Malignant bone disease is common in patients with advanced solid tumors or multiple myeloma. Bisphosphonates have been found to be important treatments for bone metastases. A positive benefit-risk ratio for bisphosphonates has been established, and ongoing clinical trials will determine whether individualized therapy is possible.
Keywords: Bisphosphonates, Neoplasm metastasis, Skeleton, Therapeutics
Abstract
Bisphosphonates are important treatments for bone metastases. Considerations for optimizing the clinical benefits of bisphosphonates include efficacy, compliance, and safety. Several bisphosphonates are approved for clinical use; however, few have demonstrated broad efficacy in the oncology setting and been compared directly in clinical trials. Among patients with bone metastases from breast cancer, the efficacy of approved bisphosphonates was evaluated in a Cochrane review, showing a reduction in the risk of skeletal-related events (SREs) ranging from 8% to 41% compared with placebo. Between-trial comparisons are confounded by inconsistencies in trial design, SRE definition, and endpoint selection. Zoledronic acid has demonstrated clinical benefits beyond those of pamidronate in a head-to-head trial that included patients with breast cancer or multiple myeloma. Compliance and adherence also have effects on treatment efficacy. In a comparison study, the adherence rates with oral bisphosphonates were found to be significantly lower compared with those of intravenous bisphosphonates. The safety profiles of oral and intravenous bisphosphonates differ. Oral bisphosphonates are associated with gastrointestinal side effects, whereas intravenous bisphosphonates have dose- and infusion rate–dependent effects on renal function. Osteonecrosis of the jaw is an uncommon but serious event in patients receiving monthly intravenous bisphosphonates or denosumab. The incidence of this event can be reduced with careful oral hygiene. A positive benefit-risk ratio for bisphosphonates has been established, and ongoing clinical trials will determine whether individualized therapy is possible.
Introduction
Malignant bone disease is common in patients with advanced solid tumors or multiple myeloma. Among patients with lung cancer, bladder cancer, or melanoma, approximately 40% develop bone metastases during the course of their disease [1]. Breast cancer (BC) and prostate cancer (PC) have an especially high potential for metastasis to bone, which occurs in approximately 75% of patients with stage IV disease [1]. Because these patients may have a median survival of several years after the development of bone metastases, they have a long-term risk of developing skeletal-related events (SREs) including pathologic fractures, spinal cord compression, the requirement for surgery (including vertebroplasty, kyphoplasty, and cementoplasty) or radiotherapy to bone, and hypercalcemia of malignancy. Indeed, in the absence of bone-specific therapies, SREs occur in 46%–68% of patients with bone metastases from solid tumors, and patients may experience multiple SREs [2–4]. Furthermore, the risk of subsequent SREs increases after the first SRE [5, 6]. These SREs can have negative consequences for patients' functional independence. Among men with PC and women with BC, there are consistent decreases in physical and emotional well-being after SREs [7, 8]. Moreover, in patients with PC or BC, pathologic fractures have been associated with reduced survival [9]. Therefore, prevention of SREs is an important therapeutic goal.
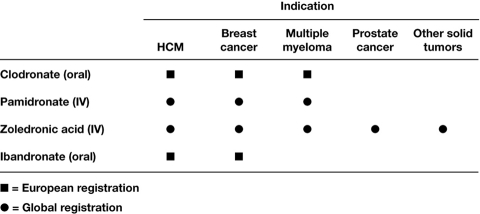
In recent years, treatment innovations have significantly extended survival, even for patients with stage III or IV lung cancer and castration-resistant PC [10, 11]. However, prolonging survival may increase the likelihood that cancer and its treatment effects on the skeleton will manifest in skeletal morbidity within patients' lifetimes. Therefore, an important goal of therapy is to preserve patients' bone health, thereby preserving their functional independence to the extent possible throughout the course of the disease. The therapeutic repertoire for managing skeletal morbidity and slowing the erosion in quality of life includes analgesics, radiotherapy, surgery, and bisphosphonates. Bisphosphonates can reduce bone pain, analgesic use, and need for radiation to bone [12]. However, bisphosphonates also treat the underlying cause of SREs—malignant osteolysis—and can therefore delay the onset and reduce the incidence of SREs [13, 14]. Although several bisphosphonates are approved for clinical use, relatively few have demonstrated efficacy for broad application in the oncology setting, and the majority of bisphosphonates are approved only for use in BC metastatic to bone (Figure 1). Moreover, few of these agents have been compared directly in clinical trials, and between-trial comparisons are confounded by inconsistencies in trial design, SRE definition, and endpoint selection. In optimizing the clinical benefits of bisphosphonates, important considerations for bisphosphonate selection include not only efficacy but also safety profiles and compliance.
Figure 1.
Approved bisphosphonate indications in the oncology setting. Abbreviations: HCM, hypercalcemia of malignancy; IV, intravenous. (Note: In the United States, prostate cancer must have progressed despite hormone therapy.)
Efficacy of Bisphosphonates
Bisphosphonates have been recommended for the treatment of primary bone lesions from multiple myeloma or bone metastases from solid tumors [15–18]. Although bisphosphonates are administered systemically, they are deposited at sites of active bone remodeling. Bisphosphonates accumulate in the bone and are ingested by osteoclasts during bone resorption, wherein they inhibit osteolysis [19]. There are two classes of bisphosphonates with different mechanisms of action: non–nitrogen-containing and nitrogen-containing [19]. Non–nitrogen-containing bisphosphonates such as clodronate are metabolized by osteoclasts to cytotoxic compounds. Nitrogen-containing bisphosphonates such as zoledronic acid, pamidronate, and ibandronate inhibit a key enzyme in the mevalonate pathway, inducing apoptosis of osteoclasts. Both classes are currently used for the treatment of bone metastases in patients with cancer. Clinical trials providing evidence for the efficacy of these agents have used similar definitions of skeletal morbidity, but not all trials have selected robust clinical endpoints that provide objective and quantitative measurements of patient benefit. For example, pain scores, analgesic use, and quality of life associated with bone metastases are difficult to objectively measure and may be confounded by observer bias [6]. These clinical endpoints are, therefore, not easily comparable in different patient populations. In contrast, SREs can be objectively measured and provide clinically relevant information for the evaluation of bisphosphonates. Indeed, the SRE has been used as an example of a clinically relevant composite endpoint by the U.S. Food and Drug Administration (FDA) [20]. However, the definition for SREs has varied. Skeletal morbidity rate ([SMR] mean SRE rate per person-year) is a less robust endpoint in clinical trials because it assumes a constant event rate for all patients and cannot adjust for inter- and intrapatient variations in SRE rates over the course of disease progression [6]. Different statistical models, based on various statistical assumptions, have been used in clinical trials, including multiple event analyses such as Andersen-Gill that provide robust methodology for reporting treatment effects by adjusting for variability in event rates over time [6].
Breast Cancer
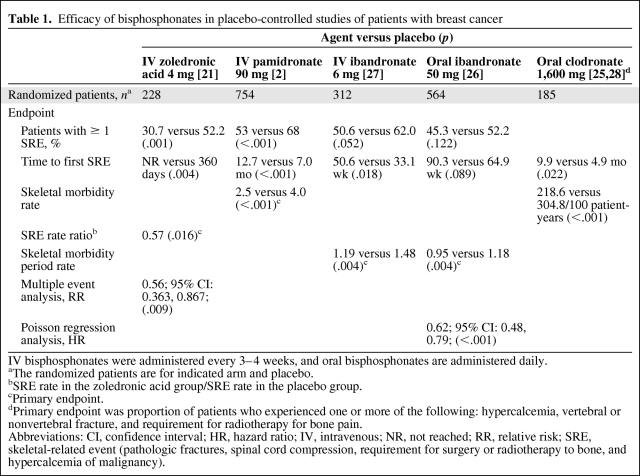
Guidelines from the American Society of Clinical Oncology (ASCO) recommend intravenous pamidronate or zoledronic acid in patients with bone metastases from BC, the only two agents approved for that indication in the United States [17]. Both have produced significant reductions in the risk of SREs compared with placebo in this setting [2, 21]. In the only head-to-head phase III trial of bisphosphonates in this setting, 4 mg of zoledronic acid demonstrated efficacy at least comparable to and some significant benefits beyond those of 90 mg of pamidronate in patients with bone lesions from multiple myeloma or BC [22, 23]. In the subset of patients with BC, Andersen-Gill multiple event analysis revealed that zoledronic acid reduced the risk of developing SREs by an additional 20% compared with pamidronate (p = .025) [3, 4, 23, 24]. The efficacy of bisphosphonates in patients with BC was also evaluated in a Cochrane review, which confirmed the utility of this class of agents to prevent SREs from bone metastases and reported a range of SRE risk reductions for bisphosphonates [25]. Versus placebo, reported risk reductions were 41% for intravenous zoledronic acid, 23% for intravenous pamidronate, and 18% and 14% for intravenous and oral ibandronate, respectively. The risk reduction with oral ibandronate fell short of statistical significance, as did the risk reduction with oral clodronate in most of the cited studies. However, differences in patient populations, trial designs, SRE definitions, and endpoint selection confound any between-trial comparisons. Overall, these data indicate a benefit for all approved agents.
Among the oral bisphosphonates studied in patients with bone metastases from BC, there was a significant reduction in the skeletal morbidity period rate (number of 12-week periods with new SREs) with ibandronate versus placebo; however, this assessment cannot be compared with SMR endpoints [26]. The remaining two clinical endpoints, proportion of patients with an SRE and time to first SRE, were not significantly different between oral ibandronate and placebo (Table 1) [2, 21, 25–28]. Oral clodronate has also demonstrated benefits in patients with metastatic BC. Efficacy results for the prevention of SREs, however, have been inconsistent between studies, especially with regard to bone pain endpoints and incidence of radiotherapy to bone [25, 28–30]. In a comparison study, intravenous pamidronate was found to be more effective than oral clodronate in improving pain scores (p < .05) [31].
Table 1.
Efficacy of bisphosphonates in placebo-controlled studies of patients with breast cancer
IV bisphosphonates were administered every 3–4 weeks, and oral bisphosphonates are administered daily.
aThe randomized patients are for indicated arm and placebo.
bSRE rate in the zoledronic acid group/SRE rate in the placebo group.
cPrimary endpoint.
dPrimary endpoint was proportion of patients who experienced one or more of the following: hypercalcemia, vertebral or nonvertebral fracture, and requirement for radiotherapy for bone pain.
Abbreviations: CI, confidence interval; HR, hazard ratio; IV, intravenous; NR, not reached; RR, relative risk; SRE, skeletal-related event (pathologic fractures, spinal cord compression, requirement for surgery or radiotherapy to bone, and hypercalcemia of malignancy).
Prostate Cancer
Among patients with bone metastases from PC, zoledronic acid is the only bisphosphonate to provide statistically significant and durable reductions in the risk of SREs versus placebo in a randomized, controlled trial and to have received widespread regulatory approval. In the phase III trial, patients with bone metastases from PC (N = 643) were randomized to receive either zoledronic acid or placebo for up to 2 years [4, 32]. At 24 months compared with placebo, zoledronic acid significantly reduced the proportion of patients with an SRE (49% versus 38%, respectively; p = .028) and the SMR (1.47 versus 0.77 SREs per year, respectively; p = .005), and increased mean time to first SRE (321 versus 488 days, respectively; p = .009) [4]. Zoledronic acid also reduced the risk of SREs by 36% versus placebo (Andersen-Gill multiple event analysis; p = .002) [3, 4, 23, 24]. Moreover, zoledronic acid provided long-term reductions in bone pain versus placebo (p < .05 at 21 and 24 months [33]. In contrast, in randomized, placebo-controlled trials, pamidronate and clodronate failed to demonstrate significant benefits in these endpoints versus placebo [34]. Similar results have been reported for oral clodronate [35].
Other Solid Tumors
Zoledronic acid has been shown to reduce SREs in patients with bone metastases from lung cancer, kidney cancer, and a broad range of other solid tumors. In the phase III trial, patients with lung cancer or other solid tumors (N = 773) were randomized to receive either zoledronic acid or placebo for up to 21 months [3]. At 21 months, 4 mg of zoledronic acid reduced the proportion of patients who developed an on-study SRE including hypercalcemia of malignancy (39% versus 48% with placebo; p = .039), significantly delayed the time to first SRE (236 days versus 155 days with placebo; p = .009), and reduced the SMR (1.74 versus 2.71 SREs per year with placebo; p = .012). In an Andersen-Gill analysis, zoledronic acid significantly reduced the risk of SREs by 31% compared with placebo (p = .003) [3, 4, 23, 24]. In a retrospective subset analysis from this trial of patients with renal cell carcinoma (n = 74), zoledronic acid significantly reduced the proportion of patients with an SRE at 9 months (37% versus 74% for placebo; p = .015) and significantly prolonged the time to first SRE (median not reached versus 72 days for placebo; p = .006) [36]. Multiple event analysis also demonstrated that zoledronic acid reduced the risk of SREs by 61% versus placebo (p = .008) [36]. Such randomized placebo-controlled data have not been reported for other bisphosphonates.
Importance of Early Treatment with Bisphosphonates
Bone pain is usually the earliest and most common symptom of bone metastases, and bone metastases often are not diagnosed until after the onset of bone pain [37]. However, bone pain can have debilitating effects on a patient's quality of life, and treatment after pain develops may not be the optimal strategy. Therefore, identification of patients at risk for bone metastases, earlier diagnosis, and earlier treatment for bone metastases may be more beneficial.
In the adjuvant BC setting, aromatase inhibitor (AI) use is increasing, and AIs have been associated with accelerated bone loss and increased fracture risk [38]. In fact, bone loss associated with AIs may occur at a twofold higher rate than that observed in healthy postmenopausal women (PMW) [39]. Among the oral bisphosphonates, 3 years of clodronate, 1,600 mg/day, reduced treatment-induced bone loss of the lumbar spine in 73 patients with BC compared with control [40]. Risedronate, 35 mg once weekly for 24 months, stabilized bone mineral density (BMD) at the hip from baseline and curtailed spinal BMD loss versus placebo (2.8% versus 4.8%, respectively, from baseline) in PMW with BC receiving an AI [41]. In another study with cyclic risedronate (30 mg/day for 2 weeks followed by 10 weeks of no drug) for 2 years in patients with BC and treatment-induced menopause, risedronate increased BMD versus placebo (p ≤ .041 for both) [42]. However, weekly risedronate failed to prevent lumbar spine BMD loss in patients with BC [43]. Ibandronate, 150 mg/day, increased BMD from baseline in osteopenic PMW with BC receiving anastrozole (n = 50) during 2 years of treatment [44]. Intravenous pamidronate (60 mg every 3 months) inhibited bone loss versus placebo in 40 premenopausal women for 1 year but did not improve BMD versus baseline [45]. Intravenous zoledronic acid (4 mg every 6 months) for 3 years stabilized BMD in premenopausal women with BC treated with endocrine therapy (n = 404) [46]. At the 5-year follow-up, BMD continued to decrease with placebo, whereas it continued to increase versus baseline with zoledronic acid. In another study with immediate or delayed treatment with zoledronic acid, 4 mg biannually, for 5 years in patients with BC receiving letrozole, immediate zoledronic acid increased BMD at 12 months compared with delayed treatment (p < .0001 for both) [47]. Therefore, bisphosphonates can reduce cancer treatment–associated bone loss in BC patients, and there are similar data for BMD preservation during androgen-deprivation therapy (ADT) for PC, although no treatments are currently approved in the United States or Europe for these specific indications [48–51].
Guidelines have been published regarding the use of bisphosphonates in BC. One expert panel recommends that bisphosphonate therapy should be started in any patient initiating or receiving AI therapy with a T-score less than −2.0 or if other risk factors are present [52]. A British expert panel recommended bisphosphonate therapy in women experiencing premature menopause receiving AIs if the annual rate of bone loss exceeds 4% at lumbar spine or total hip sites, or if there is a history of vertebral fracture or T-score less than −1.0 [53]. The recommendations for PMW were similar, but the T-score threshold was reduced to less than −2.0. An international expert panel made similar recommendations, but also recommended amino-bisphosphonates for patients with bone metastases from BC, and zoledronic acid for patients with bone metastases from other solid tumors [54].
In exploratory analyses of the phase III trials, zoledronic acid produced a more profound reduction versus placebo in patients with PC or versus pamidronate in patients with BC in the proportion of patients with one or more SREs and in patients with no pain at baseline compared with patients with pain at baseline [55, 56]. Moreover, zoledronic acid also reduced the SMR by a greater extent in patients with PC and no pain at baseline (49%) compared with patients who had pain at baseline (39%) [56]. Among patients with BC and no prior fractures at baseline, zoledronic acid reduced the SMR by a greater extent compared with patients who had a prior fracture at baseline (by 0.33 and 0.78, respectively) [21].
Pain levels often increase during disease progression and may indicate advancing bone disease. A greater number of bone metastases results in an increased risk of SREs. Indeed, patients with solid tumors and more than three bone metastases have an approximately 1.5-fold increase in the risk of SREs [57]. Furthermore, after patients experience SREs, they are at a higher risk of subsequent SREs. Among patients with bone metastases from BC, a first SRE increases the risk of a subsequent SRE twofold [58]. Moreover, pathologic fractures increase risk of death by 23%–32% in patients with bone metastases from PC or BC, respectively [9].
Bisphosphonates may have effects beyond bone health. Preclinical results demonstrated that zoledronic acid, pamidronate, clodronate, and ibandronate exhibit antitumor activity in BC cell lines and animal models of early BC disease [59, 60]. However, clinical studies have yielded mixed results. Two studies suggest a survival benefit for patients receiving clodronate as adjuvant therapy for breast cancer [61, 62]. However, a meta analysis of seven clinical studies evaluating oral clodronate (1,600 mg/day for 2–3 years) versus placebo or no additional treatment found no significant difference in overall survival or bone-metastasis–free survival in patients with either early or advanced BC [63]. Pamidronate, 90 mg every 4 weeks, has shown similar results versus placebo in patients with advanced BC [64, 65]. Overall disease benefits have not been reported with ibandronate. Zoledronic acid reduced the risk of disease progression (hazard ratio [HR] = 0.64; 95% confidence interval [CI]: 0.46, 0.91; p = .01) and produced a trend toward reduced risk of death (HR = 0.60; 95% CI: 0.32; 1.11; p =.11) versus no zoledronic acid in premenopausal patients receiving endocrine therapy for early BC [66]. A recent exploratory analysis of data from three companion studies of zoledronic acid in combination with adjuvant letrozole therapy in postmenopausal women with early BC shows significantly improved disease-free survival in the ZO-FAST study [67], but no clear benefit in the other two studies [68]. This is primarily a result of the low rates of disease recurrence, differences in the length of follow-up, and lack of follow-up after discontinuation in one study, among other confounding factors. Results from these and other clinical trials indicating that the antitumor effects of bisphosphonates may translate into clinical benefits have been recently reviewed [69].
Clinical Benefits of Continuing Bisphosphonate Treatment in Cancer Patients with Bone Metastases
Data from the placebo-controlled arms of bisphosphonate trials have revealed that patients are at risk for SREs throughout the course of their advanced disease and that long-term treatment may therefore be needed [3, 4, 23]. However, results from bisphosphonate studies that focus on clinical benefits that may occur after the first years of treatment are scarce. Intravenous ibandronate (6 mg every 3–4 weeks for up to 2 years) in patients with bone metastases from BC has significantly reduced the mean number of new SREs by 38% (p = .032) and significantly increased the time to a first new SRE (p = .018) compared with placebo [27]. However, this trial did not assess the possible benefits of ibandronate during the second year of treatment separately and, therefore, presents a limitation on assessing the clinical benefits beyond the first year. Intravenous pamidronate (90 mg every 3–4 weeks for up to 2 years) significantly reduced the incidence and delayed the onset of SREs compared with placebo in patients with bone metastases from BC, but the same assessment limitation as in the ibandronate study is present [2]. Exploratory analyses of the zoledronic acid database from the phase III efficacy trial of PC demonstrated that the risk of SREs in patients with PC receiving zoledronic acid during months 16 to 24 was significantly reduced by 53% compared with the placebo group (HR = 0.467; p = .022) [70]. This risk reduction was greater than that observed during the first 15 months of the study (HR = 0.643; p = .004). During months 16 to 24 of treatment, zoledronic acid also continued to provide significant reductions in proportion of patients with an SRE (p = .017), time to first SRE (p = .036), and SMR (p = .016) compared with placebo [70]. Exploratory analyses of the subset of patients with bone metastases from BC who entered the second year of treatment demonstrated that zoledronic acid significantly reduced the risk of developing an SRE by an additional 41% compared with pamidronate (p = .026) during the second year of treatment [71]. During the second year of therapy, zoledronic acid also continued to provide reductions in the proportion of patients with an SRE (p = .072), time to first SRE (p = .067), and SMR (p = .058) compared with pamidronate [71].
Bisphosphonate treatment continues to provide clinical benefits after a patient experiences an SRE, as shown by further exploratory analyses. A multiple event analysis of data from patients with PC receiving zoledronic acid showed that when the first SRE is excluded, there is a greater risk reduction of subsequent SREs compared with the placebo group (HR = 0.601; p = .011) [70]. Zoledronic acid also significantly reduced the proportion of patients who experienced a second SRE (p = .017) and the SMR after excluding the first event (p = .014) [70]. Furthermore, the median time to a second SRE was significantly delayed among patients with PC receiving zoledronic acid compared with the placebo group (p = .006) [70]. Among patients with PC who had experienced an SRE before study entry, zoledronic acid, 4 mg, reduced the proportion of patients who experienced an on-study SRE (41% versus 51%, respectively; p = .215) and provided a significant 65% relative reduction in mean SMR compared with placebo (0.80 versus 2.30, respectively; p = .036) [70]. Among patients with bone lesions from multiple myeloma or bone metastases from BC, zoledronic acid produced a trend toward a lower proportion of patients who experienced a second SRE (p = .170) and the SMR after excluding the first event (p = .105) compared with pamidronate [72]. However, in the patients who had experienced an SRE before study entry, zoledronic acid significantly reduced the proportion of patients who experienced an on-study SRE compared with pamidronate (54% versus 61%, respectively; p = .039) and the SMR by 24% relative to pamidronate (1.22 versus 1.61; p = .038).
Practical Considerations of Bisphosphonate Treatment
Four parameters should be considered when selecting a bisphosphonate: efficacy, compliance, adherence, and safety. Efficacy results presented in the previous sections show that, among patients with bone metastases from BC, the approved intravenous and oral bisphosphonates all reduce the risk of SRE compared with placebo [25]. However, broad generalizations regarding the relative efficacy of bisphosphonates should be avoided because direct comparative studies between bisphosphonates (other than pamidronate versus zoledronic acid) have not been done. Among patients with PC, lung cancer, or other solid tumors, zoledronic acid is the only approved bisphosphonate for the treatment of bone metastases.
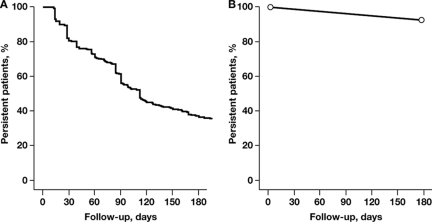
Compliance (administration regimen implemented as indicated on product label, e.g., with regard to dosing frequency), adherence (degree to which patients follow physician's advice, e.g., with regard to the duration of therapy), and convenience of administration are important considerations when selecting a bisphosphonate. For example, oral bisphosphonates may be taken at home, whereas intravenous bisphosphonates require a visit to the doctor's office or hospital. However, the tablets of oral clodronate are large and may be hard for patients to swallow, causing patients to discontinue treatment [73], but oral ibandronate tablets are smaller. Patients must also fast overnight before taking an oral bisphosphonate (because food interferes with absorption), remain upright, and continue to fast after administration from at least 30 minutes for up to 2 hours—depending on the agent—to minimize the risk of gastrointestinal adverse events [73]. The requirements for administration of oral bisphosphonates are associated with reduced compliance and may lead to reduced adherence and, therefore, to suboptimal efficacy [74, 75]. Furthermore, patient adherence with the prescribed treatment regimen cannot be adequately monitored with oral agents. A retrospective analysis of insurance claims in Germany showed that the median duration of therapy was 112 days for oral bisphosphonates [76]. After 3 months of treatment, 44% of patients had stopped therapy, and, after 6 months, 64% of patients had stopped therapy (Figure 2A) [76]. In contrast, adherence with intravenous bisphosphonates is generally high, with approximately 90% of patients remaining on treatment after 6 months (Figure 2B) [4, 23, 77]. A comparison of intravenous versus oral bisphosphonate adherence rates in patients with advanced cancer showed that at 6 months oral bisphosphonates had a significantly lower adherence rate compared with intravenous bisphosphonates (36% versus 92%; p = .0012) [77]. An intravenous treatment allows greater contact with healthcare providers and promotes adherence mostly through provider attention and proactive management. Patient adherence with an intravenous regimen is then known to the healthcare providers, and the physicians can be assured that their patients will receive the full efficacy afforded by the treatment.
Figure 2.
Adherence of approved bisphosphonate treatments by route of administration. (A): Oral bisphosphonates except for ibandronate. Adapted from Hoer A, Goethe H, Barghout V et al. Low persistency with oral bisphosphonates in cancer patients [poster]. Presented at: 5th European Oncology Nursing Society Spring Convention; April 20–22, 2006; Innsbruck, Austria; Abstract 2, with permission. (B): Intravenous bisphosphonates. Data from Mangiapane S, Hoer A, Gothe H et al. Higher persistency with i.v. bisphosphonates in patients with bone metastasis [abstract]. J Clin Oncol 2006;24(suppl):698s. Abstract 18623.
Safety considerations among patients who receive oral bisphosphonates relate to gastrointestinal side effects. In clinical trials, the incidences of diarrhea, dyspepsia, nausea, and esophagitis have typically been higher in the oral bisphosphonate group compared with the placebo group [26, 73]. In contrast, safety considerations among patients who receive intravenous bisphosphonates relate to renal function, and osteonecrosis of the jaw (ONJ). All intravenous bisphosphonates are associated with dose- and infusion rate–dependent effects on renal function, and monitoring of serum creatinine levels is recommended to ensure renal safety [74]. Therefore, the product labels for zoledronic acid and pamidronate recommend checking serum creatinine levels before each infusion [78–80]. In patients with decreased renal function, the product label for zoledronic acid recommends appropriate dose modifications (Table 2) [78]. These modifications achieve the same exposure to zoledronic acid as that in patients with a creatinine clearance of 75 ml/min. Dose adjustments for patients with reduced renal function are also included in the product label for ibandronate (Table 2) [80]. Additionally, mild to moderate acute-phase reactions (flu-like symptoms) may occur, typically only after first infusion, and patients may stop treatment. However, acute-phase reactions are usually self-limited and manageable with nonsteroidal anti-inflammatory agents or acetaminophen [81].
Table 2.
Recommended zoledronic acid and ibandronate dose reductions in patients with decreased renal function
aDoses calculated to achieve an area under the curve of 0.66 mg-h/ml with a creatinine clearance of 75 ml/min (using Cockcroft-Gault formula). Data from zoledronic acid prescribing information [78].
bAdministration every 3 to 4 weeks. Data from ibandronate summary of product characteristics [80].
Abbreviations: ZOL, zoledronic acid; IBN, ibandronate.
Recently, exposed bone in the jaw—ONJ—has been reported as an uncommon event among patients with cancer whose treatment includes a monthly intravenous bisphosphonate [81–88]. Furthermore, ONJ has been reported in a very small number of patients who were receiving oral bisphosphonates for noncancer indications [82, 86, 87]. In one retrospective study, in 4,835 patients with multiple myeloma, BC, or PC treated with intravenous bisphosphonates, 0.7% developed ONJ [89]. In another retrospective study of 1,338 patients with BC treated with intravenous bisphosphonates, 16 (1.2%) developed ONJ [84]. In two recent prospective studies in patients with solid tumors or multiple myeloma (N = 1,776) and BC (N = 2,046), the incidence of ONJ in patients receiving denosumab was similar to that of patients receiving zoledronic acid (1.1% and 1.3%, respectively, in multiple myeloma or other solid tumors; 2.0% and 1.4%, respectively, in BC) [90, 91]. Identified risks for ONJ included dental extractions (HR = 53.19; p < .0001), treatment with zoledronic acid (HR = 15.01; p = .0037), and treatment with pamidronate followed by zoledronic acid (HR = 4.00; p = .078). Other studies have shown that dental extractions or surgery may be an inciting event for ONJ [92, 93]. Moreover, a recent study showed that preventive dental measures (such as those of Weitzman et al [94]) before the initiation of bisphosphonate treatment decrease the occurrence of ONJ (0.7% versus 3.0% for “after” versus “before” the introduction of preventive dental measures, per protocol analysis) [95]. Guidelines from recent multidisciplinary panels recommend that patients with cancer have preventive dental measures before the initiation of bisphosphonate therapy and be encouraged to maintain good oral hygiene [94, 96]. Patients may have routine dental hygiene and restorative procedures during bisphosphonate therapy, but the least invasive procedures should be used. A conservative approach to the management of ONJ is recommended and includes oral rinses, antibiotics, pain control, and limited debridement by dental professionals. Current trials have been designed to monitor oral health, and additional prospective data should be forthcoming.
Discussion
An important goal of therapy for bone metastases is to preserve patients' physical functioning and quality of life by preventing SREs after diagnosis of bone metastases. Intravenous bisphosphonates allow monitoring adherence to therapy. In all solid tumors, the benefits of bisphosphonates are likely to continue throughout an approximately 2-year period. In fact, the risk reductions for SREs were even greater during patients' second year of therapy compared with those for the overall patient population in the first year of treatment in a study of zoledronic acid. Thus, ASCO guidelines for use of bisphosphonates in patients with BC recommend that treatment be continued as long as it is tolerated or until there is a substantial decline in patients' performance status [17].
Other cancer patients with bone metastases who are benefiting from effective anticancer therapies may also benefit from bisphosphonate therapy. Indeed, although the current regulatory approval for zoledronic acid in the United States stipulates that patients with bone metastases from PC must have had disease progression despite ADT, the National Comprehensive Cancer Network guidelines encourage early intervention with intravenous bisphosphonates in men receiving ADT [15]. Moreover, benefits seem to be especially profound before the onset of pain. However, the optimal timing for the initiation of bisphosphonate treatment in this setting has not been established.
The recommended dose and schedule of bisphosphonate therapy have been established in registration trials, although alternate treatment schedules for bisphosphonate therapy are under investigation. Several nonstandard, intensive treatment regimens of intravenous ibandronate have been evaluated in patients with metastatic bone disease for acute bone pain relief, followed by the approved monthly infusions [97–99]. Results from these pilot studies suggest that alternate ibandronate schedule provided acute pain relief, and that more flexible or individualized bisphosphonate treatment may provide clinical benefits. However, the efficacy of this alternate dosing schedule has not been confirmed in a large, randomized clinical trial.
An ongoing study of the cost-effective use of bisphosphonates in metastatic bone disease, a comparison of bone marker–directed zoledronic acid therapy to a standard schedule (BisMARK), will determine whether using bone marker levels to direct zoledronic acid treatment is comparable with the current fixed schedule of every 3–4 weeks in patients with BC [100]. The primary comparison is the frequency and timing of SREs. Secondary comparisons include quality of life and pharmacoeconomics. Final trial results are not expected until 2013; however, interim results are eagerly awaited. Finally, the efficacy of monthly zoledronic acid will be compared with administration every 12 weeks for up to 1 year in patients with bone metastases from BC (OPTIMIZE 2). The primary endpoint is the time to first SRE [101]. These clinical studies are evaluating whether efficacy can be maintained using alternate dosing schedules that may increase the flexibility of treatment for patients without compromising efficacy. The alternate schedules could also reduce the number of infusions, thereby improving benefit-risk ratios. Currently, only the approved doses and schedules of bisphosphonate therapy have established efficacy and safety profiles, and any other regimens are investigational.
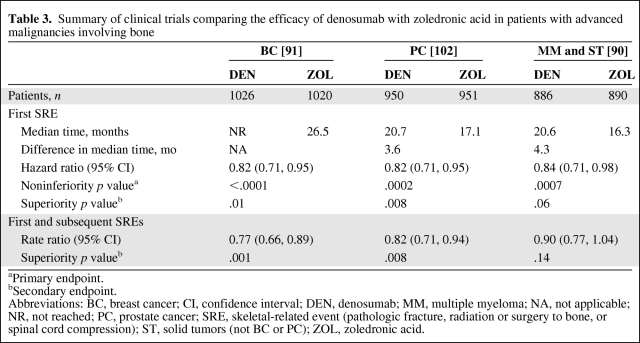
In addition to bisphosphonates, several phase III clinical trials have examined the efficacy of denosumab, a recombinant human monoclonal IgG2 antibody against the receptor activator of nuclear factor-κB ligand, for prevention of SREs in patients with bone lesions from cancer (Table 3) [90, 91, 102]. Overall, the results from three trials have shown that monthly denosumab (120 mg subcutaneous) achieved the primary endpoint of statistical noninferiority to monthly zoledronic acid (4 mg intravenous) for time to first SRE in patients with BC [91], PC [102], and multiple myeloma and other solid tumors (not BC or PC) [90], and was statistically superior to zoledronic acid in the secondary endpoints in the first two studies. Nevertheless, there was no statistical difference between denosumab and zoledronic acid for overall survival, disease progression, or bone pain improvement. Currently, in the oncology setting, the European Medicines Agency (EMA) has approved denosumab (60 mg subcutaneous every 6 months) in the treatment of bone loss associated with hormone ablation in men with PC at increased risk of fractures (defined as >70 years or <70 years with a BMD T-score at the lumbar spine, total hip, or femoral neck less than −1.0, or a history of an osteoporotic fracture) [103], based on a significant decrease in vertebral fracture risk in the Hormone Ablation Bone Loss Trial (HALT)-PC trial [104]. Denosumab also has been approved for the treatment of postmenopausal osteoporosis [103] and has shown activity for treatment of bone loss associated with AI therapy in postmenopausal women with breast cancer based on the HALT-BC trial [105]. Other oncology indications for denosumab are under review by the FDA and EMA.
Table 3.
Summary of clinical trials comparing the efficacy of denosumab with zoledronic acid in patients with advanced malignancies involving bone
aPrimary endpoint.
bSecondary endpoint.
Abbreviations: BC, breast cancer; CI, confidence interval; DEN, denosumab; MM, multiple myeloma; NA, not applicable; NR, not reached; PC, prostate cancer; SRE, skeletal-related event (pathologic fracture, radiation or surgery to bone, or spinal cord compression); ST, solid tumors (not BC or PC); ZOL, zoledronic acid.
The role of the different antiresorptive agents in oncology is likely to evolve with the emergence of denosumab and the maturation of clinical trials investigating the potential anticancer benefits of bisphosphonates, especially zoledronic acid.
Acknowledgments
Financial support for research and medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
Author Contributions
Conception/Design: Matti Aapro, Fred Saad
Provision of study material or patients: Matti Aapro, Fred Saad, Luis Costa
Collection and/or assembly of data: Matti Aapro, Fred Saad
Data analysis and interpretation: Matti Aapro, Fred Saad, Luis Costa
Manuscript writing: Matti Aapro
Final approval of manuscript: Matti Aapro, Fred Saad, Luis Costa
We thank Tamalette Loh, Ph.D., ProEd Communications, Inc., for her medical editorial assistance, for copyediting (grammatical assistance and stylistic suggestions), and for production assistance (assembling figures and tables).
References
- 1.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 2.Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski M, Rosen LS, Gordon D, et al. Zoledronic acid is superior to pamidronate in patients with breast cancer and multiple myeloma who are at high risk for skeletal complications [poster]. Presented at: Primary Therapy of Early Breast Cancer 9th International Conference; January 26–29, 2005; St. Gallen, Switzerland. Abstract 107. [Google Scholar]
- 6.Major PP, Cook R. Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am J Clin Oncol. 2002;25(suppl 1):S10–S18. doi: 10.1097/00000421-200212001-00003. [DOI] [PubMed] [Google Scholar]
- 7.Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16:579–584. doi: 10.1093/annonc/mdi122. [DOI] [PubMed] [Google Scholar]
- 8.Weinfurt KP, Castel LD, Li Y, et al. Health-related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Med Care. 2004;42:164–175. doi: 10.1097/01.mlr.0000108746.69256.45. [DOI] [PubMed] [Google Scholar]
- 9.Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 10.Mike S, Harrison C, Coles B, et al. Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst Rev. 2006:CD005247. doi: 10.1002/14651858.CD005247.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 12.Bagi CM. Targeting of therapeutic agents to bone to treat metastatic cancer. Adv Drug Deliv Rev. 2005;57:995–1010. doi: 10.1016/j.addr.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Gordon DH. Efficacy and safety of intravenous bisphosphonates for patients with breast cancer metastatic to bone: a review of randomized, double-blind, phase III trials. Clin Breast Cancer. 2005;6:125–131. doi: 10.3816/CBC.2005.n.014. [DOI] [PubMed] [Google Scholar]
- 14.Saad F, Karakiewicz P, Perrotte P. The role of bisphosphonates in hormone-refractory prostate cancer. World J Urol. 2005;23:14–18. doi: 10.1007/s00345-004-0472-2. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: prostate cancer. V. 1.2010. [Accessed March 9, 2010]. Available at http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf.
- 16.Aus G, Abbou CC, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2005;48:546–551. doi: 10.1016/j.eururo.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Berenson JR, Hillner BE, Kyle RA, et al. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002;20:3719–3736. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Green JR. Bisphosphonates: preclinical review. The Oncologist. 2004;9(suppl 4):3–13. doi: 10.1634/theoncologist.9-90004-3. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JR, Williams G, Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol. 2003;21:1404–1411. doi: 10.1200/JCO.2003.08.072. [DOI] [PubMed] [Google Scholar]
- 21.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 22.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 23.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 24.Coleman RE, Rosen L, Gleason DM, et al. Zoledronic acid has broad long-term efficacy in reducing skeletal complications in patients with bone metastases from breast cancer, prostate cancer, and other solid tumors [poster]. Presented at: What Is New In Bisphosphonates? Seventh Workshop on Bisphosphonates-From the Laboratory to the Patient; March 24–26, 2004; Davos, Switzerland. Abstract 65. [Google Scholar]
- 25.Pavlakis N, Schmidt RL, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev. 2005:CD003474. doi: 10.1002/14651858.CD003474.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Body JJ, Diel IJ, Lichinitzer M, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer. 2004;90:1133–1137. doi: 10.1038/sj.bjc.6601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Body JJ, Diel IJ, Lichinitser MR, et al. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol. 2003;14:1399–1405. doi: 10.1093/annonc/mdg367. [DOI] [PubMed] [Google Scholar]
- 28.Paterson AHG, Powles TJ, Kanis JA, et al. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol. 1993;11:59–65. doi: 10.1200/JCO.1993.11.1.59. [DOI] [PubMed] [Google Scholar]
- 29.Tubiana-Hulin M, Beuzeboc P, Mauriac L, et al. [Double-blinded controlled study comparing clodronate versus placebo in patients with breast cancer bone metastases] Bull Cancer. 2001;88:701–707. [PubMed] [Google Scholar]
- 30.Robertson AG, Reed NS, Ralston SH. Effect of oral clodronate on metastatic bone pain: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:2427–2430. doi: 10.1200/JCO.1995.13.9.2427. [DOI] [PubMed] [Google Scholar]
- 31.Jagdev SP, Purohit P, Heatley S, et al. Comparison of the effects of intravenous pamidronate and oral clodronate on symptoms and bone resorption in patients with metastatic bone disease. Ann Oncol. 2001;12:1433–1438. doi: 10.1023/a:1012506426440. [DOI] [PubMed] [Google Scholar]
- 32.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 33.Saad F. Clinical benefit of zoledronic acid for the prevention of skeletal complications in advanced prostate cancer. Clin Prostate Cancer. 2005;4:31–37. doi: 10.3816/cgc.2005.n.009. [DOI] [PubMed] [Google Scholar]
- 34.Small EJ, Smith MR, Seaman JJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277–4284. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 35.Major PP, Lipton A, Berenson J, et al. Oral bisphosphonates: a review of clinical use in patients with bone metastases. Cancer. 2000;88:6–14. [PubMed] [Google Scholar]
- 36.Lipton A, Zheng M, Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. 2003;98:962–969. doi: 10.1002/cncr.11571. [DOI] [PubMed] [Google Scholar]
- 37.Sabino MAC, Mantyh PW. Pathophysiology of bone cancer pain. J Support Oncol. 2005;3:15–24. [PubMed] [Google Scholar]
- 38.Bundred NJ. Aromatase inhibitors and bone health. Curr Opin Obstet Gynecol. 2009;21:60–67. doi: 10.1097/GCO.0b013e32831da80e. [DOI] [PubMed] [Google Scholar]
- 39.Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69:73–82. doi: 10.1016/j.critrevonc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Vehmanen L, Saarto T, Elomaa I, et al. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37:2373–2378. doi: 10.1016/s0959-8049(01)00317-3. [DOI] [PubMed] [Google Scholar]
- 41.Greenspan SL, Brufsky A, Lembersky BC, et al. Risedronate prevents bone loss in breast cancer survivors: a 2-year, randomized, double-blind, placebo-controlled clinical trial. J Clin Oncol. 2008;26:2644–2652. doi: 10.1200/JCO.2007.15.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delmas PD, Balena R, Confravreux E, et al. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: a double-blind, placebo-controlled study. J Clin Oncol. 1997;15:955–962. doi: 10.1200/JCO.1997.15.3.955. [DOI] [PubMed] [Google Scholar]
- 43.Hines SL, Mincey BA, Sloan JA, et al. Phase III randomized, placebo-controlled, double-blind trial of risedronate for the prevention of bone loss in premenopausal women undergoing chemotherapy for primary breast cancer. J Clin Oncol. 2009;27:1047–1053. doi: 10.1200/JCO.2008.19.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lester JE, Dodwell D, Purohit OP, et al. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res. 2008;14:6336–6342. doi: 10.1158/1078-0432.CCR-07-5101. [DOI] [PubMed] [Google Scholar]
- 45.Fuleihan G, Salamoun M, Mourad Y, et al. Pamidronate in the prevention of chemotherapy-induced bone loss in premenopausal women with breast cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90:3209–3214. doi: 10.1210/jc.2004-1444. [DOI] [PubMed] [Google Scholar]
- 46.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9:840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 47.Bundred NJ, Campbell ID, Davidson N, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer. 2008;112:1001–1010. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 48.Pant S, Shapiro CL. Aromatase inhibitor-associated bone loss: clinical considerations. Drugs. 2008;68:2591–2600. doi: 10.2165/0003495-200868180-00005. [DOI] [PubMed] [Google Scholar]
- 49.Saad F, Adachi JD, Brown JP, et al. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26:5465–5476. doi: 10.1200/JCO.2008.18.4184. [DOI] [PubMed] [Google Scholar]
- 50.Coleman RE, Body JJ, Gralow JR, et al. Bone loss in patients with breast cancer receiving aromatase inhibitors and associated treatment strategies. Cancer Treat Rev. 2008;34(suppl 1):S31–S42. doi: 10.1016/j.ctrv.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Brufsky AM. Cancer treatment-induced bone loss: pathophysiology and clinical perspectives. The Oncologist. 2008;13:187–195. doi: 10.1634/theoncologist.2007-0152. [DOI] [PubMed] [Google Scholar]
- 52.Hadji P, Body JJ, Aapro MS, et al. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann Oncol. 2008;19:1407–1416. doi: 10.1093/annonc/mdn164. [DOI] [PubMed] [Google Scholar]
- 53.Reid DM, Doughty J, Eastell R, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(suppl 1):S3–S18. doi: 10.1016/j.ctrv.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19:420–432. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 55.Costa L, Chen Y-M. Breast cancer patients without pain are at risk for skeletal-related events and may have better outcomes with zoledronic acid compared with pamidronate [abstract] Breast Cancer Res Treat. 2006;100(suppl 1):S62. Abstract 1071. [Google Scholar]
- 56.Saad F, Eastham J, McKiernan J, et al. Long-term reduction of bone pain and skeletal morbidity with zoledronic acid in patients with prostate cancer and bone metastases [poster]. Presented at: XXth European Association of Urology Congress; March 16–19, 2005; Istanbul, Turkey. Abstract 572. [Google Scholar]
- 57.Shirina N, Coleman RE, Chen Y-M. Effect of the number of bone lesions on efficacy of zoledronic acid for prevention of skeletal-related events in patients with bone metastases from solid tumors [abstract] J Clin Oncol. 2006;24(suppl):475s. Abstract 8529. [Google Scholar]
- 58.Aapro MS, Shirina N. Zoledronic acid is superior to pamidronate in patients with breast cancer or multiple myeloma who have had previous skeletal complications [poster]. Presented at: Multinational Association of Supportive Care in Cancer (MASCC)/International Society for Oral Oncology (ISOO) 18th International Symposium; June 22–24, 2006; Toronto, Ontario, Canada. Abstract 1625. [Google Scholar]
- 59.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34:453–475. doi: 10.1016/j.ctrv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Brufsky AM. Bone health issues in women with early-stage breast cancer receiving aromatase inhibitors. Curr Oncol Rep. 2008;10:18–26. doi: 10.1007/s11912-008-0005-z. [DOI] [PubMed] [Google Scholar]
- 61.Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19:2007–2011. doi: 10.1093/annonc/mdn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026] Breast Cancer Res. 2006;8:R13. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ha TC, Li H. Meta-analysis of clodronate and breast cancer survival. Br J Cancer. 2007;96:1796–1801. doi: 10.1038/sj.bjc.6603661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 65.Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol. 1999;17:846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 66.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 67.Eidtmann H, Bundred N, de Boer R, et al. The effect of zoledronic acid on aromatase inhibitor associated bone loss in postmenopausal women with early breast cancer receiving letrozole: 36 months follow-up of ZO-FAST [abstract]. Presented at: 31st Annual San Antonio Breast Cancer Symposium; December 10–14, 2008; San Antonio, TX. Abstract 44. [Google Scholar]
- 68.Coleman R, Bundred N, de Boer R, et al. Impact of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST, ZO-FAST, and E-ZO-FAST [poster]. Presented at: 32nd Annual San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, TX. Abstract 4082. [Google Scholar]
- 69.Holen I, Coleman RE. Bisphosphonates as treatment of bone metastases. Curr Pharm Des. 2010;16(11):1262–1271. doi: 10.2174/138161210791034003. [DOI] [PubMed] [Google Scholar]
- 70.Saad F, Chen Y-M, Gleason DM, et al. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clin Genitourin Cancer. 2007;5:390–396. doi: 10.3816/CGC.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 71.Zheng M, Rosen L, Gordon D, et al. Continuing benefit of zoledronic acid for the prevention of skeletal complications in breast cancer patients with bone metastases [poster]. Presented at: Primary Therapy of Early Breast Cancer 9th International Conference; January 26–29, 2005; St. Gallen, Switzerland. Abstract 104. [Google Scholar]
- 72.Saad F, Hirsh V, Rosen L, et al. Continuing benefit of zoledronic acid in patients with bone lesions from multiple myeloma, breast cancer, or prostate cancer who are at high risk for skeletal complications [poster]. Presented at: VI International Meeting on Cancer Induced Bone Disease; December 10–14, 2006; San Antonio, Texas. Abstract 130. [Google Scholar]
- 73.von Moos R. Bisphosphonate treatment recommendations for oncologists. The Oncologist. 2005;10(suppl 1):19–24. doi: 10.1634/theoncologist.10-90001-19. [DOI] [PubMed] [Google Scholar]
- 74.Conte P, Guarneri V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist. 2004;9(suppl 4):28–37. doi: 10.1634/theoncologist.9-90004-28. [DOI] [PubMed] [Google Scholar]
- 75.Cramer JA, Lynch NO, Gaudin AF, et al. The effect of dosing frequency on compliance and persistence with bisphosphonate therapy in postmenopausal women: a comparison of studies in the United States, the United Kingdom, and France. Clin Ther. 2006;28:1686–1694. doi: 10.1016/j.clinthera.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Hoer A, Goethe H, Barghout V, et al. Low persistency with oral bisphosphonates in cancer patients [poster]. Presented at: 5th European Oncology Nursing Society Spring Convention; April 20–22, 2006; Innsbruck, Austria. Abstract 2. [Google Scholar]
- 77.Mangiapane S, Hoer A, Gothe H, et al. Higher persistency with i.v. bisphosphonates in patients with bone metastasis [abstract] J Clin Oncol. 2006;24(suppl):698s. Abstract 18623. [Google Scholar]
- 78.East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2005. Zometa® [package insert] [Google Scholar]
- 79.West Sussex, UK: Novartis Europharm Limited; 2006. Zometa 4 mg powder and solvent for solution for infusion [summary of product characteristics] [Google Scholar]
- 80.Welwyn Garden City, UK: Roche Registration Limited; 2007. Bondronat 2 mg and 6 mg concentrate for solution for infusion [summary of product characteristics] [Google Scholar]
- 81.Tanvetyanon T, Stiff PJ. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17:897–907. doi: 10.1093/annonc/mdj105. [DOI] [PubMed] [Google Scholar]
- 82.Farrugia MC, Summerlin DJ, Krowiak E, et al. Osteonecrosis of the mandible or maxilla associated with the use of new generation bisphosphonates. Laryngoscope. 2006;116:115–120. doi: 10.1097/01.mlg.0000187398.51857.3c. [DOI] [PubMed] [Google Scholar]
- 83.Fusco V, Baraldi A, Loidoris A, et al. Jaw osteonecrosis associated with intravenous bisphosphonate: is incidence reduced after adoption of dental preventive measures? J Oral Maxillofac Surg. 2009;67:1775. doi: 10.1016/j.joms.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 84.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanat O, Ozet A, Arpaci F, et al. Bisphosphonate-associated osteonecrosis of the jaws: case reports and analysis of 184 cases [abstract] J Clin Oncol. 2006;24(suppl):695s. Abstract 18595. [Google Scholar]
- 86.Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Nase JB, Suzuki JB. Osteonecrosis of the jaw and oral bisphosphonate treatment. J Am Dent Assoc. 2006;137:1115–1119. doi: 10.14219/jada.archive.2006.0350. quiz 1169–1170. [DOI] [PubMed] [Google Scholar]
- 88.Wallace EM, Quintyne KI, Cantwell BM, et al. Osteonecrosis of the jaw associated with intravenous bisphosphonate therapy—a single institution experience [abstract] J Clin Oncol. 2006;24(suppl):692s. Abstract 18553. [Google Scholar]
- 89.Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. The Oncologist. 2008;13:911–920. doi: 10.1634/theoncologist.2008-0091. [DOI] [PubMed] [Google Scholar]
- 90.Henry D, von Moos R, Vadhan-Raj S, et al. A double-blind, randomized study of denosumab versus zoledronic acid for the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma [abstract LBA20] Eur J Cancer Suppl. 2009;7:12. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 91.Stopeck A, Body JJ, Fujiwara Y, et al. Denosumab versus zoledronic acid for the treatment of breast cancer patients with bone metastases: results of a randomized phase 3 study [abstract] Eur J Cancer Suppl. 2009;7:2. Abstract 2LBA. [Google Scholar]
- 92.Abu-Id MH, Warnke PH, Gottschalk J, et al. “Bis-phossy jaws” - high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg. 2008;36:95–103. doi: 10.1016/j.jcms.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 93.King AE, Umland EM. Osteonecrosis of the jaw in patients receiving intravenous or oral bisphosphonates. Pharmacotherapy. 2008;28:667–677. doi: 10.1592/phco.28.5.667. [DOI] [PubMed] [Google Scholar]
- 94.Weitzman R, Sauter N, Eriksen EF, et al. Critical review: updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients—May 2006. Crit Rev Oncol Hematol. 2007;62:148–152. doi: 10.1016/j.critrevonc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 95.Ripamonti CI, Maniezzo M, Campa T, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20:137–145. doi: 10.1093/annonc/mdn526. [DOI] [PubMed] [Google Scholar]
- 96.Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Practice. 2006;2:7–14. doi: 10.1200/jop.2006.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pendharkar D, Goyal H. Loading dose ibandronate in rapid pain management of metastatic bone disease (MBD) [abstract] J Clin Oncol. 2006;24(suppl):694s. Abstract 18580. [Google Scholar]
- 98.Mancini I, Dumon JC, Body J-J. Efficacy and safety of ibandronate in the treatment of opioid-resistant bone pain associated with metastatic bone disease: a pilot study. J Clin Oncol. 2004;22:3587–3592. doi: 10.1200/JCO.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 99.Heidenreich A, Elert A, Hofmann R. Ibandronate in the treatment of prostate cancer associated painful osseous metastases. Prostate Cancer Prostatic Dis. 2002;5:231–235. doi: 10.1038/sj.pcan.4500574. [DOI] [PubMed] [Google Scholar]
- 100.National Cancer Research Network. BISMARK Cost-effective use of BISphosphonates in metastatic bone disease—a comparison of bone MARKer directed zoledronic acid therapy to a standard schedule—the BISMARK trial. [Accessed March 9, 2010]. Available at http://pfsearch.ukcrn.org.uk/StudyDetail.aspx?TopicID&StudyID=1737.
- 101.U.S. National Institutes of Health. A study of the continued efficacy and safety of zoledronic acid in patients with documented bone metastases from breast cancer. [Accessed March 9, 2010]. Available at http://www.clinicaltrials.gov/ct/show/NCT00320710?order=1.
- 102.Fizazi K, Carducci MA, Smith MR, et al. A randomized phase III trial of denosumab versus zoledronic acid in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2010;28:18s. Abstract LBA4507. [Google Scholar]
- 103.European Medicines Agency. Prolia summary of product characteristics. [Accessed July 6, 2010]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001120/WC500093526.pdf. Published May 26, 2010.
- 104.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]