in heart failure: Issues of measurement and etiology. Am J Crit Care. 2002;11:520–528. [PubMed] [Google Scholar]
- 66.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised. Clin Neuropsychologist. 1998;12(1):43–55. doi: 10.1076/clin.13.3.348.1749. doi: 10.1076/clin.12.1.43.1726. [DOI] [PubMed] [Google Scholar]
- 67.Brandt J, Benedict RHB. Hopkins Verbal Learning Test – Revised. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- 68.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological Assessment. 1996;8:145–153. [Google Scholar]
- 69.Wechsler D. Administration and Scoring Manual. 3rd ed The Psychological Corporation; USA: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- 70.Benton AL, Hamsher K. Multilingual Aphasia Exam Manual. University of Iowa; Iowa City, IA: 1978. deS. [Google Scholar]
- 71.Collie A, Maruff P, Makdissi M, McCrory P, McStephen M, Darby D. CogSport: Reliability and correlation with conventional cognitive tests used in postconcussion medical evaluation. Clin J Sport Med. 2003;13:28–32. doi: 10.1097/00042752-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Maruff P. Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychology. 2009;24(2):165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- 73.Cysique LAJ, Maruff P, Darby D, Brew BJ. The assessment of cognitive function in advanced HIV-1 infection and AIDS dementia complex using a new computerized cognitive test battery. Arch Clin Neuropsychology. 2006;21:185–194. doi: 10.1016/j.acn.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 74.Darby D, Maruff P, Collie A, McStephen M. Mild cognitive impairment can be detected by multiple assessments in a single day. Neurology. 2002;59(7):1042–1046. doi: 10.1212/wnl.59.7.1042. [DOI] [PubMed] [Google Scholar]
- 75.Schinka JA, McBride A, Vanderploeg RD, Tennyson K, Borenstein AR, Mortimer JA. Florida Cognitive Activities Scale: Initial development and validity. JINS Journal of International Neuroscience. 2005;11:108–116. doi: 10.1017/S1355617705050125. [DOI] [PubMed] [Google Scholar]
- 76.Dotson VM, Schinka JA, Brown LM, Mortimer JA, Borenstein AR. Characteristics of the Florida Cognitive Activities Scale in older African Americans. Assessment. 2008;15(1):72–77. doi: 10.1177/1073191107307509. [DOI] [PubMed] [Google Scholar]
- 77.Willis SL, Marsiske M. Manual for Everyday Problems Test. Pennsylvania State University; University Park, PA: 1993. [Google Scholar]
- 78.Willis SL, Allen-Burge R, Dolan MM, Bertrand RM, Yesavage J, Taylor JL. Everyday problem solving among individuals with Alzheimer’s disease. Gerontologist. 1998;38:569–577. doi: 10.1093/geront/38.5.569. [DOI] [PubMed] [Google Scholar]
- 79.Allaire JC, Willis SL. Competence in everyday activities as a predictor of cognitive risk and mortality. Aging, Neuropsychology, & Cognition. 2006;13:207–224. doi: 10.1080/13825580490904228. [DOI] [PubMed] [Google Scholar]
- 80.Northouse LL, Walker J, Schafenacker A, Mood D, Mellon S, Galvin E, et al. A family-based program of care for women with recurrent breast cancer and their family members. OncolNurs Forum. 2002;29:1411–1419. doi: 10.1188/02.ONF.1411-1419. [DOI] [PubMed] [Google Scholar]
- 81.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: A randomized, controlled trial. J AmGeriatrSoc. 2004;52:675–684. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 82.Sochalski J, Jaarsma T, Krumholz HM, Laramee A, McMurray JJV, Naylor MD, et al. What works in chronic care management: The case of heart failure. Health Aff. 2009;28(1):179–189. doi: 10.1377/hlthaff.28.1.179. [DOI] [PubMed] [Google Scholar]
- 83.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nded Lawrence Erlbaum; Hillside, NJ: 1998. [Google Scholar]