This report summarizes the key findings of the Third Tegernsee Conference on Immunotherapy of Cancer held recently in Feldafing, Germany.
Keywords: Immunotherapy, Conferences, Cancer, Tegernsee
Abstract
Cancer immunotherapy broadly includes active immunization, as in the use of cancer vaccines, passive immunization, such as the use of adoptive cell therapy and antibodies that modulate tumor function, and immunostimulation, using antibodies and small molecules to treat malignancy by activating or unleashing an endogenous immune response against tumor cells. Currently, >100 different monoclonal antibodies are in use or under evaluation for use as therapeutic agents in various malignancies. Active stimulation of the host's immune system holds promise for achieving durable remission of malignant disease and represents a nontoxic method of therapy if tumor-specific effector cells can be selectively targeted. However, no active-specific treatment strategy (i.e., a therapeutic cancer vaccine) has yet found its way into the clinical armamentarium, although several promising recent reports suggest that, for follicular lymphoma, prostate cancer, and melanoma, clinical benefit was shown for the first time in randomized trials with a vaccine approach. Here, we report on the key findings of the Third Tegernsee Conference on Immunotherapy of Cancer (Feldafing, Germany, July 2–4, 2009) and provide short commentaries on data presented at this meeting regarding the future role of cancer vaccines, recent developments in adoptive cellular therapy, ways to improve immunotherapeutic treatment modalities (e.g., by manipulating the tumor microenvironment), and some novel targeted therapies that are well advanced in clinical testing, all of which have implications for future oncology practice.
Introduction
The Tegernsee meetings on immunotherapy of cancer have aimed to link research in the lab and the clinic more effectively and to bring together basic scientists and clinicians to promote a translational approach. The attraction of immunotherapy as a nontoxic therapy for malignant disease is great. Many different types of immunotherapeutic interventions are currently in use or under investigation for cancer. The use of monoclonal antibodies as passive immunotherapy has become a standard therapy well known to most oncologists [1–3]. The interactions between the immune system and tumor cells are complex and have not been completely elucidated to date. The clinical benefits from anticancer vaccines as an active-specific immunotherapeutic approach have not matched expectations based on their ability to induce systemic cellular (and humoral) antitumor immune responses. Nonetheless, data from recent cancer vaccine trials, the use of immune-stimulating antibodies, and the manipulation of immune suppression and the immune microenvironment hold out great hope for achieving clinical benefit. This report summarizes the key findings of the Third Tegernsee Conference on Immunotherapy of Cancer held recently in Feldafing, Germany.
Manipulating the Tumor (Micro-) Environment to Improve Immunotherapeutic Treatment Strategies
The environment in which the tumor develops and grows may inhibit the immune response against it. B.A. Fox (Portland, OR) presented both preclinical and clinical data on how to enhance antitumor immunity by manipulating regulatory T (Treg) lymphocytes. Treg are T cells that have the ability to profoundly suppress immunity, are found in high numbers in the circulation of most cancer patients, and their presence in tumors is associated with worse outcome. Vaccinating a reconstituted-lymphopenic host, a strategy that depletes Treg and promotes homeostasis-driven T cell proliferation, resulted in higher frequencies of tumor-specific effector T cells in preclinical murine models. Clinical application of this concept in prostate cancer patients resulted in a higher prostate-specific antigen doubling time and an induction of humoral antibody responses as identified on protein arrays. The use of the fusion toxin denileukin difitox (Ontak®; Ligand Pharmaceuticals, Inc., San Diego), an interleukin (IL)-2-toxin molecule that binds to the IL-2 receptor highly expressed on Treg, has been shown to induce regression in melanoma and ovarian cancer, indicating that elimination of these cells has important clinical implications [4]. However, the rapid recovery of Treg within several weeks following reconstitution of lymphopenic patients may hamper the induction of a cellular immune response. To further reduce the number of Treg, the next step within clinical trials will include antibody-mediated depletion of CD25+ natural Treg and/or total CD4+ cells and blockade of the immune-suppressive transforming growth factor (TGF)-β and IL-10 that are generated by these cells.
Cytotoxic T lymphocyte antigen (CTLA-4) is a member of the immunoglobulin superfamily that is expressed predominantly on activated CD4 T cells transferring inhibitory signals to cytotoxic T lymphocytes (CTLs) (reviewed in [5]). Use of the anti–CTLA-4 antibody ipilimumab (Bristol-Myers Squibb, Princeton, NJ) has led to excellent 1-, 2-, and 3-year survival rates in clinical trials for unresectable stage IV melanoma patients, and for patients with resected stage IV disease (data presented by J.S. Weber, Tampa, FL). About 30% of patients treated for advanced melanoma with ipilimumab experienced a long-term survival benefit, according to recently updated results from three pivotal phase II studies. The 18-month survival rates in these studies were in the range of 34.5%–39.4% for previously treated patients [6]. However, the exact mechanism of action of CTLA-4–abrogating antibodies remains to be elucidated. The current data suggest that nonspecific alterations in immunity, possibly related to more activation markers on CD4 T cells, changes in total lymphocyte counts, and dendritic cell (DC) expression of indoleamine dioxygenase, may be associated with clinical activity. Ipilimumab has been shown to have activity in the central nervous system, can be used to retreat patients who have progressed after an initial response to the drug, and may enter the oncologic armamentarium for melanoma within 1–2 years if the pivotal randomized trial in untreated stage IV disease is positive.
M. Lotze (Pittsburgh, PA) reported on damage-associated molecular-pattern molecule (DAMP) release during cytolysis of tumor cells that are impaired in their ability to undergo apoptosis. DAMPs such as heat-shock proteins or high-mobility group box 1 (HMGB1) may enhance autophagy, a cellular self-destruction mechanism that is deficient in cancer, which, in turn, promotes tumor cell growth and aggressive behavior. Future clinical applications of these findings may therefore aim at (a) preventing DAMP release (proapoptotic therapies, ethyl pyruvate to induce necrosis-to-apoptosis switch), (b) neutralizing DAMPs extracellularly (anti-HMGB1), or (c) blocking DAMP receptors or their signaling (DAMP receptor antibodies, receptor for advanced glycation end products antagonists). These data may promote a whole new series of antitumor drugs that take advantage of this novel mechanism.
D. I. Gabrilovich (Tampa, FL) demonstrated how to increase the susceptibility of tumor cells to the effector mechanisms of CTLs by combining therapeutic vaccination or adoptive T cell transfer with chemotherapy. Granzymes are serine proteases that are released by cytoplasmic granules within CTLs and natural killer cells to induce apoptosis in target cells such as tumor cells or virus-infected cells. Pretreatment or concomitant treatment with standard chemotherapeutic agents resulted in a dramatic increase in permeability to granzyme B released by CTLs in the presence of tumors. This suggests that small numbers of CTLs may be sufficient to induce a potent antitumor effect when combined with chemotherapy, establishing a path forward for developing vaccines and chemotherapy.
Another way to directly activate immune stimulatory signals within the tumor microenvironment is toll-like receptor (TLR) agonists such as RNA oligonucleotides (presented by S. Endres, Munich, Germany). RNA oligonucleotides are able to simultaneously activate both the innate and adaptive arms of the immune system by stimulating DCs, inducing T helper type 1 cytokines, and triggering an antigen-specific CTL and IgG2a response (reviewed in [7]). They may have a role as vaccine adjuvants, and have already shown effectiveness when added to vaccines in animal tumor models.
Commentary
Manipulation of the tumor microenvironment is a promising approach to improving the efficacy of most immunotherapeutic strategies, especially cancer vaccines. Immune modulators must overcome multiple mechanisms employed by tumor cells to escape immune recognition and destruction, such as induction of Treg, alterations in antigen presentation, induction of T cell apoptosis, and production of immunosuppressive cytokines such as TGF-β. Counteracting these escape mechanisms will be a major challenge in developing immunotherapies for cancer beyond the first encouraging steps seen in prostate cancer, lymphoma, and melanoma [8–10]. Some immunomodulatory agents, such as lenalidomide, already clinically used by oncologists for multiple myeloma, may act directly within the tumor microenvironment, for example, by inhibiting immune-suppressive hypoxia-inducible factor-1α by epithelial tumor cells, including prostate, breast, and pancreatic cancer cells [11].
Will There Be a Role for Vaccines in Anticancer Therapy?
Active-specific stimulation of the host's own immune system still holds great promise for achieving nontoxic and durable antitumor responses despite, to date, sobering lack of clinical success. W. J. Urba (Portland, OR) confirmed the overall very low objective response rate (<5%) in clinical trials using therapeutic vaccines despite the induction of a measurable systemic immune response. Autophagy, or autophagocytosis, is a catabolic process involving the degradation of a cell's own components through the lysosomal machinery. The tumor cells' autophagosomes may be efficient carriers of (short-lived) antigens vital to crosspriming antigen-specific T cells normally not observed in peptide-based cancer vaccines. Urba and colleagues found a way to increase the number of autophagosomes in tumor cell lines by inducing autophagy with proteasome inhibitors (while inhibiting autophagosome maturation). The resulting vaccine contained more short-lived proteins, including defective ribosomal products, and was highly efficient at stimulating antigen-specific CD4 and CD8 T cells both in vitro and in vivo. A clinical trial using this approach in stage IIIB and stage IV non-small cell lung cancer (NSCLC) patients who have undergone one or fewer prior chemotherapy regimens for metastatic disease is under way (ClinicalTrials.gov identifier, NCT00850785).
C. L. Slingluff (Charlottesville, VA) and colleagues have directed their attention to understanding molecular and cellular events at the vaccination site when using a therapeutic cancer vaccine. The vaccine site microenvironment plays an important role in both T cell activation and regulation of the immune response. Adjuvants to boost vaccine effects, such as incomplete Freund's adjuvant or GM-CSF, which are given with many cancer vaccines, may play a distinct role in this context, although significant concerns have arisen over the potential suppressive effects of GM-CSF.
Licia Rivoltini (Milan, Italy) suggested that antitumor vaccines should primarily be used in the settings of minimal residual disease and early disease, when the immune functions of patients are mostly conserved. She presented data from a phase II clinical trial in stage IIB–IIC/III melanoma patients using a multipeptide, modified epitope vaccine emulsified in Montanide ISA 51 VG. A specific immune response was observed in >90% of patients. However, no impact was detected on clinical endpoints such as disease-free survival. Possibly, this may be explained by poor crossrecognition of primed T cells between modified vaccine peptides and native epitopes, resulting in a very limited ability to recognize tumor cells.
Transient lymphodepletion, alleviation of immune suppression, direct positive effects on immune effectors, and rendering tumor cells more immunogenic are some examples of the effects that standard-dose chemotherapy may exert on immune stimulation. Leisha A. Emens (Baltimore, MD) and colleagues combined low-dose cyclophosphamide (CY), doxorubicin (DOX), and an allogeneic, human epidermal growth factor receptor (HER)-2+, GM-CSF–secreting breast tumor vaccine in 28 patients with metastatic breast cancer. She demonstrated that certain chemotherapy regimens (such as CY + DOX) resulted in optimal stimulation and that low-dose chemotherapy can be combined with tumor vaccines to augment immunity, but the therapeutic window for a positive effect seemed narrow [12].
J. J. Mulé (Tampa, FL) and colleagues aimed to improve DC-based cancer vaccines by adding the chemokine secondary lymphoid tissue chemokine (SLC/CCL-21), which has been shown to create lymph node–like structures at the vaccine site to enhance host antitumor immunity, or by blocking macrophage receptor with collagenous structure (MARCO) in order to optimize the migration of DCs from vaccine sites to peripheral lymphoid tissue. This strategy may allow the oncologist to create an immune reaction in isolation from the tumor-suppressive influence of a tumor cell–infiltrated lymph node.
Melanoma antigen A3 (MAGE-A3) is a tumor-associated antigen of the germline (cancer testis) antigen family encoded on the X chromosome that is developmentally regulated and shut off in embryonic life but can be expressed by a large variety of cancers, including NSCLC, with no expression in normal adult cells other than placenta and germ cells. This tissue distribution indicates that it is an excellent candidate as a vaccine target. J.-F. Baurain (Brussels, Belgium) presented recent data from a phase II clinical trial in cutaneous melanoma patients (unresectable or in-transit stage III or stage IV M1a) using MAGE-A3 recombinant protein combined with two different adjuvants. With the adjuvant AS15 containing a QS-21 based adjuvant with a TLR-9 agonist CpG compound, three complete responses and one partial response were observed in 36 patients, with very little toxicity. Thus, the adjuvant preparation containing the CpG motif was also used for a randomized phase III trial in resected stage III melanoma patients (Adjuvant Immunotherapy with MAGE-A3 in Melanoma, called DERMA; 1,300 patients; ClinicalTrials.gov identifier, NCT00796445) that is currently under way, as is a trial in lung cancer patients after treatment with chemotherapy (Table 1). Most recently, data presented at the 2009 Annual Meeting of the American Urological Association revealed a 4.1 months longer median survival time for patients with advanced prostate cancer treated with the therapeutic cancer vaccine sipuleucel-T (Provenge®; Dendreon Corp., Seattle, WA) than for patients given placebo (Table 1) [8]. Recently, other approaches using active-specific immunotherapy in metastatic melanoma and follicular lymphoma have also shown signs of clinically relevant outcome improvements in randomized, controlled phase III trials [9, 10]. More large phase III clinical studies are currently under way. Table 1 summarizes recent and ongoing phase III trials of cancer vaccines. With the recent promising evidence from large phase III cancer vaccine trials, it is possible that the first therapeutic cancer vaccine will receive regulatory approval and will find its way into daily clinical routine. However, cancer vaccines may turn out to have their strengths in disease settings with low tumor burden or as maintenance therapy following standard treatment such as chemotherapy and radiotherapy. Cancer vaccines will also need to be combined with other immune-stimulating therapies and/or other targeted approaches. Figure 1 illustrates the proposed mechanism of action of cancer vaccines on the cellular level.
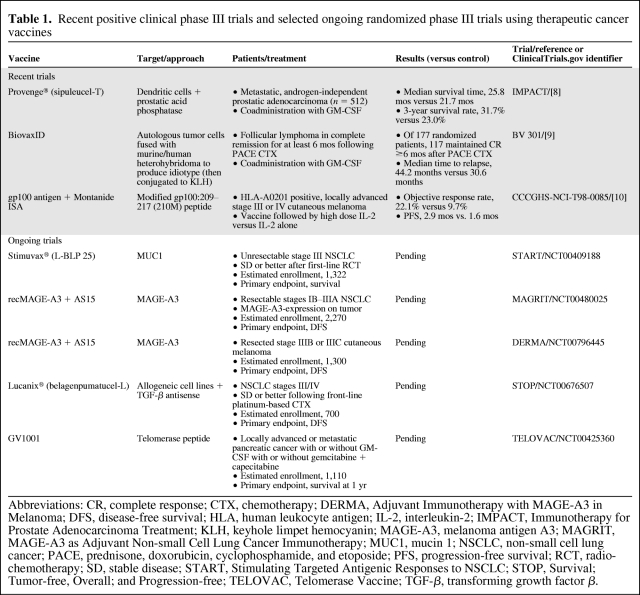
Table 1.
Recent positive clinical phase III trials and selected ongoing randomized phase III trials using therapeutic cancer vaccines
Abbreviations: CR, complete response; CTX, chemotherapy; DERMA, Adjuvant Immunotherapy with MAGE-A3 in Melanoma; DFS, disease-free survival; HLA, human leukocyte antigen; IL-2, interleukin-2; IMPACT, Immunotherapy for Prostate Adenocarcinoma Treatment; KLH, keyhole limpet hemocyanin; MAGE-A3, melanoma antigen A3; MAGRIT, MAGE-A3 as Adjuvant Non-small Cell Lung Cancer Immunotherapy; MUC1, mucin 1; NSCLC, non-small cell lung cancer; PACE, prednisone, doxorubicin, cyclophosphamide, and etoposide; PFS, progression-free survival; RCT, radio-chemotherapy; SD, stable disease; START, Stimulating Targeted Antigenic Responses to NSCLC; STOP, Survival; Tumor-free, Overall; and Progression-free; TELOVAC, Telomerase Vaccine; TGF-β, transforming growth factor β.
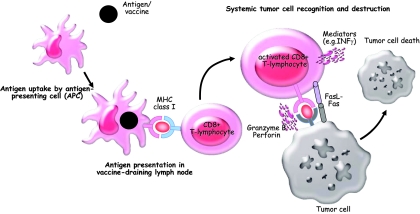
Figure 1.
Proposed mechanism of action for cancer vaccines. Tumor antigens (e.g., administered as proteins, peptides, or whole tumor cells) are taken up and processed by specialized antigen-presenting cells (APCs) such as dendritic cells (DCs). DCs migrate to the vaccine-draining lymph nodes and present relevant antigens to CD8+ T lymphocytes, which, in turn, are able to recognize tumor cells throughout the body and destroy them by several effector mechanisms such as the perforin/granzyme pathway, direct cell–cell interaction (e.g., Fas/Fas ligand), or certain mediators (e.g., INFγ). Not shown but also of importance are B lymphocytes, CD4+ T helper cells and cells of the innate immune system such as natural killer cells and macrophages.
Abbreviations: INF, interferon; MHC, major histocompatibility complex.
Optimizing Adoptive Cell Therapy
The use of immune cells with antitumor activity that are expanded ex vivo and transferred into a tumor-bearing host is referred to as adoptive cellular immunotherapy. Generating and identifying T cells specific for tumor-associated antigens (TAAs) remains difficult because such TAAs often represent self-peptides derived from overexpressed proteins presented by self–major histocompatibility complex (MHC) molecules. Dolores J. Schendel (Munich, Germany) and colleagues used autologous DCs loaded with RNA encoding a TAA and an allogeneic MHC molecule to tap unselected T cell repertoires. This approach may allow highly avid T cells with very strong receptor specificities for common TAAs to be easily obtained for direct adoptive therapy or as a source of therapeutic transgenic T cell receptor (TCR) sequences. Helga Bernhard (Darmstadt, Germany) demonstrated that primary T cells transduced with a HER-2–specific TCR for patients with HER-2–overexpressing breast cancer could be expanded for adoptive transfer. In preclinical studies, the HER-2–reactive TCR was conserved following transduction into primary T cells, and an additional crossrecognition with HER-3 and HER-4 was observed. This strategy is due for its first clinical test, especially because crossrecognition with HER-3 may turn out to be beneficial because HER-2– and HER-3–overexpressing tumors are particularly aggressive. R.A. Morgan (Bethesda, MD) described a clinical trial with 36 melanoma patients who received autologous peripheral lymphocytes genetically engineered to express TCRs highly reactive to melanoma/melanocyte antigens. Objective cancer regressions were observed in 30% of patients who received the human TCR-transduced T cells. Adoptively transferred T cells persisted at high levels in the peripheral blood of all patients 1 month after treatment. However, patients also exhibited destruction of normal melanocytes in the skin, eye, and ear resulting in vitiligo, hearing loss, and retinal pigment loss. This strategy is undergoing testing in other cancers as well.
Commentary
For a highly selected population of metastatic melanoma patients, adoptive transfer of autologous tumor-infiltrating lymphocytes has shown objective tumor regressions in up to 50% of patients [13]. When total-body irradiation (TBI) to eliminate Treg and deplete endogenous lymphocytes was added to the preparative chemotherapy in this nonrandomized study, objective tumor response rates were higher, up to 72% with the highest dose of TBI [14]. Even though these results provide optimism, common epithelial cancers have not been treated with similar success using adoptive T cell transfer. The biggest challenge for a broader application of adoptive cell therapy arises from the highly personalized character of this treatment, which is labor intensive and does not easily fit into current modes of oncological practice. Nevertheless, these studies serve as proof of concept that transferred T cells are capable of mediating the regression of large, metastatic tumors that have failed previous treatments, and the practicing oncologist will be seeing more of these early clinical trials performed at academic centers.
Targeted and Novel Immunotherapeutic Approaches
Selected monoclonal antibodies can mediate clinically significant antitumor effects in many cancers. Numerous advances in molecular biology have made possible the identification of new tumor targets and the genetic and structural manipulation of antibodies and their recombinant production. OX40 (CD134) is a member of the tumor necrosis factor receptor family that functions as a molecule to costimulate T cells, that is, to amplify the signal delivered by the TCR–antigen interaction. A. Weinberg (Portland, OR) and colleagues found that OX40 is expressed on lymphocytes infiltrating tumors in several human solid malignancies. They generated an agonist mouse anti-human OX40 antibody, which is currently in phase I clinical testing. Four of 20 patients showed evidence of tumor regression, with an overall good safety profile with this antibody. Interestingly, anti-OX40 treatment resulted in a dose-dependent increase in proliferation of both CD4 and CD8 T cells.
Tumor escape mechanisms may limit immunotherapeutic approaches that aim to elicit or support tumor-specific T cell responses. C. Itin (Bethesda, MD) presented data using the bispecific T cell engager (BiTE®) class, a novel approach directly recruiting T cells independently of antigen presentation or specificity of CTLs. BiTE antibodies function as adaptors that physically link T cells and tumor cells and, at the same time, potently trigger the signaling cascade of the TCR complex and might, therefore, be less limited by tumor escape mechanisms. Two BiTE antibodies are currently in clinical trials, with blinatumumab (CD3/CD19) being the most advanced with very promising early results.
Catumaxumab (Removab®; Fresenius Biotech, Munich, Germany) is another bispecific antibody, albeit with an intact Fc region and of murine origin, which binds and activates Fcγ+ accessory cells such as macrophages and natural killer cells. Diane Seimetz (Munich, Germany) described the pathway from the preclinical development of epithelial cell adhesion molecule/CD3-bispecific catumaxumab to approval by the European Commission in April 2009. The molecule was approved for i.p. treatment of malignant ascites, and has entered the oncologic repertoire in Europe. Catumaxumab was able to prolong “puncture-free survival” by 35 days in patients with malignant ascites from ovarian cancer and different gastrointestinal malignancies.
Receptors for the immune regulatory cytokine IL-13 are overexpressed on various solid malignancies, such as head and neck and ovarian tumors. R. K. Puri (Bethesda, MD) introduced two approaches to target IL-13 for cancer therapy: (a) active immunization against IL-13Rα2, a high-affinity receptor, and (b) a recombinant fusion cytotoxin composed of IL-13 and a mutated form of Pseudomonas exotoxin (IL13-PE). Interestingly, normal cells lacking IL-13Rα2 are spared from the cytotoxic effects of IL13-PE.
Commentary
Monoclonal antibodies have emerged as effective therapeutic agents for a number of tumors [1–3], albeit in a limited number of indications. New antibody-based treatment modalities such as radioimmunotherapy, drug conjugates, and immunotoxins are currently under development or already approved by the U.S. Food and Drug Administration (e.g., tositumomab, gemtuzumab). Other approaches aim at modifying the structure of monoclonal antibodies, as is the case with blinatumumab, a bispecific single-chain antibody construct, resulting in objective response rates of up to 100% in non-Hodgkin's lymphoma patients (mainly follicular and mantle cell lymphoma) [15]. Nevertheless, especially in the antibody-based treatment of solid tumors, many obstacles remain, such as overly rapid clearance that restricts antibody access to tumors and the difficulty of obtaining effective concentrations at the tumor site.
Conclusion
Monoclonal antibodies, which are a form of immunotherapy, have become a standard part of treatment regimens for some malignancies. Initial phase III results using cancer vaccines for several different histologies showed promising results. Other immune approaches, such as adoptive cell transfer, may be the most effective treatment modalities for selected cancer patients. Favorable reports presented at this meeting on the results of immunotherapy for cancer in phase II and phase III clinical trials raise the hope that it is only a matter of time before therapeutic cancer vaccines and immune-stimulatory antibodies become standard treatment options for the oncologist. Intensified scientific interactions between basic researchers and clinical investigators, such as those at this meeting, will hopefully accelerate the development of novel and successful immunotherapies for cancer.
Acknowledgments
This work was supported by the Chiles Foundation, Portland, OR, the German Research Foundation (DFG), Bonn, Germany, and the Walter-Schulz-Foundation, Munich, Germany.
Author Contributions
Conception/Design: Dominik Rüttinger, Bernard A. Fox, Jeffrey S. Weber
Provision of study material or patients: Dominik Rüttinger, Hauke Winter, Natasja van den Engel, Rudolf A. Hatz, Karl-Walter Jauch, Bernard A. Fox, Jeffrey S. Weber
Collection and/or assembly of data: Dominik Rüttinger, Hauke Winter, Natasja van den Engel, Rudolf A. Hatz, Karl-Walter Jauch, Bernard A. Fox, Jeffrey S. Weber
Data analysis and interpretation: Dominik Rüttinger, Hauke Winter, Natasja van den Engel, Rudolf A. Hatz, Karl-Walter Jauch, Bernard A. Fox, Jeffrey S. Weber
Manuscript writing: Dominik Rüttinger, Hauke Winter, Natasja van den Engel, Rudolf A. Hatz, Karl-Walter Jauch, Bernard A. Fox, Jeffrey S. Weber
Final approval of manuscript: Dominik Rüttinger, Hauke Winter, Natasja van den Engel, Rudolf A. Hatz, Karl-Walter Jauch, Bernard A. Fox, Jeffrey S. Weber
References
- 1.Peeters M, Price T, Van Laethem JL. Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: Where are we today? The Oncologist. 2009;14:29–39. doi: 10.1634/theoncologist.2008-0167. [DOI] [PubMed] [Google Scholar]
- 2.Wheatly-Price P, Shepherd FA. Targeting angiogenesis in the treatment of lung cancer. J Thorac Oncol. 2008;3:1173–1184. doi: 10.1097/JTO.0b013e318187220f. [DOI] [PubMed] [Google Scholar]
- 3.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. The Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 4.Ruter J, Barnett BG, Kryczek I, et al. Altering regulatory T cell function in cancer immunotherapy: A novel means to boost the efficacy of cancer vaccines. Front Biosci. 2009;14:1761–1770. doi: 10.2741/3338. [DOI] [PubMed] [Google Scholar]
- 5.Korman A, Yellin M, Keler T. Tumor immunotherapy: Preclinical and clinical activity of anti-CTLA4 antibodies. Curr Opin Investig Drugs. 2005;6:582–591. [PubMed] [Google Scholar]
- 6.O'Day S, Weber J, Lebbe C, et al. Effect of ipilimumab treatment on 18-month survival: Update of patients with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials [abstract 9033] J Clin Oncol. 2009;27(15 suppl) [Google Scholar]
- 7.Smits EL, Ponsaerts P, Berneman ZN, et al. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. The Oncologist. 2008;13:859–875. doi: 10.1634/theoncologist.2008-0097. [DOI] [PubMed] [Google Scholar]
- 8.Schellhammer PF, Higano C, Berger ER, et al. A randomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen independent prostatic adenocarcinoma (AIPC) [abstract LBA 9] Proc Am Urol Assoc. 2009;104 [Google Scholar]
- 9.Schuster SJ, Neelapu SS, Gause BL, et al. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma in first complete remission: Phase III clinical trial results [abstract 2] J Clin Oncol. 2009;27(18 suppl) [Google Scholar]
- 10.Schwartzentruber DJ, Lawson D, Richards J, et al. A phase III multi-institutional randomized study of immunization with the gp100:209–217 (210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma [abstract CRA9011] J Clin Oncol. 2009;27(18 suppl) [Google Scholar]
- 11.Lu L, Schafer P, Bartlett JB. Inhibition by lenalidomide of growth factor and hypoxia-induced signaling in endothelial and epithelial tumor cells, and effects with the tumor cell microenvironment [abstract e14620] J Clin Oncol. 2009;27 [Google Scholar]
- 12.Emens LA, Asquith JM, Leatherman JM, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: A chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]