This article systematically reviews the currently available evidence on the clinical effectiveness and cost-effectiveness of particle therapy in lung cancer.
Keywords: Hadron, Heavier charged particles, Carbon ion, Proton, Lung cancer, Radiotherapy
Learning Objectives
After completing this course, the reader will be able to:
Evaluate the published results of particle therapy in the treatment of lung cancer and discuss their implications for the treatment of stage I and stage III NSCLC.
Using the available evidence to date, assess the current role of particle therapy in the treatment of lung cancer.
Identify relevant outstanding issues and address these issues with an action plan for further research.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
The societal burden of lung cancer is high because of its high incidence and high lethality. From a theoretical point of view, radiotherapy with beams of protons and heavier charged particles, for example, carbon ions (C-ions), should lead to superior results, compared with photon beams. In this review, we searched for clinical evidence to justify implementation of particle therapy as standard treatment in lung cancer.
Methods.
A systematic literature review based on an earlier published comprehensive review was performed and updated through November 2009.
Results.
Eleven fully published studies, all dealing with non-small cell lung cancer (NSCLC), mainly stage I, were identified. No phase III trials were found. For proton therapy, 2- to 5-year local tumor control rates varied in the range of 57%–87%. The 2- and 5-year overall survival (OS) and 2- and 5-year cause-specific survival (CSS) rates were 31%–74% and 23% and 58%–86% and 46%, respectively. Radiation-induced pneumonitis was observed in about 10% of patients. For C-ion therapy, the overall local tumor control rate was 77%, but it was 95% when using a hypofractionated radiation schedule. The 5-year OS and CSS rates were 42% and 60%, respectively. Slightly better results were reported when using hypofractionation, 50% and 76%, respectively.
Conclusion.
The present results with protons and heavier charged particles are promising. However, the current lack of evidence on the clinical (cost-)effectiveness of particle therapy emphasizes the need to investigate the efficiency of particle therapy in an adequate manner. Until these results are available for lung cancer, charged particle therapy should be considered experimental.
Introduction
The societal burden of lung cancer is high because of its high incidence and high mortality in all countries worldwide [1–3]. The majority of all lung cancers (80%) are categorized as non-small cell lung cancer (NSCLC).
Different treatment options are available and often combined treatment is suggested. For stage I–II NSCLC, surgery, in some subgroups followed by chemotherapy, is the first treatment choice. For stage III NSCLC, chemoradiation is standard, which is, in some cases, combined with surgery. There has been significant progress with radiotherapy (RT) in the past years, as a result of more advanced technology, better staging, insights into radiation (molecular) biology, and combined modality approaches.
A major problem in the treatment of lung cancer remains its poor local tumor control [4, 5]. Theoretically, an RT dose >84 Gy, delivered in 2-Gy fractions, is necessary to obtain local tumor control in 50% of patients [6]. However, delivery of high-dose RT is limited because of complications in the surrounding normal tissues.
RT can be delivered with photons (x-rays) from linear accelerators [7] or with charged particles. The present paper deals only with beams of protons [8, 9] or carbon ions (C-ions) [10–12], because these are the particles most commonly used in clinical practice. In the literature, these particles are called charged particles or sometimes also hadrons.
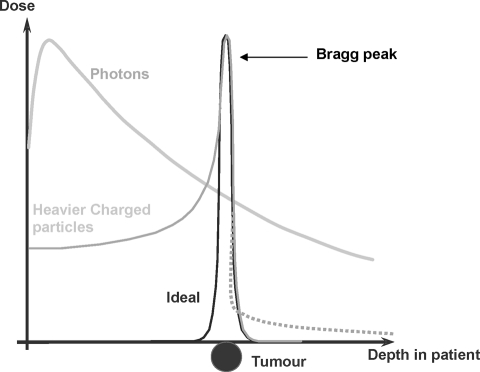
From a theoretical point of view, because of the shape of their dose distribution, a therapeutic gain should be achieved with charged particles. This has been shown for virtually any localized tumor that is rather large or has an irregular shape [13–24]. Accordingly, particle therapy should help minimize the incidence and severity of pulmonary, heart, and esophageal injury. Charged particle beams, as compared with conventional photon beams, have a completely different dose distribution. Initially, there is little buildup of dose with depth and then, rapidly, the dose builds to a peak. This effect of energy deposition toward the end of the beam path is known as the Bragg peak and is characteristic of protons and carbon ions (Fig. 1).
Figure 1.
Simplified depth dose distributions of photons and heavier charged particles compared with the ideal dose distribution. Heavier charged particles like protons and carbon ions (C-ions) show an inverted depth-dose profile compared with photons exhibiting a maximum (Bragg peak) at the end of the range, approaching the ideal dose profile. Beyond this Bragg peak, the dose decreases within a few millimeters to a low value. In contrast to protons, C-ions exhibit a dose tail (dashed line) at the distal side of the Bragg peak.
The dose delivered with particles is prescribed in Gray equivalents (GyE) or cobalt Gray equivalents (CGE), often used with protons. GyE and CGE are equal to the measured physical dose in Grays multiplied by the relative biological effectiveness (RBE) specific for the beam used. The RBE is the ratio of the dose of radiation required to produce a certain biological effect with photons relative to the dose required to produce the same effect with another form of ionizing radiation, such as protons or heavier ions. An RBE value of 1.1 is generally accepted for clinical use with proton beams. The RBE of C-ions is not a constant value but depends on a variety of factors (e.g., dose, energy, tissue, etc.), and for dose-reporting purposes a value of 3 is often used [25]. The depth of the Bragg peak depends on the particle type and its energy. The Bragg peak associated with charged particle beams is advantageous when attempting to treat a tumor that directly overlies vulnerable normal tissue.
Therapy with protons or C-ions could benefit all patients referred for RT, and even more for those patients with comorbidities such as lung or heart disease. However, at present, the precise role of particle therapy in lung treatment is still unclear. This article, therefore, aims to systematically review the currently available evidence on the clinical effectiveness and cost-effectiveness of particle therapy in lung cancer.
Methods
Search Strategy and Selection Criteria
The present review is based on a comprehensive systematic literature review by Lodge et al. [26] updated for lung cancer by Pijls-Johannesma et al. [27] through July 2007, and updated through November 2009 for the present article. The following electronic databases were used: CINAHL, EMBASE, and MEDLINE. Search terms (using free text words as well as MESH terms) related to lung cancer and charged particle treatment were used alone or in combination. These included the following terms: neoplasm, cancer, carcinoma, lung cancer, proton, ion, charged particle, and hadron. There was no limit applied to publication year, language, or study design. Studies on animals only were excluded. After identifying search results, only studies in the English, French, or German language that investigated protons and/or C-ions in the treatment of lung cancer with at least 20 patients and a follow up of 2 years were included. In addition, the literature on cost issues was systematically reviewed. The search methodology is described elsewhere [28] and has been updated through November 1, 2009.
Key Outcomes and Quality Assessment
Two independent reviewers assessed the trials, both for the quality of the methods and for the results of key outcomes, which were identified and tabulated. Two reviewers (M.P.J. and J.G.) extracted data independently to ensure validity, whereas a third reviewer (D.D.R.) was responsible for resolving discrepancies.
We attempted to collect the following data from study reports: study design, particle type, initial disease stage, total tumor dose, fractionation, overall RT treatment time, local tumor control, survival, side effects (including the scoring system used), and quality of life (QoL) assessment.
A detailed listing of patient, treatment, and outcome aspects was performed with the literature reporting patient series of at least 20 patients and a follow-up period ≥24 months. In cases in which publications were entirely or partly based on the same patient data, only the largest patient population was further analyzed.
Results
Search Results
The original search retrieved 5,089 search hits. Of those hits, 185 relevant papers regarding different types of cancer were identified as potentially relevant [26]. Of those 185 references, 134 duplicates and clearly irrelevant references were excluded. Through manual searches of the reference list and specialist journals and correspondence with authors, no additional references were identified. After we applied the above-mentioned restrictions, and selected only studies dealing with lung cancer, 11 references [8–12, 29–33] were retrieved. An update of the literature [28] on the cost aspects of particle therapy yielded another five papers [34–38]. However, none of those papers reported cost-effectiveness of particle therapy. Because no new data were found, this subject is not discussed further in this paper.
Description of the Studies
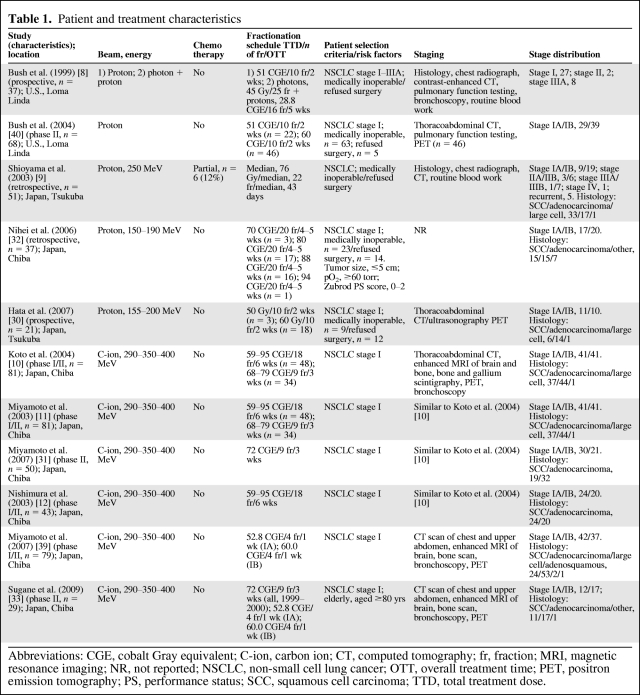
Eleven studies were identified, all for histologically proven NSCLC [8–12, 29–33]. Of these 11 studies, five (n = 214) investigated protons—one phase II study [29], two prospective studies [8, 30], and two retrospective studies [9, 32]. The remaining six studies, all prospective and all performed at the same institution, investigated C-ions (n = 210) [10–12, 31, 33, 39]. Study characteristics are tabulated in Table 1.
Table 1.
Patient and treatment characteristics
Abbreviations: CGE, cobalt Gray equivalent; C-ion, carbon ion; CT, computed tomography; fr, fraction; MRI, magnetic resonance imaging; NR, not reported; NSCLC, non-small cell lung cancer; OTT, overall treatment time; PET, positron emission tomography; PS, performance status; SCC, squamous cell carcinoma; TTD, total treatment dose.
The prospective study of Bush et al. [8] had two treatment groups. One group of patients was treated with protons only, whereas the other was treated with both photons and protons. Thirty-seven stage I-IIIA patients were assigned to one of the treatment groups according to pulmonary reserve and cardiac function. Patients in the first group of the study were given proton beam RT that covered only the gross tumor volume as identified on the planning computed tomography scan. Those patients received 10 daily fractions of 5.1 CGE for a total of 51 CGE in 2 weeks. The second group of the study included patients judged suitable for elective mediastinal irradiation (45 Gy) based on their lung function. A lung capacity test revealed that these patients had a forced expiratory volume in 1 second >1 liter and no evidence of severe cardiac insufficiency. Concomitant with the last 16 photon treatments, a proton boost to the primary tumor was given, with a total tumor dose of 73.8 CGE in 5 weeks.
The phase II study of Bush et al. [40] included only patients with stage I disease (n = 68). They were given 10 proton fractions in 2 weeks, with a total dose of 51 CGE for the first 22 patients and 60 CGE for the last 46 patients.
One retrospective study from Japan [9] evaluated 51 stage I–IV patients. Fractionation regimens for proton therapy varied as a result of irregularly allocated beam time. The median fraction size and total doses were 3.0 CGE (range, 2–6 CGE) and 76 CGE (range, 49–93 CGE), respectively. The median overall treatment time (OTT) was 43 days (range, 10–76 days). The other retrospective study, also from Japan, evaluated 37 stage I patients [32]. The tumor size of all patients was ≤5 cm in diameter and all patients had a Zubrod performance status score of 0–2. Ten of those 37 patients were enrolled in an earlier phase I dose-escalation study (70–90 CGE in 20 fractions) at the same institution. The remaining 27 patients were treated with either a dose of 88 CGE (20 fractions of 4.4 CGE; n = 13) or a dose of 80 CGE (20 fractions of 4 CGE; n = 14).
A recent prospective study from Japan [30] investigated 21 stage I NSCLC patients. The first three patients were treated with a total dose of 50 CGE in 10 fractions over 2 weeks. When no therapy-related toxicities of grade ≥3 were observed for 3 months, the total dose was escalated to 60 CGE, also in 10 fractions over 2 weeks. The survival, local progression-free survival (PFS), and disease-free survival (DFS) rates were calculated according to the Kaplan–Meier method.
Koto et al. [10] conducted a prospective phase I/II dose-escalation study with C-ions in stage I NSCLC patients to investigate the incidence of in-field recurrences. The study was carried out using two different protocols. The first-stage phase I/II trial used 18 fractions over 6 weeks in 47 patients and the second one delivered nine fractions over 3 weeks in 34 patients. Dose escalations from 59.4 CGE to 95.4 CGE in incremental steps of 10% and from 68.4 CGE to 79.2 CGE in 5% increments were done in the two stages of the trial, respectively.
From the latter study, Miyamoto et al. [11] reported results for local control (LC) and survival, which were obtained using the Kaplan–Meier method.
In an update of that phase II trial [31] of 50 patients receiving nine fractions of 8 CGE over 3 weeks, survival, local PFS, and DFS rates were also calculated according to the Kaplan–Meier method.
The same study population was also used in a study by Nishumura [12]. We included that study because the report focused on the pulmonary side effects of C-ion therapy, which were not mentioned in the previous studies.
Finally, Sugane et al. [33] performed a subgroup analysis of the above study population in which only patients aged ≥80 years were included.
LC and Survival
The 2-year LC and overall survival (OS) rates in the first published prospective study [8] with protons were 87% and 31%, respectively (Table 2). The DFS rate at 2 years was 63% for the total group, 85% for stage I patients, and 19% for stage IIIA patients. The phase II study of Bush et al. [40], with an OTT of 2 weeks, reported a 3-year LC rate of 74%, which differed between stage IA (87%) and stage IB (49%) tumors. Overall, the OS rate at 3 years was 44%, whereas the OS rate was 27% in the patient group that received 51 CGE and 55% in the patient group receiving 60 CGE. The disease-specific survival (DSS) rate at 3 years was 72%.
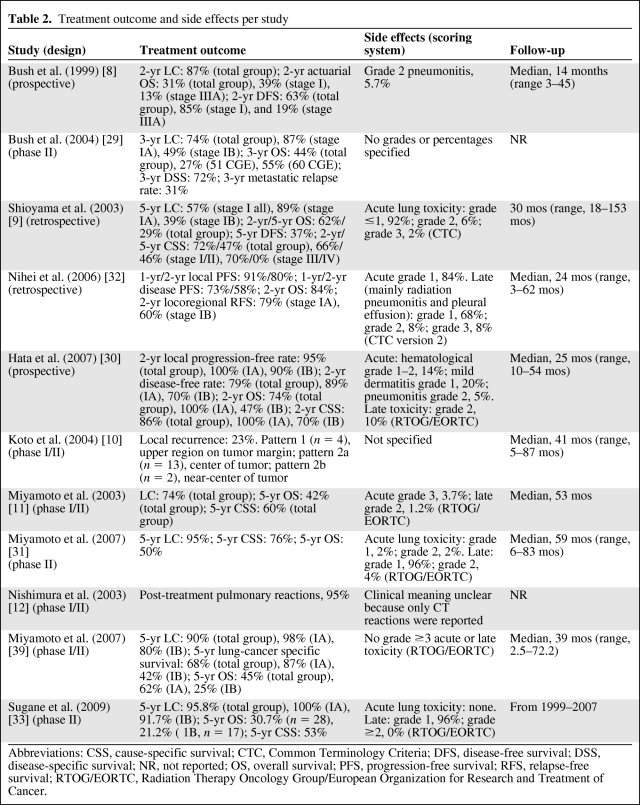
Table 2.
Treatment outcome and side effects per study
Abbreviations: CSS, cause-specific survival; CTC, Common Terminology Criteria; DFS, disease-free survival; DSS, disease-specific survival; NR, not reported; OS, overall survival; PFS, progression-free survival; RFS, relapse-free survival; RTOG/EORTC, Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer.
In a retrospective study by Shioyama et al. [9], the 5-year LC rate was 57% for stage I NSCLC patients (n = 28). The LC rate for the remaining patients with stage II–IV NSCLC in that study was unclear. For all patients, the 5-year OS and DFS rates were 29% and 37%, respectively, whereas the 5-year DSS rate was 46% for 37 stage I–II patients and the 2-year DSS rate was 70% for nine stage III–IV patients. In another retrospective study, Nihei et al. [32] reported 2-year locoregional PFS rates of 79% in stage IA NSCLC patients and 60% in stage IB NSCLC patients, whereas the 2-year local PFS and OS rates were 80% and 84%, respectively. Hata et al. [30], who also used an RT schedule with an OTT of 2 weeks, found a local PFS rate at 2 years of 95% in stage I patients. The reported 2-year OS and cause-specific survival (CSS) rates were 74% and 86%, respectively.
The LC and 5-year OS/CSS rates in the Miyamoto et al. [11] study with C-ions, which only included stage I patients (n = 81), were 74% and 42%/60%, respectively. Koto et al. [10] concluded that LC was dose dependent and determined the optimal therapeutic dose based on their findings. With a median follow-up period of 40.6 months (range, 4.9–86.9 months), the survival rate was 45.7% for all stage I patients.
In an update of their study (n = 50), Miyamoto et al. [31] reported 5-year LC and OS/CSS rates of 95% and 50%/76%, respectively.
Within the same study population as that evaluated by Miyamoto et al. [31], Sugane et al. [33] investigated a cohort of elderly patients (aged ≥80 years; n = 29). The 5-year LC rates were 95.8% for the total group) and 100%/91.7% for stage IA/stage IB NSCLC patients. The OS and CSS rates were 30.7% and 53%, respectively.
Side Effects and QoL
Treatment-related toxicity is depicted in Table 2. After proton therapy, acute grade 2 pneumonitis was reported in two studies [8, 30] at rates of 5% and 5.7%, whereas one study reported grade 3 toxicity for only one patient (2%) [9]. The incidence of late grade 2 toxicity was in the range of 8%–10% [30, 32] and grade 3 toxicity was reported in one study (8%) [32].
After C-ion treatment, Miyamoto et al. [11] reported acute side effects (grade ≥3) in 3.7% of the patients and late side effects (grade ≥3) in 1.2% of the patients, according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer scoring system. In an update of that study [31], no acute severe lung toxicity was reported and grade 2 late lung toxicity was found in only 4% of patients.
Sugane et al. [33], who investigated elderly patients aged ≥80 years, reported no severe acute or late side effects in that subgroup of patients (n = 28).
QoL was not reported in any of the above-mentioned studies.
Discussion
For theoretical advantages, protons and C-ions show great promise, because they may enable lowering the dose to normal tissues or allow radiation dose escalation and hence yield higher tumor control probabilities [41]. On top of that, C-ions, having a higher RBE than protons or photons, may further increase the effectiveness of charged particles beyond that of protons [42]. Although new health technologies are ultimately aimed at improving treatment outcome, the ultimate goal from a health economist point of view is to improve efficiency over standard care (i.e., better LC and/or survival, less morbidity/better QoL against acceptable costs) [43].
In this review, we searched for clinical evidence that protons or C-ions would benefit patients with lung cancer. Using a systematic approach, 11 fully published studies, all dealing with NSCLC (mainly stage I), were identified [8–12, 29–33]. Studies on proton therapy were performed in Japan and the U.S., whereas only data from Japan are available for studies on C-ions.
In the identified studies, a wide variety of RT schedules was used, making comparisons of results difficult. Furthermore, in all identified studies, systemic chemotherapy appeared to not be a part of the treatment package, which could indicate that tumor volumes and consequently treatment volumes were small. In addition, the use of different staging and follow-up procedures, more specifically the use of positron emission tomography imaging, or not, increases the complexity of these comparisons. Nevertheless, the results, at least for stage I NSCLC, seem to be better than what is generally achieved by conventional photon RT [44–46]. However, they seem inferior to what may be achieved with hypofractionated stereotactic RT (SRT) using photons (see Table 3), with which LC rates ≥90% were reported in several phase I/II studies [47–51]. Nevertheless, because LC is defined differently in different studies, comparison of this outcome among different studies is difficult, and any comparisons should be interpreted with caution. In general, the LC rates for proton therapy of 87% (2-year) [8], 74% (3-year) [29], and 57% (5-year) [9] are not higher than those achieved with SRT with photons [47–51]. Yet the local PFS rate of 95% that was reported by Hata et al. [30] using protons is equivalent to the results of Timmerman et al. [52] using photons (updated by Fakiris et al. [47]). The CSS and OS rates achieved with proton therapy are equivalent to, or even inferior to, those reported in SRT series using photons [47, 49–51], with weighted means for 2-year and 5-year CSS rates of 80% and 72% and for 2-year and 5-year OS rates of 59% and 44%, respectively [26]. The better results with protons reported by Hata et al. [30], compared with the other proton series described in this review paper, could be explained by a higher biological dose resulting from the hypofractionated RT schedule, with high fraction doses (5–6 CGE) and a short OTT (2 weeks). An equivalent RT schedule was used by Bush et al. [40]. The 2-year CSS rate from this latter study was also better than those from other proton series (72% versus 58%–66%). This result is in line with earlier publications on small cell lung cancer showing advantages using schedules with a short OTT or short time from the start of any treatment until the end of radiotherapy [53, 54].
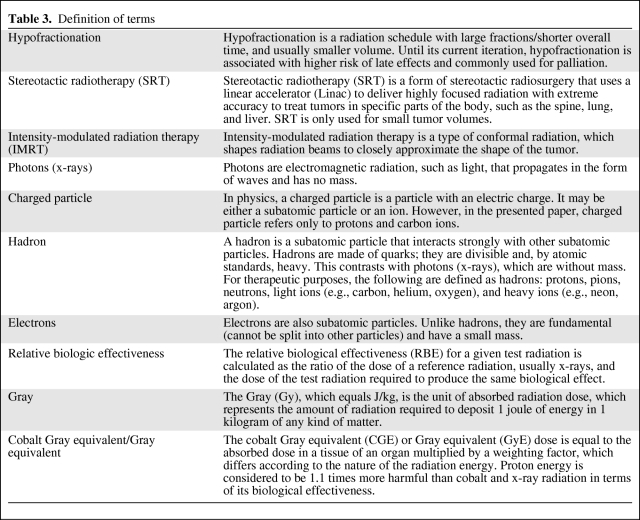
Table 3.
Definition of terms
Treatment with C-ions, in particular, as reported in the recent study of Miyamoto et al. [31], seems to result in better LC (5-year LC rate, 95%). Their 5-year OS rate is somewhat higher than that of photon studies [50, 51] (50% versus 30% and 47%, respectively), and the 5-year CSS rate is comparable with the best achievements in photon therapy [51] (76% versus 78%). However, because these C-ion outcomes were found in only one study with 50 patients, they should be interpreted with caution.
In all the included studies, the incidence of reported severe side effects was relatively low. Taking the most recent publications into account, the percentages of late toxicities were similar in the group of patients receiving proton therapy (and even lower after treatment with C-ions) and in the group of patients receiving SRT— proton therapy, 10% (grade 2) [30]; C-ion therapy, 4% (grade 2) [31, 39]; photon SRT, 9%(grade 2–5; range, 5%–27%) [47, 52, 55, 56]. Even in a subgroup of elderly patients, C-ion radiotherapy seemed to be a safe treatment modality [33]. Because of the relatively low number of patients evaluated so far, the results regarding late severe toxicities after particle therapy should be interpreted with caution.
We could not draw any conclusions about QoL, because it was not reported in any of the included studies.
The cost of proton therapy is about 2.4 times higher than that of conventional RT with photons [57]. C-ions are more expensive than protons, mainly because of the considerably higher investment cost of a C-ion facility (about 1.5 times higher than a proton facility). However, because of the possibility of treating with fewer fractions in the case of lung cancer, the treatment costs for lung cancer with C-ions are not necessarily higher than the treatment costs for proton therapy [58]. Nevertheless, as compared with conventional RT with photons, it is necessary to determine whether these higher costs are worthwhile in light of the expected advantages [28]. Because decisions need to be made on whether or not to invest in proton or C-ion centers, evidence is needed on whether, and for which indications, particle therapy is a cost-effective treatment modality. This puts the introduction of new (expensive) irradiation treatments, such as particle therapy, in a difficult situation: firm data on effectiveness can be obtained only after the implementation and clinical evaluation of the treatment, which in the case of RT often implies that important capital investments must be made. Yet data on effectiveness will only become available years after the necessary investment in equipment, buildings, and highly qualified personnel. As mentioned above, effectiveness data on particle therapy treatments are lacking; regardless, sufficient financing is necessary to perform clinical research and ensure the eventual dissemination of evidence-based treatments into clinical practice.
Recently presented results are promising. In our opinion, we mainly expect a gain in delivering high doses to the tumor volume while optimally sparing the surrounding tissues.
Preliminary results from a group of stage III NSCLC patients treated with chemotherapy and concurrent proton beam therapy showed that they had less toxicity than patients treated with photon intensity-modulated radiation therapy (IMRT) (Table 3) [59]. There was significantly less fatigue in patients treated with protons when all grades of fatigue were compared (p = .04), as well as when the analysis was limited to grade ≥2 fatigue (p < .001). The authors suggested that the greater conformality with proton therapy than with IMRT may result in less myelotoxicity. Distant disease is a major problem in advanced lung cancer, and by limiting the myelosuppression associated with RT it may be possible to decrease the number of patients who require a break from chemotherapy or a dose reduction. It may also be possible to escalate the concurrent dose of chemotherapy or allow other agents (e.g., biological agents) to be added to the chemoradiation, which could potentially improve outcomes. Pneumonitis is another important toxicity that could potentially be decreased with the use of protons. From the results of a subsequent study, it appears that protons do reduce the irradiated volume of the normal lung and have the potential to decrease the rate of radiation-induced pneumonitis over that seen with IMRT [60]. These results suggest that protons could reduce the risk for radiation-induced pneumonitis and may again allow further dose escalation, thereby leading to better LC. It would be interesting to extend the current review with these data as soon as follow-up data are available.
Because of the overwhelming theoretical data on the beneficial properties of protons and light ions [13–18, 20–23], as well as some promising recent results, further investment in the infrastructure needed to perform large trials in patients with different lung cancer stages is warranted. According to Brada et al. [61], the current lack of evidence for the benefit of charged particles should provide a stimulus for an effort to identify suitable tumor targets with the greatest potential benefit in measurable and, particularly, clinically relevant endpoints. Well-designed model-based trials using validated models are essential and can predict the magnitude of the benefit. The perceived and largely theoretical benefit should be confirmed by clinical evidence from well-designed prospective studies, convincingly demonstrating superior outcomes. Prospective data collection should start now in the few operational centers in order to determine the results with regard to side effects, QoL, and other endpoints achieved with the currently used RT techniques.
In the meantime, the complexity introduced by tumor motion and radiation-induced tumor volume changes must be recognized [62–65].
In this era of evidence-based medicine, more evidence is required before particle therapy can become the standard treatment for (subsets of) lung cancer patients.
Conclusion
The present results with protons and heavier charged particles are promising regarding better LC and less RT-induced side effects. However, because of a lack of evidence on the clinical efficacy and cost-effectiveness of proton and C-ion therapy, there is a current need to investigate efficiency in an adequate manner with high-quality methodology. Because it is doubtful whether randomized clinical trials are possible or even ethical [43, 66], well-designed model-based trials using validated models should be performed to predict the magnitude of the benefit. Until more evidence is available, proton and C-ion therapy in lung cancer should be considered experimental.
Author Contributions
Conception/Design: Madelon Pijls-Johannesma, Dirk De Ruysscher, Janneke Grutters, Philippe Lambin
Collection and/or assembly of data: Madelon Pijls-Johannesma, Dirk De Ruysscher, Janneke Grutters, Philippe Lambin
Data analysis and interpretation: Madelon Pijls-Johannesma, Dirk De Ruysscher, Janneke Grutters, Philippe Lambin
Manuscript writing: Madelon Pijls-Johannesma, Dirk De Ruysscher, Janneke Grutters, Philippe Lambin, Frank Verhaegen
Final approval of manuscript: Madelon Pijls-Johannesma, Dirk De Ruysscher, Janneke Grutters, Philippe Lambin, Frank Verhaegen
References
- 1.Shibuya K, Mathers CD, Boschi-Pinto C, et al. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer. 2002;2:37. doi: 10.1186/1471-2407-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 4.Kim YS, Yoon SM, Choi EK, et al. Phase II study of radiotherapy with three-dimensional conformal boost concurrent with paclitaxel and cisplatin for stage IIIB non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2005;62:76–81. doi: 10.1016/j.ijrobp.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Farray D, Mirkovic N, Albain KS. Multimodality therapy for stage III non-small-cell lung cancer. J Clin Oncol. 2005;23:3257–3269. doi: 10.1200/JCO.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer. 1999;24:31–37. doi: 10.1016/s0169-5002(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 7.De Jaeger K, Seppenwoolde Y, Boersma LJ, et al. Pulmonary function following high-dose radiotherapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:1331–1340. doi: 10.1016/s0360-3016(02)04389-4. [DOI] [PubMed] [Google Scholar]
- 8.Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest. 1999;116:1313–1319. doi: 10.1378/chest.116.5.1313. [DOI] [PubMed] [Google Scholar]
- 9.Shioyama Y, Tokuuye K, Okumura T, et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;56:7–13. doi: 10.1016/s0360-3016(02)04416-4. [DOI] [PubMed] [Google Scholar]
- 10.Koto M, Miyamoto T, Yamamoto N, et al. Local control and recurrence of stage I non-small cell lung cancer after carbon ion radiotherapy. Radiother Oncol. 2004;71:147–156. doi: 10.1016/j.radonc.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto T, Yamamoto N, Nishimura H, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer. Radiother Oncol. 2003;66:127–140. doi: 10.1016/s0167-8140(02)00367-5. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura H, Miyamoto T, Yamamoto N, et al. Radiographic pulmonary and pleural changes after carbon ion irradiation. Int J Radiat Oncol Biol Phys. 2003;55:861–866. doi: 10.1016/s0360-3016(02)04495-4. [DOI] [PubMed] [Google Scholar]
- 13.Krengli M, Hug EB, Adams JA, et al. Proton radiation therapy for retinoblastoma: Comparison of various intraocular tumor locations and beam arrangements. Int J Radiat Oncol Biol Phys. 2005;61:583–593. doi: 10.1016/j.ijrobp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 14.St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional x-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727–734. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 15.Weber DC, Trofimov AV, Delaney TF, et al. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004;58:1596–1606. doi: 10.1016/j.ijrobp.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 16.Baumert BG, Lomax AJ, Miltchev V, et al. A comparison of dose distributions of proton and photon beams in stereotactic conformal radiotherapy of brain lesions. Int J Radiat Oncol Biol Phys. 2001;49:1439–1449. doi: 10.1016/s0360-3016(00)01422-x. [DOI] [PubMed] [Google Scholar]
- 17.Zurlo A, Lomax A, Hoess A, et al. The role of proton therapy in the treatment of large irradiation volumes: A comparative planning study of pancreatic and biliary tumors. Int J Radiat Oncol Biol Phys. 2000;48:277–288. doi: 10.1016/s0360-3016(00)00522-8. [DOI] [PubMed] [Google Scholar]
- 18.Miralbell R, Crowell C, Suit HD. Potential improvement of three dimension treatment planning and proton therapy in the outcome of maxillary sinus cancer. Int J Radiat Oncol Biol Phys. 1992;22:305–310. doi: 10.1016/0360-3016(92)90047-l. [DOI] [PubMed] [Google Scholar]
- 19.Miralbell R, Lomax A, Russo M. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuro-ectodermal tumors: Spinal theca irradiation. Int J Radiat Oncol Biol Phys. 1997;38:805–811. doi: 10.1016/s0360-3016(97)00005-9. [DOI] [PubMed] [Google Scholar]
- 20.Cozzi L, Fogliata A, Lomax A, et al. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol. 2001;61:287–297. doi: 10.1016/s0167-8140(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Tait D, Nahum AE, et al. Comparison of proton therapy and conformal x-ray therapy in non-small cell lung cancer (NSCLC) Br J Radiol. 1999;72:1078–1084. doi: 10.1259/bjr.72.863.10700825. [DOI] [PubMed] [Google Scholar]
- 22.Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol. 2002;62:137–145. doi: 10.1016/s0167-8140(01)00476-5. [DOI] [PubMed] [Google Scholar]
- 23.Isacsson U, Montelius A, Jung B, et al. Comparative treatment planning between proton and x-ray therapy in locally advanced rectal cancer. Radiother Oncol. 1996;41:263–272. doi: 10.1016/s0167-8140(96)01851-8. [DOI] [PubMed] [Google Scholar]
- 24.Isacsson U, Hagberg H, Johansson KA, et al. Potential advantages of protons over conventional radiation beams for paraspinal tumours. Radiother Oncol. 1997;45:63–70. doi: 10.1016/s0167-8140(97)00097-2. [DOI] [PubMed] [Google Scholar]
- 25.Tsujii H, Mizoe J, Kamada T, et al. Clinical results of carbon ion radiotherapy at NIRS. J Radiat Res (Tokyo) 2007;48(suppl A):A1–A13. doi: 10.1269/jrr.48.a1. [DOI] [PubMed] [Google Scholar]
- 26.Lodge M, Pijls-Johannesma M, Stirk L, et al. A systematic literature review of the clinical and cost-effectiveness of hadron therapy in cancer. Radiother Oncol. 2007;83:110–122. doi: 10.1016/j.radonc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Pijls-Johannesma M, Grutters JP, Lambin P, et al. Particle therapy in lung cancer: Where do we stand? Cancer Treat Rev. 2008;34:259–267. doi: 10.1016/j.ctrv.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Pijls-Johannesma M, Pommier P, Lievens Y. Cost-effectiveness of particle therapy: Current evidence and future needs. Radiother Oncol. 2008;89:127–134. doi: 10.1016/j.radonc.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Bush DA, Slater JD, Shin BB, et al. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest. 2004;126:1198–1203. doi: 10.1378/chest.126.4.1198. [DOI] [PubMed] [Google Scholar]
- 30.Hata M, Tokuuye K, Kagei K, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: Preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys. 2007;68:786–793. doi: 10.1016/j.ijrobp.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto T, Baba M, Yamamoto N, et al. Curative treatment of stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2007;67:750–758. doi: 10.1016/j.ijrobp.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Nihei K, Ogino T, Ishikura S, et al. High-dose proton beam therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:107–111. doi: 10.1016/j.ijrobp.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Sugane T, Baba M, Imai R, et al. Carbon ion radiotherapy for elderly patients 80 years and older with stage I non-small cell lung cancer. Lung Cancer. 2009;64:45–50. doi: 10.1016/j.lungcan.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Brower V. Carbon ion therapy to debut in Europe. J Natl Cancer Inst. 2009;101:74–76. doi: 10.1093/jnci/djn496. [DOI] [PubMed] [Google Scholar]
- 35.Greco C, Wolden S. Current status of radiotherapy with proton and light ion beams. Cancer. 2007;109:1227–1238. doi: 10.1002/cncr.22542. [DOI] [PubMed] [Google Scholar]
- 36.Jäkel O, Karger CP, Debus J. The future of heavy ion radiotherapy. Med Phys. 2008;35:5653–5663. doi: 10.1118/1.3002307. [DOI] [PubMed] [Google Scholar]
- 37.Jalali R. Particle therapy in clinical practice: Is there enough evidence to justify the current surge in interest? J Cancer Res Ther. 2008;4:54–56. doi: 10.4103/0973-1482.42250. [DOI] [PubMed] [Google Scholar]
- 38.Jones B. The case for particle therapy. Br J Radiol. 2006;79:24–31. doi: 10.1259/bjr/81790390. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto T, Baba M, Sugane T, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol. 2007;2:916–926. doi: 10.1097/JTO.0b013e3181560a68. [DOI] [PubMed] [Google Scholar]
- 40.Bush DA, Hillebrand DJ, Slater JM, et al. High-dose proton beam radiotherapy of hepatocellular carcinoma: Preliminary results of a phase II trial. Gastroenterology. 2004;127:S189–S193. doi: 10.1053/j.gastro.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 41.Chang JY, Liu HH, Komaki R. Intensity modulated radiation therapy and proton radiotherapy for non-small cell lung cancer. Curr Oncol Rep. 2005;7:255–259. doi: 10.1007/s11912-005-0047-4. [DOI] [PubMed] [Google Scholar]
- 42.Orecchia R, Zurlo A, Loasses A, et al. Particle beam therapy (hadrontherapy): Basis for interest and clinical experience. Eur J Cancer. 1998;34:459–468. doi: 10.1016/s0959-8049(97)10044-2. [DOI] [PubMed] [Google Scholar]
- 43.Bentzen SM. Randomized controlled trials in health technology assessment: Overkill or overdue? Radiother Oncol. 2008;86:142–147. doi: 10.1016/j.radonc.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sirzén F, Kjellén E, Sörenson S, et al. A systematic overview of radiation therapy effects in non-small cell lung cancer. Acta Oncol. 2003;42:493–515. doi: 10.1080/02841860310014453. [DOI] [PubMed] [Google Scholar]
- 45.Jeremic B, Classen J, Bamberg M. Radiotherapy alone in technically operable, medically inoperable, early-stage (I/II) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:119–130. doi: 10.1016/s0360-3016(02)02917-6. [DOI] [PubMed] [Google Scholar]
- 46.Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer. 2003;41:1–11. doi: 10.1016/s0169-5002(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 47.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 48.Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–1955. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 49.Uematsu M, Shioda A, Suda A, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: A 5-year experience. Int J Radiat Oncol Biol Phys. 2001;51:666–670. doi: 10.1016/s0360-3016(01)01703-5. [DOI] [PubMed] [Google Scholar]
- 50.Nyman J, Johansson KA, Hultén U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer—mature results for medically inoperable patients. Lung Cancer. 2006;51:97–103. doi: 10.1016/j.lungcan.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 52.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 53.De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006;24:1057–1063. doi: 10.1200/JCO.2005.02.9793. [DOI] [PubMed] [Google Scholar]
- 54.Pijls-Johannesma MC, De Ruysscher D, Lambin P, et al. Early versus late chest radiotherapy for limited stage small cell lung cancer. Cochrane Database Syst Rev. 2005;(1):CD004700. doi: 10.1002/14651858.CD004700.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(suppl 3):S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann FB, Geinitz H, Schill S, et al. Stereotactic hypofractionated radiotherapy in stage I (T1-2 N0 M0) non-small-cell lung cancer (NSCLC) Acta Oncol. 2006;45:796–801. doi: 10.1080/02841860600913210. [DOI] [PubMed] [Google Scholar]
- 57.Goitein M, Jermann M. The relative costs of proton and x-ray radiation therapy. Clin Oncol (R Coll Radiol) 2003;15:S37–S50. doi: 10.1053/clon.2002.0174. [DOI] [PubMed] [Google Scholar]
- 58.Perrier L, Combs S, Auberger T, et al. A decision-making tool for a costly innovative technology: The case of carbon ion radiotherapy. J Econ Med. 2007;25:367–380. [Google Scholar]
- 59.Komaki R, Sejpal S, Wei X, et al. O10. Vol. 14. Jacksonville, Florida: PTCOG 47; 2008. Reduction of Bone Marrow Suppression for Patients With Stage III NSCLC Treated by Proton and Chemotherapy Compared With IMRT and Chemotherapy. Available at http://ptcog.web.psi.ch/PTCOG47/presentations/4_Meeting_Thursday/Session_2/abstr_komaki.pdf. [Google Scholar]
- 60.Sejpal S, Komaki R, Wei X, et al. O17. Vol. 22. Jacksonville, Florida: PTCOG 47; 2008. Does Proton Beam Radiotherapy (PBT) Reduce Treatment Related Pneumonitis (TRP) Compared to Intensity Modulated Radiation Therapy (IMRT) in Patients with Locally Advanced Non-Small Cell Lung Cancer (NSCLC) Treated with Concurrent Chemotherapy? Available at http://ptcog.web.psi.ch/PTCOG47/presentations/5_Meeting_Friday/Session_3/abstr_sejpal.pdf. [Google Scholar]
- 61.Brada M, Pijls-Johannesma M, De Ruysscher D. Current clinical evidence for proton therapy. Cancer J. 2009;15:319–324. doi: 10.1097/PPO.0b013e3181b6127c. [DOI] [PubMed] [Google Scholar]
- 62.Bush DA, Slater JD, Garberoglio C, et al. A technique of partial breast irradiation utilizing proton beam radiotherapy: Comparison with conformal x-ray therapy. Cancer J. 2007;13:114–118. doi: 10.1097/PPO.0b013e318046354b. [DOI] [PubMed] [Google Scholar]
- 63.Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol Biol Phys. 2006;64:1589–1595. doi: 10.1016/j.ijrobp.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 64.Kang Y, Zhang X, Chang JY, et al. 4D Proton treatment planning strategy for mobile lung tumors. Int J Radiat Oncol Biol Phys. 2007;67:906–914. doi: 10.1016/j.ijrobp.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 65.Mori S, Endo M, Komatsu S, et al. Four-dimensional measurement of lung tumor displacement using 256-multi-slice CT-scanner. Lung Cancer. 2007;56:59–67. doi: 10.1016/j.lungcan.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Glimelius B, Montelius A. Proton beam therapy: Do we need the randomised trials and can we do them? Radiother Oncol. 2007;83:105–109. doi: 10.1016/j.radonc.2007.04.009. [DOI] [PubMed] [Google Scholar]