Abstract
Background
Ethanol modulates glutamate and GABA function. However, little is known about the acute pharmacologic effects of ethanol on levels of GABA, glutamate, and other metabolites measurable in the human cortex in vivo using 1H magnetic resonance spectroscopy (MRS).
Methods
Eleven healthy social drinkers received two intravenous ethanol infusions that raised breath alcohol levels to a clamped plateau of 60 mg/dL over 60–70 minutes. The first infusion established tolerability of the procedure, and the second procedure, conducted 15±12 days later, was performed during 1H MRS of occipital GABA, glutamate, and other metabolites.
Results
The time course of brain ethanol approximated that of breath ethanol, but venous ethanol lagged by about 7 minutes. GABA fell 13±8% after 5 minutes of the ethanol infusion and remained reduced (p=0.003) throughout the measurement. The combination of N-acetylaspartate and N-acetylaspartyl glutamate (summed as NAA) fell steadily during the infusion by 8±3% (p=0.0036).
Conclusions
Ethanol reduced cortical GABA and NAA levels in humans. Reductions in GABA levels are consistent with facilitation of GABAA receptor function by ethanol. The gradual decline in NAA levels suggests inhibition of neural or metabolic activity in the brain.
Keywords: 1H MRS, GABA, alcohol, NAA, glutamate, brain
Introduction
Alcohol has a complex pharmacology at concentrations that constitute legal intoxication in many countries (blood alcohol concentration: 0.04–0.08%) (1). Its acute facilitation of GABAA receptor function and blockade of NMDA receptors have received intensive study (2, 3). The facilitation of GABAA receptor function by ethanol is complex and involves possible direct effects on extrasynaptic GABAA receptors and indirect effects mediated by increases in the release of GABA and GABAA receptor-modulating neurosteroids (4–7). There currently is little understanding of the acute effects of ethanol on cortical GABA or glutamate levels in humans, as well as other relevant metabolite levels that may be measured in humans using proton magnetic resonance spectroscopy (1H-MRS) (8–10). One study of acute ethanol effects on frontal cortical metabolites in humans measured with MRS found reductions in creatine, choline, inositol, and aspartate, but no effects on levels of NAA (11).
1H MRS provides measurements of several neurochemicals implicated in neural and glial structure and function (12). Levels of the metabolite NAA, which is found in neurons (13), may change with alterations in neural activity and integrity and may be related to mitochondrial energetics or neuronal health. A signal that represents choline, phosphocholine, and glycerophosphocholine is generally believed to represent active synthesis or breakdown of membranes. Creatine and phosphocreatine, which are involved in high-energy phosphate metabolism, are unresolved in the brain at most magnetic field strengths. Myoinositol has a prominent signal and has been identified as a major brain osmolyte (14) (14). Metabolites involved in amino acid neurotransmission, including GABA, glutamate, and glutamine, are also quantifiable with MRS. Understanding the acute effects of ethanol may shed light on the pattern of metabolite changes measured with 1H MRS in heavy social drinkers and alcohol dependent patients (15). These studies report reductions in NAA (10, 16–18) and total choline levels (Cho) (16, 17, 19, 20) with little or no effect on other metabolites such as creatine, myoinositol, or aspartate (20). In recovering alcohol dependent patients, lower levels of GABA and/or higher levels of glutamate appear to be associated with the duration of abstinence, the severity of dependence, and the presence of comorbid smoking (9, 21).
Reports that ethanol potentiates the GABAergic system and that the brain adapts acutely and chronically to the presence of ethanol led us to hypothesize that the concentration of GABA will change when ethanol is administered. It could be that GABA would drop as ethanol’s potentiation of the GABA receptors requires less GABA transmission, or it could be that GABA would rise as less is released. The current study evaluated changes in GABA, glutamate, and other neurochemicals in the brains of subjects receiving continuous infusions of ethanol to shed light on the neuro-metabolic effects of ethanol in healthy humans. To minimize the impact of the variable pharmacokinetics of oral ethanol administration, this study employed an intravenous (IV) ethanol infusion paradigm (22) to clamp breath alcohol at 60 mg/dL.
Methods and Materials
Subjects
Healthy human subjects were recruited from the community via local advertisements. Prior to their participation, all subjects provided written informed consent based on a protocol approved by the Yale Human Investigations Committee (New Haven, CT). Alcohol-naïve individuals, pregnant women (confirmed by urine pregnancy testing), adoptees unable to provide information regarding their family history of alcoholism, and people with a history of neurological disorders, ethanol intolerance, or contraindications for MR were excluded. Individuals were also excluded on the basis of a structured diagnostic interview that revealed a psychiatric or substance dependence history (other than tobacco smoking) that met diagnostic criteria (23). Other exclusionary criteria included unwillingness to remain alcohol-free for 48 hours before test days, or a history of psychosis or bipolar disorder in a first- or second-degree relative. Subjects completed a physical exam and routine laboratory testing, including urine toxicology. On the evening prior to ethanol administration, subjects were instructed to fast after 10pm to reduce aspiration risk.
Administration of Ethanol
The Investigational Drug Service prepared the ethanol infusate with a 6% (volume/volume) alcohol concentration in 0.9% saline. The time course of the infusion rate was calculated based on the subject’s height, weight, age, and gender, using a model of ethanol uptake and clearance developed at Indiana University-Purdue University Indianapolis (24) with the specified target of a 20-minute rise in breath alcohol concentration (BAC) to 60 mg/dL and a plateau of 40–50 minutes. This level approximates ethanol levels commonly achieved through social ethanol consumption. An antecubital IV line was placed in each arm, one for infusion of ethanol and the other for drawing of blood samples. Venous blood samples were obtained every 10 minutes during the infusion and measured using the Analox GM7 (Analox Instruments USA, Inc., Lunenburg, Massachusetts).
The procedure was performed twice for each subject. The first infusion was in the Hospital Research Unit to fine-tune the computer-generated infusion profile in an environment where BAC could be measured with a 6510 Alcotest breathalyzer (Draeger Safety Diagnostics, Inc., Irving, Texas). Breath samples were obtained every minute until the plateau was reached and every two minutes thereafter for the duration of the infusion. Self-assessments were completed at baseline and again over the course of the alcohol infusion: the Similarity Scale (25), the Number of Drinks Scale (25) and the Biphasic Alcohol Effects Scale (26). Before and during the infusion, subjects were administered the Mini-Mental Status Exam (27) and then completed the Grooved Peg Board Task to assess coordination.
After waiting at least one week, the fine-tuned infusion was repeated in a 4-Tesla magnet (Bruker Instruments, Billerica, MA, U.S.A.) in the Yale Magnetic Resonance Research Center while MRS was performed. Two IV lines were placed, as was done for the first infusion. Subjects lay in the scanner for 10–20 minutes while positioning and scanner adjustments were performed, and then a 20-minute baseline MRS scan was acquired, followed by the start of the infusion and the acquisition of 60–70 minutes of MRS data. When the study was designed, we estimated that one hour would suffice to see possible changes in GABA, based on microdialysis measurements of GABA in the extracellular fluid of rats infused with ethanol, in which effects were seen at 30 minutes and continued through 90 minutes and beyond (28).
1H MRS
Gradient-echo scout images were acquired for subject positioning. For high sensitivity and temporal resolution, a surface coil was placed against the back of the head. A voxel of 3.0×3.0×1.5 cm3 was selected in the occipital cortex in or overlapping V1. Non-iterative, localized shimming was performed (29), and the radiofrequency power was calibrated to optimize volume selection and editing efficiency over the volume of interest. Localization was achieved using a spin-echo sequence to select two dimensions, with Shinar-LeRoux excitation and a sinc refocusing pulses. 3D localization was superimposed with adiabatic outer volume suppression. Water suppression was performed with adiabatic Chemical-Shift Selective (CHESS) (30). The J-editing technique for isolation of GABA (31) relied on pairs of spectra acquired over a 20-minute period, acquiring 1024 complex data points over a 410-ms acquisition window, with a repetition time of 2.5 seconds. In half the scans, a frequency-selective editing pulse was applied at 1.89 ppm, and in the other half, at 1.31 ppm, which, in conjunction with the echo time of 68 ms, led to a phase difference of the GABA resonance at 3.0 ppm. Upon subtraction of the spectral pairs, the GABA was separated from the larger, nearby signals for creatine and choline. The levels of glutamate, glutamine, NAA, myoinositol, total creatine, total choline, scylloinositol, and ethanol were measured in the subspectrum that was acquired with the 1.31-ppm editing pulse. Glutamate and glutamine that appeared co-edited in the difference spectrum were not analyzed, because of the power-dependence of the frequency spread of the editing pulse profile away from 1.89 ppm.
Each subject’s data was grouped into 10-minute averages to evaluate the metabolite time courses. Spectral fitting was performed using a measured basis set that included aspartate, GABA, glutamate, glutamine, creatine, myoinositol, choline, phosphorylcholine, and glycerophosphorylcholine. Phosphocreatine was simulated by shifting the creatine methyl resonance, N-acetylaspartyl glutamate (NAAG) was simulated by shifting the NAA methyl resonance, and scylloinositol was simulated by shifting the creatine methyl resonance. The final analyses yielded NAA as a sum with NAAG, creatine with phosphocreatine, and the three species of choline, because of insufficient resolution to determine their levels individually. Glutamate and glutamine could be measured separately at the echo time of 68 ms, with the center peaks up and the outer wings of each triplet down. At 68 ms, the aspartate resonance was low in amplitude and poorly determined. Uncertainties in individual measurements were determined by Monte-Carlo analysis in which the least-squares spectral fits were treated with random Gaussian noise whose standard deviation was equal to that of the raw data and refitted, using 20 repetitions, from which the standard deviations of the uncertainty for each metabolite was determined. For each metabolite, a threshold for rejection was set at twice the average noise-based standard deviation of the respective metabolite. GABA levels whose uncertainties were greater than 11%, glutamine levels whose uncertainties were greater than 20%, and glutamate levels whose uncertainties were greater than 16% were not included in subsequent analysis. Results were expressed relative to the initial tissue water. The ethanol time course for each subject was normalized to the individual’s final ethanol signal.
In addition, six subjects were studied in paired sessions without ethanol to evaluate macromolecular contamination. In one session, metabolite nulling (32, 33) was done with a double inversion-recovery that nulled the metabolite resonances but yielded full recovery of macromolecular resonances, and the editing pulse was placed at 1.89 ppm and 6.65 ppm, symmetrically around the water resonance, acquiring 432 transients in total for each subject. Then, in the same group of six subjects, a second approach was used, comparing the difference spectrum with the editing pulse placed symmetrically about water with the result from symmetric placement of the editing pulse around the 1.6-ppm macromolecular resonance (34), with 432 transients recorded in each condition. Both cases revealed no detectible macromolecular contamination of the edited GABA resonance.
Safety Measures
GABA editing requires minimal head motion, which is typically achieved by padding the head firmly. With a subject supine, the primary safety concern was aspiration. A nurse or physician was in the scan room at all times with the subject, the head holder was easily releasable by the subject, and subjects were instructed to open the doors holder immediately upon any sensation of nausea. No subject reported nausea.
Because the ethanol was delivered from two one-liter bags of a 6% solution, the most pure ethanol that could be delivered was 4 fluid ounces, although far less than this was administered for this procedure. After the infusion, subjects were discharged from the Magnetic Resonance Research Center or the Hospital Research Unit when their breath alcohol had fallen to ≤ 20 mg/dL, which is below the threshold of BAC associated with impairment in locomotor coordination or judgment in humans (35).
Statistical Analysis
Linear mixed models, which included time (0, 5, 15, 25, 35, 45, 55, 65 min) as a within-subjects factor, was used to evaluate GABA levels over time. The best fitting variance-covariance structure was assessed using information criteria. The mixed effects approach is advantageous in that it is unaffected by randomly missing data and allows greater flexibility in modeling the correlation structure of repeated measures data. Significant time effects were interpreted using appropriate post-hoc tests and graphical displays. Similar models were used for glutamate, glutamine, NAA, creatine, myoinositol, and choline, with a Bonferroni adjustment of 6 multiple comparisons. Scylloinositol, whose signal is very low, was evaluated as an exploratory aim. Correlation analysis was used to examine potential associations between subjective measures associated with ethanol and metabolite levels. All tests were two-sided and considered significant at the alpha=.05 threshold.
Results
Table 1 describes participants in the ethanol administration study. The sample was predominately a group of well-educated healthy females in their mid-twenties who exhibited light and infrequent social drinking.
Table 1.
Demographic data and drinking history from subjects (n=11) in this study.
| Characteristic | Measure | % | Number of Subjects |
|---|---|---|---|
| Gender | women/men | 73 / 27 | 8/3 |
| Age (mean SD) | 26.4 4.1 years | - | - |
| Years of education (mean SD) | 16.6 1.0 years | - | - |
| Ethnicity | Caucasian | 100 | 11 |
| In past year, frequency of drinking | 0 drinks 3–11 times/year 2–3 times/month 2 times/week |
18 27 45 9 |
2 3 5 1 |
| On a typical drinking occasion, number of drinks consumed | 0 1 2 3–4 |
18 9 36 36 |
2 1 4 4 |
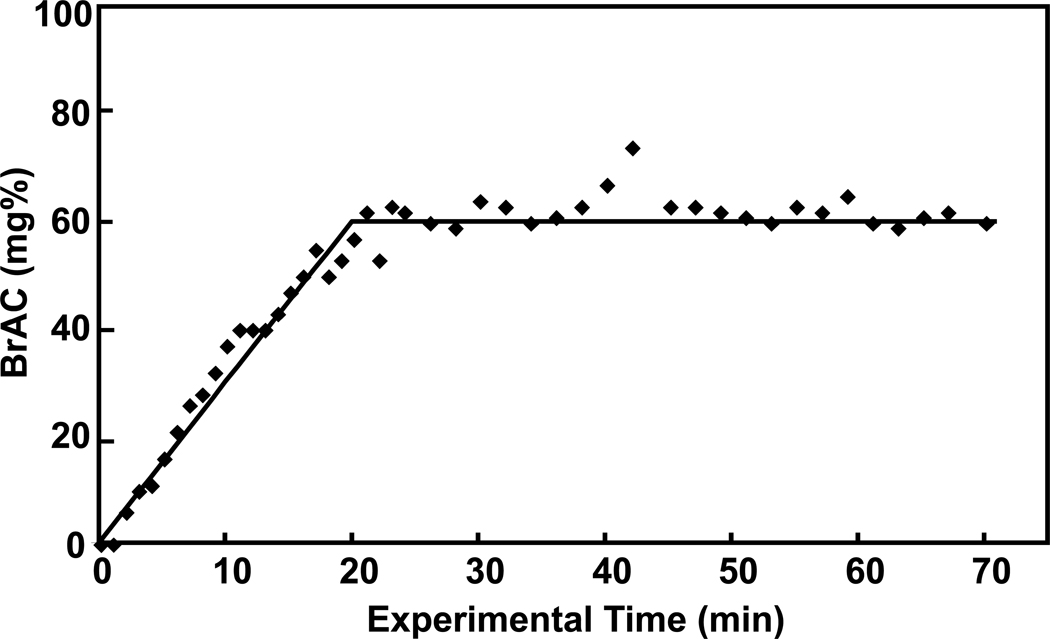
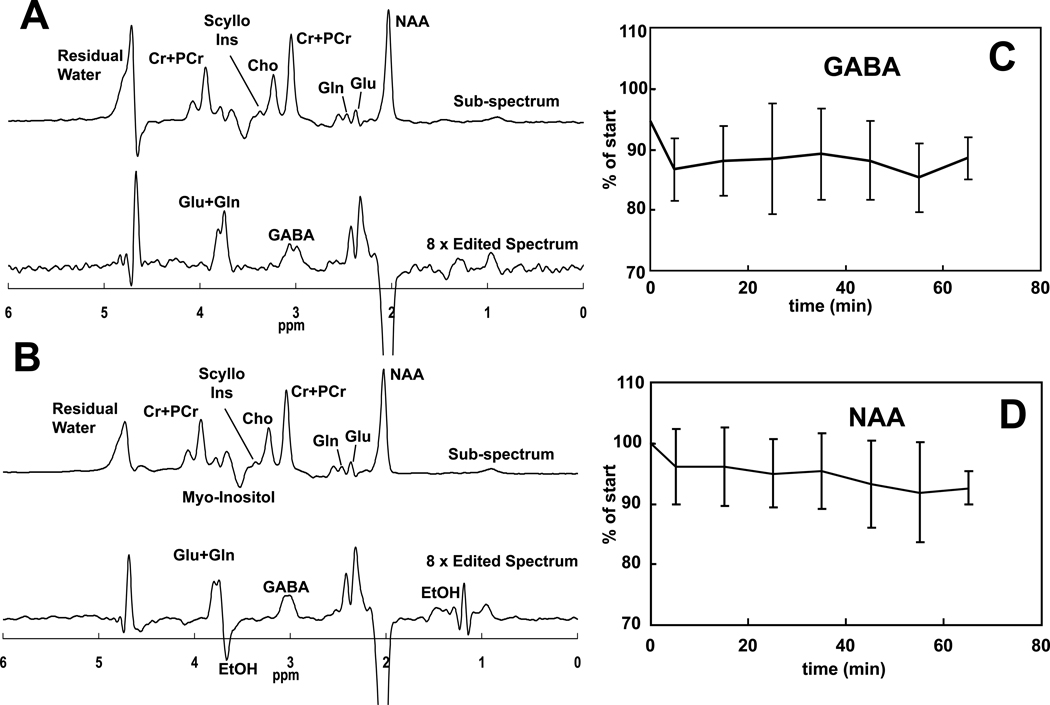
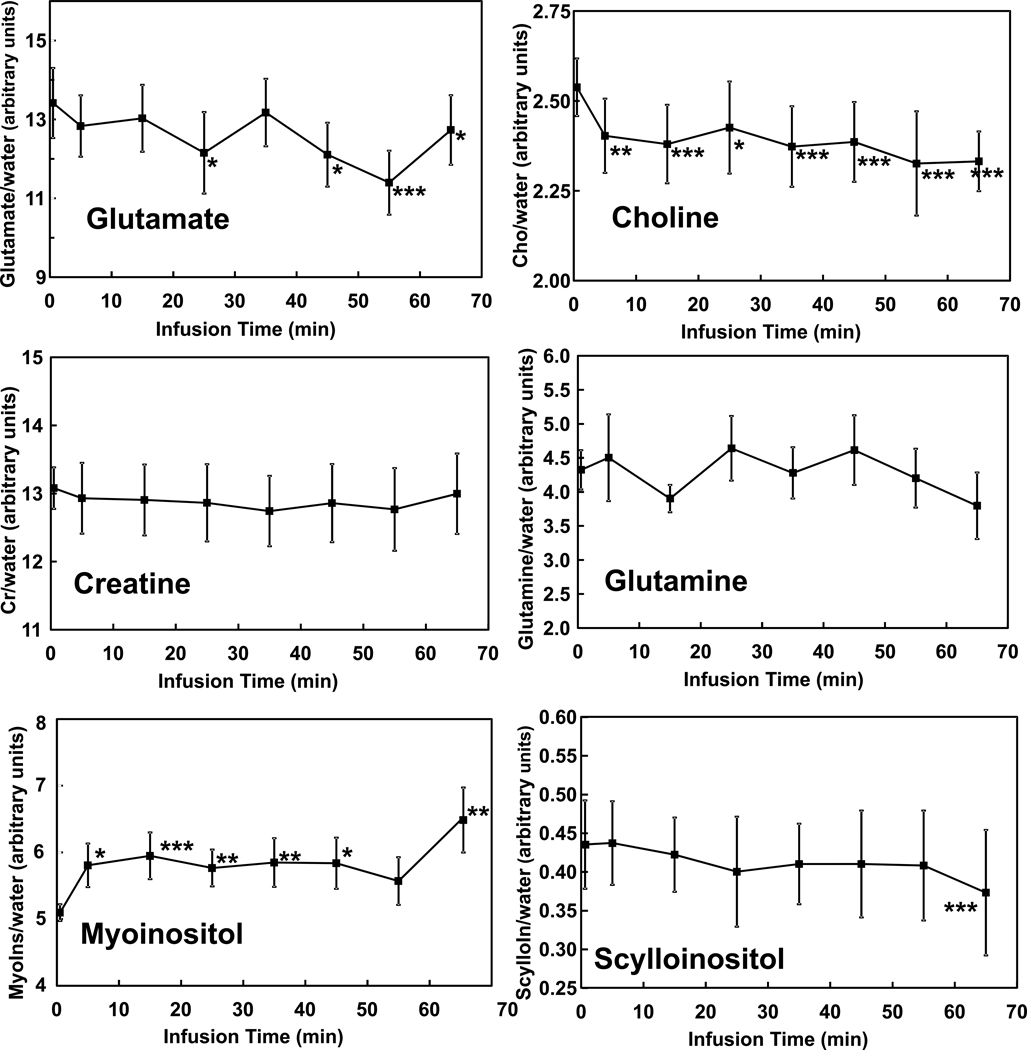
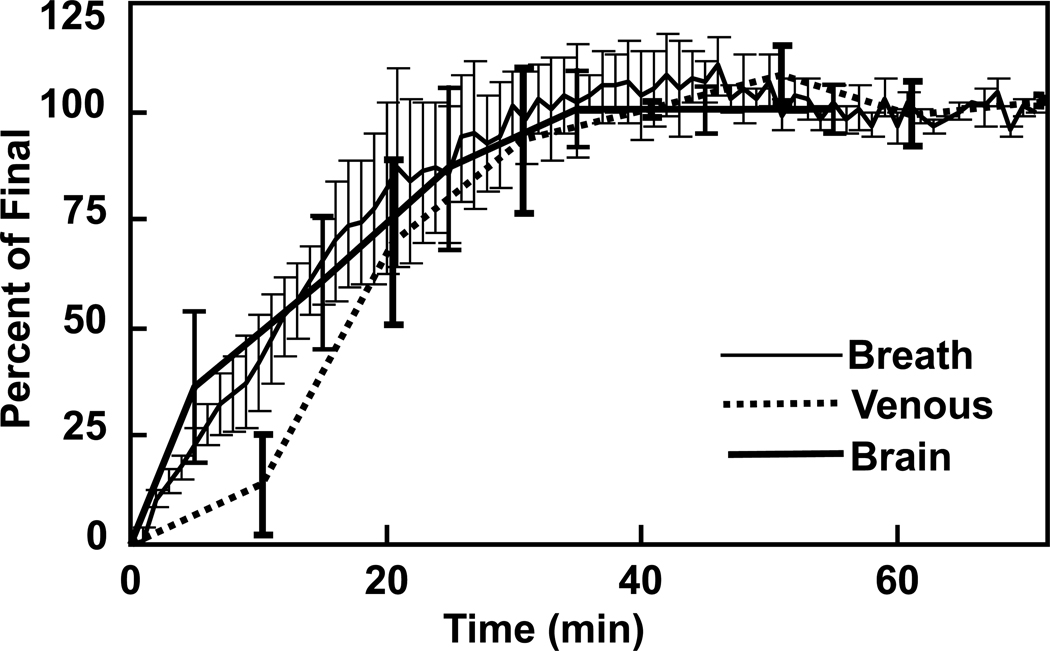
Ethanol infusion increased breath alcohol levels from 0 to 60 mg% in approximately 20 minutes and maintained it there for the duration of the study (Figure 1). Figure 2 contains spectra acquired before and during administration of ethanol, as well as time courses of GABA and NAA during the ethanol infusion. The ethanol resonance at 1.18 ppm was not seen in the sub-spectrum because placement of the editing pulse at 1.31 ppm generates nearly a signal null for that resonance. A signal from ethanol is present at 3.68 ppm in the sub-spectrum but is not obvious due to overlap with myoinositol. A significant effect of infusion time on levels of GABA (F(7,60)=3.57, p=0.003) was observed. GABA decreased rapidly by 13 ± 8% during the first 10 minutes of the infusion (t(60)=3.82, p=0.0003). The decrease was maintained after blood alcohol concentration reached the target of 60mg% and remained there for the duration of the study. NAA also showed a significant effect of time after Bonferroni correction (F(7,60)=3.45, p=0.004). When evaluated by individual time points, NAA decreased by 4 ± 6% in the first 10 minutes (t(60)=3.85, p = 0.0003) and then decreased steadily over the rest of the hour, to 8 ± 3% below the pre-infusion value by the end of the infusion (all points p<.05). Figure 3 shows the effects on glutamate and other MRS measurements over time. The effect of time on glutamate was significant before Bonferroni correction (F(7,60)=2.63, p=0.019) with reductions observed at most times. Choline also changed significantly before Bonferroni correction (F(7,60)=2.65, p=0.019), with decreases observed at all infusion time points, which might be consistent with reports of low brain choline levels in early sobriety (16, 17, 19, 20). Creatine was consistent over time (F(7,60)=0.57, p=0.78), while scylloinositol, a metabolite of unknown function, showed a gradual decrease that after the Bonferroni correction did not reach significance (F(7,60)=2.35, p=0.035). Although a time effect was not observed for glutamine (F(7,60)=1.47, p=0.19), glutamine was determined less precisely than glutamate due to its lower concentration and overlap with other metabolites. A trend time effect was observed for myoinositol levels (F(7,60)=2.08, p=0.059) with significant increases observed post-hoc. Brain ethanol rose with a time course that was almost identical to that of breath ethanol, but venous ethanol lagged the brain and breath by ~7 minutes (Figure 4).
Figure 1.
Breath alcohol concentrations (BAC; diamonds) from subjects during continuous infusions of ethanol 6% (v/v) compared to the desired profile (solid line). Breath samples were obtained every minute until the plateau was reached and then every 1–2 minutes thereafter for the duration of the experiment.
Figure 2.
Spectra obtained before (A) and during (B) IV administration of ethanol show resonances that include GABA and ethanol. In each set, the upper spectrum represents the subspectrum obtained without the J-editing pulse applied, and the lower spectrum shows the difference spectrum where ethanol and GABA are clearly visible. Figures C and D show the time courses of GABA and NAA over the course of the ethanol infusion. (****p<0.001; ***p<0.01; **p<0.02; *p<0.05)
Figure 3.
Time courses of brain metabolites before (t=0) and during the ethanol clamp. (****p<0.001; ***p<0.01; **p<0.02; *p<0.05)
Figure 4.
Average time courses of ethanol observed in the brain, breath, and blood during the IV alcohol infusions.
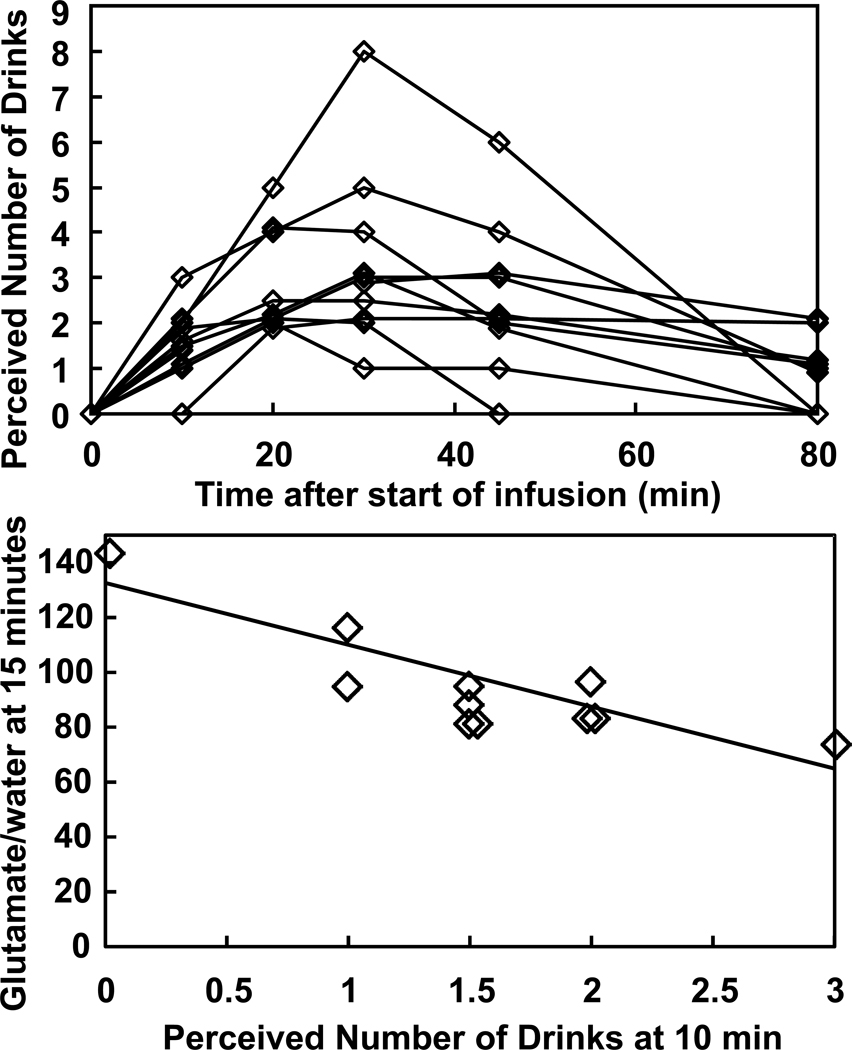
With only three male subjects, sex effects on metabolite levels could not be explored. Subjects were not blinded to the fact that they were receiving ethanol, and they judged the IV ethanol effects to be very similar to their previous experiences with alcohol. The perceived number of drinks changed significantly with time during the infusion (F(5,44)=14.5, p<0.0001), with an inverted U-shape, so that the number of drinks perceived decreased after 30 minutes of ethanol administration, even though the breath alcohol level remained constant (Figure 5). The number of drinks perceived at 10 minutes, when subjects began to perceive the drinks, was negatively correlated with the concentration of glutamate at 15 min (R2=0.74, p=0.0014) (Figure 5), although after correcting for the many correlations that were tested, the significance did not remain. The two subjects who reported consuming no alcohol in the previous year appeared unremarkable in all subjective and MRS parameters when compared to the rest of the group.
Figure 5.
(A) Number of drinks perceived by the subjects over time during the infusion. (B) Relationship between the perceived number of drinks at 10 minutes and the level of glutamate at 15 minutes, which is actually the glutamate signal averaged from 10 to 20 minutes (R2=0.74; p=0.0014).
Discussion
The principal finding of this study was that a one-hour infusion of ethanol decreased cortical GABA and NAA levels in a group of healthy young adults, predominately females. Changes in NAA, glutamate, and other metabolites were also observed in this acute study.
GABA
These data suggest that acute ethanol administration powerfully regulates GABA neuronal function, although they are limited in their capacity to specify how. The reduction in brain GABA levels by ethanol is consistent with its known facilitation of GABAA receptor function (36). In the rodent hypothalamus, acute ethanol administration suppressed the activity of the GABA synthesizing enzyme, glutamic acid decarboxylase (GAD) (37). The conversion of GAD from the active holoenzyme to inactive apoenzyme can occur in minutes (38, 39), so there is potential for changes on the time scale of the rapid rise of ethanol early in the infusion. It might also be important that the time is similar to how long it takes people to begin to feel that they have had a drink (Fig. 5). Meanwhile, ethanol does not acutely modulate the activity of GABA transaminase, the principal GABA catabolic enzyme (40), so changes in GABA level are expected to be mediated by GAD. These data are also consistent with the finding that GABAA receptor stimulation with alprazolam similarly produced a rapid reduction in human cortical GABA levels (41), attributed to a reduction in GAD activity, although that study made treatment observations only 90 minutes after oral drug administration. The possibility that ethanol directly or indirectly inhibits GAD activity may have implications for risk of developing alcoholism. Variance in the human GAD1 gene has been associated with subjective response to ethanol and the age of onset of alcoholism (42). Variation in the GAD2 gene also has been associated with alcoholism risk (43).
N-Acetylaspartate (NAA)
This study found that ethanol acutely depressed NAA levels, a mitochondrial product synthesized in neurons that is sometimes utilized as a measure of neuronal viability. While changes in NAA levels might reflect a neuronal ethanol effect, it is also possible that ethanol enhances the activity of acetylaspartylase, a glial enzyme that degrades NAA (44, 45). In the context of alcohol dependence, decreases in NAA levels may signal reversible brain metabolic compromise that reflects neural injury or even death. However, in this study NAA reductions are likely to reflect a reversible metabolic consequence of a relatively low alcohol dose. Furthermore, it is possible that the NAA effects are region-dependent. For example, a prior study of frontomesial and cerebellar cortices did not find that ethanol lowered NAA levels (11). However, there were other differences with the prior study, including methods of the NAA measurement, the proportion of males and females in the study sample, the way that alcohol was administered, and the possibility that breath alcohol levels differed. A recent study with gavage administration of ethanol in rats (46) showed in their control group reductions of 9% and 5% in NAA and Cho, respectively, compared to 8% and 7% in Cho in the present data, similar to the present results.
Other Metabolites
The decreases in choline (p = 0.019) and scylloinositol (p = 0.035) did not remain significant after the correction for multiple comparisons. Likewise, the decrease in glutamate (p = 0.019) the p-value of 0.019 for glutamate did not remain significant after Bonferroni adjustment, nor did the correlation of glutamate with the perceived number of drinks (p = 0.0014) A future study with an a priori hypothesis of changes in glutamate and glutamine could assess these observations and possibly employ 13C-MRS to measure the turnover rates for glutamate and glutamine directly may help to inform our understanding of ethanol effects on human cortical glutamatergic function (47, 48).
Comparisons of Ethanol in Brain, Venous Blood, and Breath
The time courses of brain and breath ethanol differed insignificantly, but venous blood ethanol levels lagged by ~7 minutes. Given a recent report that capillary and breath alcohol are very similar, while venous ethanol lags significantly (49), the current observations suggest that brain ethanol is determined primarily by rapid transfer from the capillary beds. Rapid perfusion is consistent with MRS measurements in rats (50) and with other measurements in organs with high blood flow, including brain (51). This conclusion is based on ethanol signals normalized by the final values, because absolute quantification was not performed due to power-dependent bandwidths of the J-editing pulse at the 1.18-ppm position of ethanol. Similar conclusions were reached on the basis of measurements at steady-state following oral administration of ethanol (52). Within each session, the same power was used for all measurements, and the ethanol signals were internally consistent and could therefore be normalized to the end point.
Acute ethanol tolerance
This study replicated the observation of acute tolerance to the subjective effects of ethanol made possible by the improved technique for holding blood alcohol levels steady using the IV alcohol clamp procedure (53). However, we were not able to identify a neuro-metabolic signature of the acute tolerance to ethanol effects.
Macromolecular measurements
Quantification of the small edited GABA signal can be affected significantly by even a little contamination from macromolecular resonances. The echo delay of 68 ms reduces their signal sufficiently that they are normally ignored, their impact on edited GABA was measured and found to be undetectable. Because the resonances are farther apart at higher field strength, the finite bandwidth of the GABA editing pulse has less effect on the macromolecular resonance at 1.6 ppm. Still, to reduce contamination that might potentially occur, the GABA editing scheme of symmetry about the 1.6-ppm resonance was used (34).
Limitations
This pilot study was associated with a number of limitations that affect the ability to draw firm conclusions from the results. First, despite efforts to reduce clinical confounds, the small study sample limited our ability to control for sex, age, menstrual cycle, extent of prior alcohol consumption, and family history of alcoholism. It also limited the statistical power to explore correlations between metabolite changes and subjective effects. Second, the use of a surface coil placed over occipital cortex made it possible to make the GABA measurements with high sensitivity and time resolution in this study, but it limited the brain regions that could be studied. Third, this pilot study did not employ a placebo infusion, raising the possibility that this report does not control adequately for some experimental confounds. Fourth, the safety measure of fasting overnight has the potential to affect ethanol’s effects, such as the euphoric response (54), but information about the effects of a brief period of fasting on ethanol are limited. Last, unsuppressed water was only measured with the first acquisition, pre-ethanol administration, so water cannot be used to evaluate a possible change in the MRS detection efficiency in the volume of interest as an explanation for the decrease in GABA and other metabolites. However, the fact that creatine was very stable, myoinositol rose, and the patterns of decrease in GABA, glutamate, and choline differed from one another suggests that it is unlikely that the decreases resulted from changes in detection efficiency (Figure 3).
Summary
In conclusion, GABA was observed to decrease significantly within the first few minutes of IV ethanol administration and remained low, while NAA fell more gradually over the course of the infusion. Ethanol in the brain had a time course that was similar to that of the breath but faster than that seen in the venous blood. These findings provide a neuro-metabolic profile of the acute effects of ethanol in humans that may inform subsequent studies of human ethanol intoxication and the risk for alcoholism.
Acknowledgements
Professor Sean O’Connor of Indiana University-Purdue University Indianapolis deserves our special thanks for instruction, training, gift of software, and loan of equipment for the intravenous infusion of ethanol. Mark Abildgaard and Peter Brown of the Yale Magnetic Resonance Research Center developed the quick-release head-holder for safety during the MRS studies. This work was supported by the National Institutes of Health via P50 AA12870 (GFM, ILP, JFK), R01 DA021785 (GFM), R21 AA018210 (GFM), 2R01AA011321 (GFM, EG, GS, ILP, JHK), KO5 AA 14906 (JHK), K02 MH076222 (GS), and CTSA Grant UL1 RR024139. It also received support from the Department of Veterans Affairs via the VA Alcohol Research Center (I.P., J.H.K.), the State of Connecticut via its support for the Abraham Ribicoff Research Facilities (EG, GS, JHK), the Conselho Nacional de Desenvolvimento Científico e Tecnológico of Brazil (RG), and the Dana Foundation (GFM).
Financial information for John Krystal
Individual consulting arrangements, which all total less than $10,000 per year:
Aisling Capital, LLC, AstraZeneca Pharmaceuticals, Brintnall & Nicolini, Inc., Easton Associates, Gilead Sciences, Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Inc., Merz Pharmaceuticals, MK Medical Communications, F. Hoffmann-La Roche Ltd, SK Holdings Co., Ltd, Takeda Industries, Teva Pharmaceutical Industries, Ltd.
Scientific Advisory Board
Abbott Laboratories, Bristol-Myers Squibb, Eisai, Inc., Eli Lilly and Co., Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Naurex, Inc., Pfizer Pharmaceuticals, Shire Pharmaceuticals.
Exercisable Warrant Options
Tetragenex Pharmaceuticals (value less than $150)
Board of Directors
Coalition for Translational Research in Alcohol and Substance Use Disorders
President Elect
American College of Neuropsychopharmacology (12-2010)
Research Support to Department of Veterans Affairs
Janssen Research Foundation (Provided drug and some study support to the Department of Veterans Affairs)
Editorial Board
Income Greater than $10,000:
Editor - Biological Psychiatry
Employment
Yale University School of Medicine
VA CT Healthcare System
Patents and Inventions
Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent #:5,447,948. September 5, 1995
Co-inventor with Dr. Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1).
Intranasal Administration of Ketamine to Treat Depression (pending)
Financial information for Gerard Sanacora
Individual Consulting Arrangements
Abbott, AstraZeneca, Bristol-Myers Squibb, Evotec, Eli Lilly & Co., Hoffman La-Roche, Johnson & Johnson, Novartis, Noven Pharmaceuticals, Inc.
Grant Support
AstraZeneca, Bristol-Myers Squibb, Hoffman La-Roche, Merck & Co., Sepracor Inc (Sunovion).
Patents and Inventions
Co-inventor with Dr. John Krystal on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1).
Financial information for Stuart Weinzimer
Individual Consulting Arrangements within the last 12 months:
Animas, Bayer, Becton-Dickinson, Eli Lilly, Medtronic, Novo Nordisk.
Grant Support
Medtronic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Krystal JH, Tabakoff B. Ethanol abuse, dependence, and withdrawal: neurobiology and clinical implications. In: Davis KL, Charney DS, Coyle JT, Nemeroff C, editors. Psychopharmacology: a fifth generation of progress. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 1425–1443. [Google Scholar]
- 2.Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcoholism: Clinical & Experimental Research. 1998;22:3–9. [PubMed] [Google Scholar]
- 3.Krystal JH, D'Souza DC, Gallinat J, Driesen N, Abi-Dargham A, Petrakis I, et al. The vulnerability to alcohol and substance abuse in individuals diagnosed with schizophrenia. Neurotoxicity Research. 2006;10:235–252. doi: 10.1007/BF03033360. [DOI] [PubMed] [Google Scholar]
- 4.Golovko AI, Golovko SI, Leontieva LV, Zefirov SY. The influence of ethanol on the functional status of GABA(A) receptors. Biochemistry-Russia. 2002;67:719–729. doi: 10.1023/a:1016354420673. [DOI] [PubMed] [Google Scholar]
- 5.Krystal JH, Madonick S, Perry E, Gueorguieva R, Brush L, Wray Y, et al. Potentiation of low dose ketamine effects by naltrexone: potential implications for the pharmacotherapy of alcoholism. Neuropsychopharmacology. 2006;31:1793–1800. doi: 10.1038/sj.npp.1300994. [DOI] [PubMed] [Google Scholar]
- 6.Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Research - Brain Research Reviews. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- 7.Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacology & Therapeutics. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Ende G, Walter S, Welzel H, Demirakca T, Wokrina T, Ruf M, et al. Alcohol consumption significantly influences the MR signal of frontal choline-containing compounds. Neuroimage. 2006;32:740–746. doi: 10.1016/j.neuroimage.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, et al. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biological Psychiatry. 2006;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, et al. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcoholism: Clinical & Experimental Research. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biller A, Bartsch AJ, Homola G, Solymosi L, Bendszus M. The effect of ethanol on human brain metabolites longitudinally characterized by proton MR spectroscopy. Journal of Cerebral Blood Flow & Metabolism. 2009;29:891–902. doi: 10.1038/jcbfm.2009.12. [Erratum appears in J Cereb Blood Flow Metab. 2009 Jun;29(6):1227] [DOI] [PubMed] [Google Scholar]
- 12.Mason GF, Sanacora G, Krystal JH. Nuclear magnetic resonance imaging and spectroscopy: basic principals and recent findings in neuropsychiatric disorders. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan & Sadock's Comprehensive Textbook of Psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. pp. 248–273. [Google Scholar]
- 13.Urenjak J, Williams SR, Gadian DG, Noble M. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem. 1992;59:55–61. doi: 10.1111/j.1471-4159.1992.tb08875.x. [DOI] [PubMed] [Google Scholar]
- 14.Ross BD, Jacobson S, Villamil F, Korula J, Kreis R, Ernst T, et al. Subclinical hepatic encephalopathy: proton MR spectroscopic abnormalities. Radiology. 1994;193:457–463. doi: 10.1148/radiology.193.2.7972763. [DOI] [PubMed] [Google Scholar]
- 15.Mason G, Bendszus M, Meyerhoff D, Hetherington H, Schweinsburg B, Ross B, et al. Magnetic resonance spectroscopic studies of alcoholism: from heavy drinking to alcohol dependence and back again. Alcohol Clin Exp Res. 2005;29:150–158. doi: 10.1097/01.alc.0000150010.72739.58. [DOI] [PubMed] [Google Scholar]
- 16.Bartsch AJ, Homola G, Biller A, Smith SM, Weijers H-G, Wiesbeck GA, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- 17.Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, et al. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. Ajnr: American Journal of Neuroradiology. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- 18.Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, et al. Effects of alcoholism and gender on brain metabolism. American Journal of Psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- 19.Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, et al. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biological Psychiatry. 2005;58:974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, et al. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcoholism: Clinical & Experimental Research. 2002;26:1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- 21.Behar K, Rothman D, Petersen K, Hooten M, Namanworth S, Delaney R, et al. Preliminary evidence of reduced cortical GABA levels in localized 1H NMR spectra of alcohol dependent and hepatic encephalopathy patients. Am J Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- 22.Ramchandani VA, O'Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr, et al. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcoholism: Clinical & Experimental Research. 1999;23:1320–1330. [PubMed] [Google Scholar]
- 23.First M, Spitzer R, Gibbon M, Williams J. Structured Clinincal Interview for DSM-IV-TR Axis I Disorders (SCID-I/NP) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- 24.O'Connor S, Ramchandani VA, Li TK. PBPK modeling as a basis for achieving a steady BrAC of 60 +/− 5 mg% within ten minutes. Alcoholism: Clinical & Experimental Research. 2000;24:426–427. [PubMed] [Google Scholar]
- 25.Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, et al. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Archives of General Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- 26.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical & Experimental Research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Piepponena TP, Kiianmaab K, Ahtee L. Effects of ethanol on the accumbal output of dopamine, GABA and glutamate in alcohol-tolerant and alcohol-nontolerant rats. Pharmacol Biochem Behav. 2002;74:21–30. doi: 10.1016/s0091-3057(02)00937-1. [DOI] [PubMed] [Google Scholar]
- 29.Shen J, Rycyna RE, Rothman DL. Improvements on an in vivo automatic shimming method [FASTERMAP] Magnetic Resonance in Medicine. 1997;38:834–839. doi: 10.1002/mrm.1910380521. [DOI] [PubMed] [Google Scholar]
- 30.Haase A, Frahm J, Hanicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Physics Med Biol. 1985;30:341–344. doi: 10.1088/0031-9155/30/4/008. [DOI] [PubMed] [Google Scholar]
- 31.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magnetic Resonance in Medicine. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 33.Rothman DL, Behar KL, Petroff OAC. Improved quantitation of short TE 1H NMR human brain spectra by removal of short T1 macromolecule resonances. Society for Magnetic Resonance in Medicine. 1994:47. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 34.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magnetic Resonance in Medicine. 2001;45:517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara SD, Zancaner S, Giorgetti R. Low blood alcohol concentrations and driving impairment. A review of experimental studies and international legislation. International Journal of Legal Medicine. 1994;106:169–177. doi: 10.1007/BF01371332. [DOI] [PubMed] [Google Scholar]
- 36.Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, et al. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Archives of General Psychiatry. 2006;63:957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- 37.Seilicovich A, Duvilanski B, Gonzalez NN, Rettori V, Mitridate de Novara A, Maines VM, et al. The effect of acute ethanol administration on GABA receptor binding in cerebellum and hypothalamus. Eur J Pharmacol. 1985;111:365–368. doi: 10.1016/0014-2999(85)90643-0. [DOI] [PubMed] [Google Scholar]
- 38.Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends in Pharmacological Sciences. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- 39.Porter TG, Martin DL. Stability and activation of glutamate apodecarboxylase from pig brain. J Neurochem. 1988;51:1886–1891. doi: 10.1111/j.1471-4159.1988.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 40.Sherif FM, Tawati AM, Ahmed SS, Sharif SI. Basic aspects of GABA-transmission in alcoholism, with particular reference to GABA-transaminase. Eur Neuropsychopharmacol. 1997;7:1–7. doi: 10.1016/s0924-977x(96)00383-5. [DOI] [PubMed] [Google Scholar]
- 41.Goddard AW, Mason GF, Rothman DL, Behar KL, Krystal JH. Reduced cortical GABA neuronal response to benzodiazepine administration in panic disorder. Am J Psychiatry. 2004;161:2186–2193. doi: 10.1176/appi.ajp.161.12.2186. [DOI] [PubMed] [Google Scholar]
- 42.Kuo PH, Kalsi G, Prescott CA, Hodgkinson CA, Goldman D, Alexander J, et al. Associations of glutamate decarboxylase genes with initial sensitivity and age-at-onset of alcohol dependence in the Irish Affected Sib Pair Study of Alcohol Dependence. Drug Alcohol Depend. 2009;101:80–87. doi: 10.1016/j.drugalcdep.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lappalainen J, Krupitsky E, Kranzler HR, Luo X, Remizov M, Pchelina S, et al. Mutation screen of the GAD2 gene and association study of alcoholism in three populations. Am J Med Genet B Neuropsychiatr Genet. 2007;144:183–192. doi: 10.1002/ajmg.b.30377. [DOI] [PubMed] [Google Scholar]
- 44.Truckenmiller ME, Namboodiri MA, Brownstein MJ, Neale JH. N-Acetylation of L-aspartate in the nervous system: differential distribution of a specific enzyme. J Neurochem. 1985;45:1658–1662. doi: 10.1111/j.1471-4159.1985.tb07240.x. [DOI] [PubMed] [Google Scholar]
- 45.Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem. 2001;78:736–745. doi: 10.1046/j.1471-4159.2001.00456.x. [DOI] [PubMed] [Google Scholar]
- 46.Zahr NM, Mayer D, Rohlfing T, Hasak MP, Hsu O, Vinco S, et al. Brain Injury and Recovery Following Binge Ethanol: Evidence from In Vivo Magnetic Resonance Spectroscopy. Biol Psychiatry. 2010;67:846–854. doi: 10.1016/j.biopsych.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, et al. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci USA. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridder TD, Ver Steeg BJ, Laaksonen BD. Comparison of spectroscopically measured tissue alcohol concentration to blood and breath alcohol measurements. Journal of Biomedical Optics. 2009;14:054039. doi: 10.1117/1.3253353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adalsteinsson E, Sullivan EV, Mayer D, Pfefferbaum A. In vivo quantification of ethanol kinetics in rat brain. Neuropsychopharmacology. 2006;31:2683–2691. doi: 10.1038/sj.npp.1301023. [DOI] [PubMed] [Google Scholar]
- 51.Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcoholism: Clinical & Experimental Research. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 52.Fein G, Meyerhoff DJ. Ethanol in human brain by magnetic resonance spectroscopy: correlation with blood and breath levels, relaxation, and magnetization transfer. Alcoholism: Clinical & Experimental Research. 2000;24:1227–1235. [PMC free article] [PubMed] [Google Scholar]
- 53.O'Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22:202–210. [PubMed] [Google Scholar]
- 54.Morgan CJ, Badaway AA-B. Alcohol-induced euphoria: exclusion of serotonin. Alcohol Alcoholism. 2001;36:22–25. doi: 10.1093/alcalc/36.1.22. [DOI] [PubMed] [Google Scholar]