This review evaluates the available data to help guide patients and caregivers when developing treatment plans for women diagnosed with breast cancer during pregnancy.
Keywords: Breast cancer, Pregnancy, Chemotherapy, Prognosis, Birth defects, Teratogenicity
Abstract
The treatment of breast cancer diagnosed during pregnancy presents a challenging situation for the patient, family, and caregivers. Case series have demonstrated the efficacy and safety of using anthracycline-based chemotherapy during the second and third trimesters. Additionally, patients should be seen, evaluated, and treated in a multidisciplinary setting with facilitated communication among the medical oncologist, surgical oncologist, obstetrician, radiation oncologist, pathologist, and radiologist. This review details the available data regarding the diagnosis and management of the pregnant breast cancer patient.
Introduction
A simultaneous diagnosis of breast cancer during pregnancy adds complexity to cancer treatment recommendations. However, available data demonstrate that the pregnant breast cancer patient can receive timely standard therapies in a multidisciplinary environment with a medical oncologist, surgical oncologist, radiation oncologist, and maternal–fetal medicine specialist participating in her care. Much of these data are retrospective in nature, from case reports and small case series. However, because more women are delaying childbirth, the incidence of breast cancer diagnosed during pregnancy, and women choosing treatment during pregnancy rather than termination of pregnancy or treatment delays, is expected to increase. This review evaluates the available data to help guide patients and caregivers when developing treatment plans for women diagnosed with breast cancer during pregnancy.
Epidemiology
There are several estimates of the frequency of breast cancer diagnosed during pregnancy. Smith et al. [1] report, from the California Obstetrics registry for 1992–1997, that the incidence is 13 cases per 100,000 live births. This registry, however, tracks pregnancies that went on to delivery and therefore may underestimate incidence if women chose to terminate a pregnancy. The Surveillance Epidemiology and End Results estimate of breast cancer diagnosed in women aged ≤44 years is 215.8 per 100,000 [2]. Because women delay childbirth into their third and fourth decades [3, 4], the incidence of breast cancer diagnosed during pregnancy and in the immediate postpartum period appears to be increasing. A study from the Swedish National Health Registry demonstrated that the incidence of pregnancy-associated breast cancer (PABC), which encompasses diagnoses during pregnancy and within 1 year postpartum, increased between 1963 and 2002 from 16.0 to 37.4 per 100,000 deliveries [5].
Diagnosis and Staging of Breast Cancer During Pregnancy
Pregnancy causes multiple changes within the breast, including increased glandularity, size, and density of the breast tissue, which may obscure a mass [6]. Masses may also be assumed to be related to the pregnancy; however, in a pregnant patient, a new palpable mass that does not resolve within 2 weeks should be investigated further [7].
Imaging
Mammography has been successfully used during pregnancy, when done with proper fetal shielding. The fetal radiation exposure is estimated to be 0.4 mrad, which is less than the 5 rad level known to be associated with fetal malformations [8–10]. However, the increased density of the breast during pregnancy may lower the sensitivity of mammography. Ultrasonography is an attractive choice because there is no radiation exposure risk for the fetus and it is able to distinguish solid from cystic structures. Yang and colleagues were successfully able to identify 100% of breast masses and axillary masses in 18 of 20 women diagnosed with breast cancer during pregnancy [11]. Breast magnetic resonance imaging (MRI) requires the use of gadolinium for best imaging of the breast. There have been studies in animal models showing that gadolinium crosses the placenta and is associated with fetal abnormalities [12]. However, there are emerging, but scant, data on the safe use of gadolinium during pregnancy for nonbreast MRI [13–15].
Until more safety data are available, the use of gadolinium-enhanced MRI should be reserved until after delivery. The interpretation of breast MRI during pregnancy may also be problematic. Imaging of the breast mass should include tumor measurements and nodal basin evaluations as well as clip placement at the time of biopsy if the patient is going to receive preoperative chemotherapy.
Biopsy
Imaging and biopsies can be performed at a single session as appropriate. Although there has been a case report of a milk fistula with core needle biopsy [16], Dominici et al. [17] reported on 67 patients with a diagnosis of breast cancer during pregnancy, 35 of whom had core needle biopsies with no complications. It is important for the pathologist to be aware that the biopsy specimen is from a pregnant breast. As in nonpregnant patients, estrogen receptor, progesterone receptor, and human epidermal growth factor receptor (HER)-2 should be assessed. The stage of disease and tumor characteristics guide the treatment plan.
Staging Evaluations During Pregnancy
Obtaining maximal information about disease extent in the mother while limiting fetal exposure to radiation is the goal when choosing staging evaluations. A complete history and physical examination, CBC, and comprehensive metabolic panel should be done prior to initiating therapy. Consideration of an echocardiogram prior to anthracycline-based chemotherapy is warranted. Because the major sites of metastatic disease are bone, lung, and liver, guidelines have been established for ordering liver ultrasound, MRI without contrast of the spine, and chest x-ray with fetal shielding to evaluate for metastatic disease in women with suspected stage II or greater cancers [7]. Computed Tomography scans and bone scans are not recommended for routine staging studies in the pregnant patient because of concerns of fetal radiation exposure. Sites concerning for metastatic disease should be biopsied whenever possible and safe to confirm distant metastases.
An evaluation of the fetus should be made by an obstetrician prior to initiation of therapy. Additional ultrasounds to evaluate fetal growth, amniotic fluid, and placental function should be done at regular intervals if the patient is receiving chemotherapy.
Locoregional Therapy
Surgery During Pregnancy
Several case series have evaluated the risk to the fetus during surgery. Mazze and Källén reported that when any surgical intervention was completed in 5,406 pregnant women, the observed risk for fetal malformation was not greater than in 720,000 nonpregnant controls [18]. A higher incidence of low birth weight in this population was attributed to the underlying cause of the emergency surgery during pregnancy. Duncan et al. [19] reported on 2,565 women who underwent surgery during pregnancy. They demonstrated that the incidence of fetal abnormalities was not higher than in pregnant women who did not undergo surgery.
Dominici et al. [17] reported on 67 women who were diagnosed with breast cancer during pregnancy at The University of Texas M.D. Anderson Cancer Center. All 67 women received chemotherapy during pregnancy; 45% received preoperative chemotherapy. Forty-seven women underwent mastectomy and 20 underwent breast-conserving therapy. Of these 67 women, six had postoperative complications, all of which were managed as an outpatient. Four women had had a modified radical mastectomy and were treated for postoperative cellulitis. Of the two women who underwent segmental mastectomies, one had an axillary abscess and the other had a postoperative hematoma. The authors concluded that both mastectomy and breast-conserving surgery were feasible with minimal postoperative complications.
Recently, Cardonick et al. [20] reported their series of women who participated in a voluntary registry of PABC patients. One hundred thirty were diagnosed with breast cancer during pregnancy. Ninety-five women underwent surgery during pregnancy—38 in the first trimester, 48 in the second trimester, and nine in the third trimester. Fifty-four women had a mastectomy, 34 had a lumpectomy, and 15 had an excisional biopsy with no further surgery. Three of the patients miscarried after first trimester surgery (7%). Postoperative complications were not described.
Limited data exist regarding the use of sentinel lymph node biopsies during pregnancy. In the M.D. Anderson surgical cohort, it was noted that sentinel lymph node biopsy was done in three women during pregnancy. There were no noted complications [17, 21]. The estimated radiation exposure to the fetus with the use of technetium is low at 4.3 mGy [21]. There is some concern regarding the use of the isosulfan blue dye because of unknown fetal effects and the risk for anaphylaxis for the patient [22]. However, anaphylactic risk has been significantly decreased since the addition of preprocedure steroid use [23]. Cardonick et al. [20] described sentinel lymph node biopsy in 30 patients, most of whom did not receive isosulfan blue dye. Of this group, two of the patients who had the procedure in the first trimester had a miscarriage and three children had a low birth weight. Two children had congenital malformations, asymptomatic pulmonary artery fistula, and hydrocephalus, but they had other drug exposures during the pregnancy. More safety data on sentinel lymph node biopsies are warranted prior to making this a recommended procedure during pregnancy.
Radiation Therapy
Recommendations for the use of radiation therapy should be done as per guidelines based on the patient's stage of disease and tumor characteristics. Radiation during pregnancy risks exposure of the fetus to the radiation field [10]. Because surgery and the initiation of systemic therapies are often completed prior to the initiation of radiation, radiation therapy can be delayed until after delivery.
Systemic Therapies
Chemotherapy
The decision to use chemotherapy in a pregnant breast cancer patient should depend upon the patient's disease stage and tumor characteristics. Generally these are the same indications as in a nonpregnant breast cancer patient. Most chemotherapy agents are rated pregnancy category D (Table 1). Because treating cancer during pregnancy is a relatively rare situation, the available data are limited and consist of case reports, case series, and retrospective registries, and there can be no account or knowledge of unreported cases. From the data that are available, emerging trends in the systemic treatment of breast cancer during pregnancy are reviewed.
Table 1.
U.S. Food and Drug Administration pregnancy category definitions
Reports of fetal malformations have been in the range of 14%–19% when chemotherapy has been given in the first trimester. The reported frequency decreases to 1.3% during the second and third trimesters [24]. Cardonick et al. [20] described 130 women diagnosed with breast cancer during pregnancy. Of these, 104 received chemotherapy during pregnancy. They reported a fetal malformation rate of 3.8%, which was not higher than that reported in the general population. Chemotherapy is typically administered without dose modifications in the pregnant woman and adjusted and dosed per actual weight and body surface area [25, 26].
Anthracyclines
Multiple case series report the use of anthracycline-based chemotherapy during the second and third trimesters of pregnancy. The M.D. Anderson Cancer Center has been prospectively treating women diagnosed with breast cancer during pregnancy with a standardized protocol of 5-fluorouracil (500 mg/m2 i.v. on days 1 and 4), doxorubicin (50 mg/m2 given by continuous infusion over 72 hours), and cyclophosphamide (500 mg/m2 given i.v. on day 1) the FAC regimen, since 1989 [26, 27]. Premedications include dexamethasone, lorazepam, and ondansetron for nausea control. Hahn et al. [26] reported on 57 women treated up to 2006, and the median number of FAC cycles given during pregnancy was four (range, 1–6). The mean gestational age at delivery was reported as 37 weeks. Chemotherapy was held after the 35th week in order to avoid a hematologic nadir at the time of delivery. All the women had live births. One immediate maternal postpartum death was reported, resulting from the complication of a pulmonary embolus after a cesarean delivery. Of the children, three had reported congenital malformations. One neonate was born with Down syndrome, one was born with ureteral reflux, and a third was born with club foot.
Cardonick et al. [20] reported, from their registry cohort, on 104 women who received chemotherapy during pregnancy at a mean gestational age of 20.4 ± 5.4 weeks. Sixty-nine percent received an anthracycline and cyclophosphamide, but the dosing schedule and dosages were not described. Other regimens included FAC or 5-fluorouracil, epirubicin, and cyclophosphamide, and several included taxanes. Toxicity data collected showed very limited chemotherapy side effects, with the most common being neutropenia (n = 5), mouth ulcers (n = 3), and constipation, tachycardia, anaphylaxis, and cellulitis of the arm (each with n = 2). Fetal complications included intrauterine growth retardation (IUGR) in eight neonates, pulmonary complications at birth in five neonates, hyperbilirubinemia in three neonates, and placental complications in two pregnancies, and one child developed a severe autoimmune disorder and died at 13 months.
Other series include a French National Survey that retrospectively identified 20 women treated for breast cancer with chemotherapy during pregnancy. No consistent chemotherapy regimen was used. Two women received chemotherapy during the first trimester and both had a spontaneous abortion. Another neonate died at 8 days without the cause of death being determined [28]. Van Calsteren et al. [29] reported on 215 pregnant cancer patients, 99 of whom had breast cancer. Although there were no reported congenital malformations, there was a high rate of neonatal complications resulting from early delivery. Deliveries were induced in 71.7% of cases and 54.2% of deliveries were preterm in the entire cohort. Mir et al. [30] reported on a literature search of 50 women who received epirubicin-based chemotherapy during pregnancy. Two of the three women treated during the first trimester had a spontaneous abortion, and three of the 47 women treated during the second and third trimesters had complications, including significant fetal complications.
Ring et al. [31] evaluated 28 women for breast cancer during pregnancy: 24 received chemotherapy for early breast cancer and four received chemotherapy for metastatic cancer. Twelve of the women received cyclophosphamide, methotrexate, and 5-fluorouracil, with one spontaneous abortion. The remaining patients received anthracycline-based chemotherapy. Given that methotrexate is a known abortifacient, its use is contraindicated during pregnancy. Complications included IUGR and placental insufficiency, and two newborns experienced neonatal respiratory distress [31].
Taxanes
Use of taxanes (paclitaxel and docetaxel) during pregnancy has been described in several case series that include patients with breast and gynecological cancers. Often, the use of taxanes is delayed until after delivery because the anthracycline portion of the chemotherapy is frequently initiated during pregnancy as a result of more published experience with anthracyclines. Mir et al. [32] published a systematic review of the use of taxanes during pregnancy. They were able to identify taxane use in 40 patients. Of these, 21 received paclitaxel, 16 received docetaxel, and three received both. There were no reported congenital malformations, except for the possibility of pyloric stenosis in one infant who had been exposed to multiple chemotherapeutic agents [20]. There were no intrauterine deaths.
Concern of the effectiveness of taxanes during pregnancy has been raised because the cytochrome P-450 system is upregulated by 50%–100% during the third trimester of pregnancy, thus potentially increasing drug metabolism [33]. Additionally, Lycette et al. [34] reported a case in which pharmacokinetics were measured during pregnancy and showed a lower peak paclitaxel concentration and total exposure than in historical controls.
Biological Agents
Trastuzumab therapy is standard care for women with HER-2/neu overexpressing breast cancers. To date, there have been 11 case reports of its use during pregnancy [35–44]. In seven of these, trastuzumab was given for metastatic breast cancer, whereas two women had stage II cancer and one had stage III disease. No stage was identified in one woman who became pregnant during the year of maintenance trastuzumab and tamoxifen. Five of the pregnancies were found to have oligohydramnios and two had anhydramnios. Two of the fetuses were found to have renal dysfunction. Four pregnancies had no complications identified. One of the newborns developed respiratory failure, capillary leak syndrome, and enterocolitis and died from multiorgan failure at 21 weeks old [43]. Given the concern for oligo- and anhydramnios, trastuzumab therapy should be delayed until after delivery.
There has been only one report of lapatinib exposure in a woman who conceived while on lapatinib [45]. Lapatinib was discontinued after the first 11 weeks of pregnancy and the delivery was uncomplicated with a healthy newborn. Given the lack of data for lapatinib, its use is not recommended until after delivery.
Endocrine Therapy
Tamoxifen therapy is not recommended during pregnancy because it has been associated with birth defects. Although several case reports exist with tamoxifen exposure and healthy neonatal outcomes [46], it has also been associated with malformations in up to 20% of exposures, including Goldenhar's syndrome [47], ambiguous genitalia, vaginal bleeding, and spontaneous abortion [48–50]. Aromatase Inhibitors are contraindicated as single-agent endocrine therapy in premenopausal women.
Supportive Medications
Antiemetic agents, such as ondansetron and granisetron, are rated as pregnancy risk factor B and should be used to manage nausea in pregnant women receiving chemotherapy. Dexamethasone can also be used for anthracycline premedication for nausea prophylaxis [26]. For neutropenia prophylaxis, there are no randomized trials evaluating the usage of G-CSF in pregnant breast cancer patients. However, G-CSF has been used to treat neonatal neutropenia and/or sepsis [51, 52] and its use during pregnancy has been reported [53]. There are no available data regarding pegfilgastrim in pregnancy.
Prognosis
Several case series describe outcomes in pregnant patients. Conflicting conclusions may be the result of the reports including PABC patients diagnosed both during and within 1 year after delivery. Advanced stages of diagnosis as well as delays in treatment initiation may influence the outcomes of these analyses as well. Ribeiro et al. [54] detailed worse outcomes in 178 women with PABC, 121 diagnosed during pregnancy. Most women in that series delayed therapy until after delivery and there was no information regarding chemotherapy given during pregnancy. Tretli et al. [55] reported on 35 women diagnosed with PABC, 20 of whom were pregnant at the time of diagnosis. They were compared with nonpregnant women randomly assigned with two sets of controls. The estimate was a 3.1× higher risk for death in the PABC cohort. However, there is no description of the treatment received or treatment delay in these patients. Bonnier et al. [56] evaluated 154 women with PABC in a retrospective multicenter trial and compared them with non-PABC patients. They found that pregnancy was an adverse prognostic factor. However, in that series, chemotherapy was given to only 63.6% of the PABC patients, and the dosages, schedules, and delays in treatment initiation were not detailed.
More recent case series have described no significant difference. Beadle et al. [57] evaluated 652 women diagnosed with breast cancer at age ≤35 years who were either pregnant at the time of diagnosis or diagnosed within 1 year postpartum. There was no difference in locoregional recurrence, distant metastasis, or overall survival when compared with non-PABC controls. A Saudi-Arabian study evaluated 28 pregnant breast cancer patients matched by age and stage to nonpregnant controls and no difference in terms of relapse-free or overall survival was identified [58]. Other studies from Japan, Canada, and New Zealand also showed no differences in outcomes [6, 59, 60]. At the 2010 American Society of Clinical Oncology Annual Meeting, Murphy et al. [61] reported on 99 patients with PABC matched 1:2 with nonpregnant breast cancer patients by age at diagnosis and year of diagnosis. Of these, 36 were diagnosed during pregnancy and the rest were diagnosed within 1 year after delivery. They found that the PABC tumors had worse biological features, such as stage, grade, and a higher percentage of hormone receptor negativity; however, PABC was not found to be an independent predictor of worse overall survival on multivariate analysis. Therefore, when women receive timely standard therapy regimens there appears to be no significant difference in outcomes.
Outcomes in the Children Exposed to Chemotherapy in Utero
Data on long-term outcomes in children exposed to chemotherapy in utero for breast cancer are very limited. Hahn et al. [26] described 57 children exposed in utero to chemotherapy. Ten percent of these deliveries were complicated by reversible breathing difficulties in the newborns. One child developed a subarachnoid hemorrhage 2 days postpartum and recovered. One child had Down syndrome and two children had congenital malformations (bilateral ureteral reflux and club foot). At the time of the administration of the health survey, the ages of the children were in the range of 2–157 months, and overall all the children were doing well, with only two of them requiring special educational needs.
Cardonick et al. [20] reported from their voluntary registry of 113 women who continued their pregnancy, and the mean gestational age at delivery was 36 weeks. Eight of the women delivered infants with birth weights <10% for gestational age. There were pregnancy complications in 19 of the patients and a malformation rate of 3.8%, not significantly different from that in the general population [62]. Queisser-Luft et al. [62] estimated the rate of general congenital malformations to be 6.9% in a German birth registry for 1990–1998. Follow-up for 93 of the children was provided by the treating pediatricians, with a median age at follow-up of 41.8 months. Medical issues included speech delay in two children, gastrointestinal reflux, pneumonia, corneal abrasion, and IgA deficiency.
Additional long-term outcomes of children exposed to chemotherapy in utero were provided by Avilés et al. [63, 64], who followed 84 children whose mothers were given chemotherapy during pregnancy for a hematologic malignancy. They reported no significant cardiac, physical, neurologic, or psychological abnormalities. Reynoso et al. [65] described seven cases of eight children (one twin pregnancy) exposed to chemotherapy in utero for acute leukemia. One child in the twin pregnancy was born with multiple congenital abnormalities and eventually developed thyroid cancer and neuroblastoma. The remaining children had no reported abnormalities.
Recently, Zoccarato et al. [66] reported on a retrospective multicenter trial of 30 patients diagnosed with cancer during pregnancy, with 27 women who continued their pregnancy. Fifty-nine percent had cesarean deliveries, with 63% of the neonates with a low birth weight resulting from premature delivery. No excess in congenital malformations was noted.
Other Considerations
Termination of the Pregnancy
The decision to terminate a pregnancy during breast cancer is a highly personal decision to be made by an informed woman. A woman may be advised to terminate a pregnancy if it is felt that her life expectancy may not be longer than gestation, and historically it was often assumed that termination was warranted in all cases. One recent series described 30 of 130 patients who were advised to terminate their pregnancy. These recommendations did not change significantly between 1996 and 2003, but more recently the frequency of the recommendation for termination has been decreasing [20]. Most likely this is a result of more information becoming available regarding the similar prognosis for the pregnant patient and the safety of surgery and chemotherapy during pregnancy. In addition, termination of the pregnancy has not been shown to improve survival, and several reports suggest a trend toward shorter survival [67–69].
Lactation and Breast Feeding
Chemotherapy when completed prior to delivery has been shown to significantly decrease milk production, with one report estimating that only 55% of women would be able to successfully breast feed [20]. Additionally, chemotherapy has been shown to be excreted into the breast milk. Neutropenia has been described in a nursing infant whose mother was receiving cyclophosphamide [53, 70]. Therefore, pregnant breast cancer patients should be prepared to use formula after delivery, and breast feeding is contraindicated in women who are concomitantly receiving chemotherapy.
Hereditary Breast Cancer Syndromes
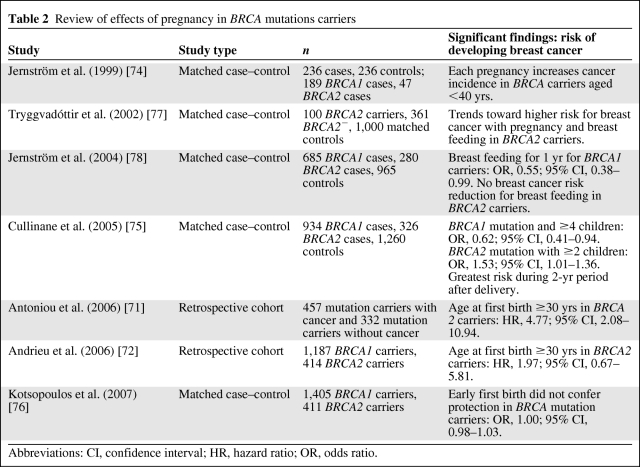
Women with strong family histories or known BRCA1 or BRCA2 mutations are more likely to develop breast cancer at a younger age, when pregnancy is more likely. There appears, however, to be a difference between BRCA1 and BRCA2 mutations in relation to pregnancy. Antoniou et al. [71] evaluated 457 mutation carriers who developed breast cancer and compared them with 332 mutation carriers who did not develop cancer. The protective effects of pregnancy were seen only among carriers who were >40 years old. Increasing age at first birth was associated with a higher risk in BRCA2 carriers but not in BRCA1 carriers. Andrieu et al. [72] also demonstrated a similar higher breast cancer risk with later ages of parity in BRCA2 mutation carriers. Tryggvadottir et al. [73] determined that an increasing number of births decreased breast cancer risk in BRCA2− women but not in women with deleterious BRCA2 mutations. Jernström et al. [74] demonstrated, in a case-control study, that mutation carriers of both BRCA1 and BRCA2 had a higher risk for breast cancer with a higher number of births. Cullinane et al. [75] reported on 1,260 pairs of women with known BRCA1 and BRCA2 mutations and compared them with matched nonmutation carriers. Women with a greater number of children had a lower breast cancer risk if they had a BRCA1 mutation (odds ratio [OR], 0.62; 95% confidence interval [CI], 0.41–0.94), but BRCA2 mutation carriers appeared to have a greater risk with greater parity (OR, 1.53; 95% CI, 1.01–1.36). Also, in the 2-year period after a birth, BRCA2 carriers had a trend for a higher risk for developing breast cancer (OR, 1.70; 95% CI, 0.97–3.0). Still, Kotsopoulos et al. [76] reported on a multicenter trial in 11 different countries for 7,243 mutation carriers and matched controls showing no significant difference in cancer rates in BRCA mutation carriers when stratified for mutation, number of pregnancies, or age at first pregnancy. Also, the noted protective role of an early first pregnancy did not appear to confer the same protection in BRCA mutation carriers. (Table 2).
Table 2.
Review of effects of pregnancy in BRCA mutations carriers
Abbreviations: CI, confidence interval; HR, hazard ratio; OR, odds ratio.
Conclusion
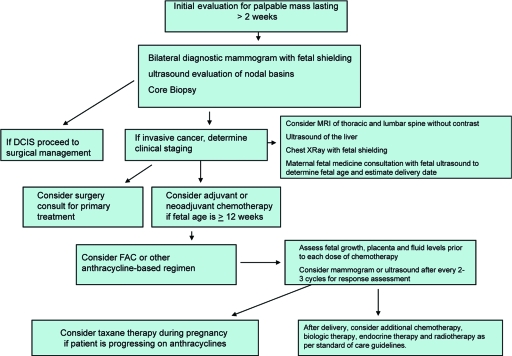
The treatment of breast cancer during pregnancy should proceed with a multidisciplinary approach and with direct communication among the treating oncologists and the obstetrician or maternal fetal specialists (Fig. 1). Increasingly data are becoming available concerning the safety and efficacy of standard treatments, and therefore these should be initiated with minimal delay in the pregnant breast cancer patient for optimal cancer control. Prospective evaluations and long-term follow-up of children are necessary, but to date there are no significant long-term health concerns identified in children exposed to chemotherapy in utero. In order to provide further information for this challenging clinical situation, further collaborations between registries and cancer centers is needed for long-term follow-up for both the patient and the children.
Figure 1.
Evaluation and management of breast cancer during pregnancy.
Abbreviations: DCIS, ductal carcinoma in situ; FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide; MRI, magnetic resonance imaging.
Author Contributions
Conception/Design: Jennifer K. Litton
Provision of study material or patients: Jennifer K. Litton, Richard Lee Theriault
Collection and/or assembly of data: Jennifer K. Litton
Data analysis and interpretation: Jennifer K. Litton
Manuscript writing: Jennifer K. Litton
Final approval of manuscript: Jennifer K. Litton, Richard Lee Theriault
References
- 1.Smith LH, Dalrymple JL, Leiserowitz GS, et al. Obstetrical deliveries associated with maternal malignancy in California, 1992 through 1997. Am J Obstet Gynecol. 2001;184:1504–1512. doi: 10.1067/mob.2001.114867. discussion 1512–1513. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review 1975–2007. Bethesda, MD: National Cancer Institute; [accessed November 5, 2010]. based on November 2009 SEER data submission, posted to the SEER web site, 2010. Available at http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 3.Stensheim H, Moller B, van Dijk T, et al. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: A registry-based cohort study. J Clin Oncol. 2009;27:45–51. doi: 10.1200/JCO.2008.17.4110. [DOI] [PubMed] [Google Scholar]
- 4.Martin JA, Kochanek KD, Strobino DM, et al. Annual summary of vital statistics–2003. Pediatrics. 2005;115:619–634. doi: 10.1542/peds.2004-2695. [DOI] [PubMed] [Google Scholar]
- 5.Andersson TM, Johansson AL, Hsieh CC, et al. Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol. 2009;114:568–572. doi: 10.1097/AOG.0b013e3181b19154. DOI: 10.1097/AOG.0b013e3181b19154. [DOI] [PubMed] [Google Scholar]
- 6.Ishida T, Yokoe T, Kasmu F, et al. Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: Analysis of case-control study in Japan. Jpn J Cancer Res. 1992;83:1143–1149. doi: 10.1111/j.1349-7006.1992.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loibl S, von Minckwitz G, Gwyn K, et al. Breast carcinoma during pregnancy. Cancer. 2006;106:237–246. doi: 10.1002/cncr.21610. [DOI] [PubMed] [Google Scholar]
- 8.Nicklas AH, Baker ME. Imaging strategies in the pregnant breast cancer patient. Semin Oncol. 2000;27:623–632. [PubMed] [Google Scholar]
- 9.Brent RL. The effect of embryonic and fetal exposure to x-ray, microwaves, and ultrasound: Counseling the pregnant and nonpregnant patient about these risks. Semin Oncol. 1989;16:347–368. [PubMed] [Google Scholar]
- 10.Mazonakis M, Varveris H, Damilakis J, et al. Radiation dose to conceptus resulting from tangential breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:386–391. doi: 10.1016/s0360-3016(02)04206-2. [DOI] [PubMed] [Google Scholar]
- 11.Yang WT, Dryden MJ, Gwyn K, et al. Imaging of breast cancer diagnosed and treated with chemotherapy during pregnancy. Radiology. 2006;239:52–60. doi: 10.1148/radiol.2391050083. [DOI] [PubMed] [Google Scholar]
- 12.Novak Z, Thurmond AS, Ross PL, et al. Gadolinium DTPA transplacental transfer and distribution in fetal tissue in rabbits. Invest Radiol. 1993;28:828–830. [PubMed] [Google Scholar]
- 13.DeSantis M, Lucchese A, DeCarolis S, et al. Metastatic breast cancer in pregnancy: First case of chemotherapy with docetaxel. Eur J Cancer Care. 2000;9:235–237. doi: 10.1046/j.1365-2354.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- 14.Birchard KR, Brown MA, Hyslop WB, et al. MRI of acute abdominal and pelvic pain in pregnant patients. Am J Roentgenol. 2005;184:452–458. doi: 10.2214/ajr.184.2.01840452. [DOI] [PubMed] [Google Scholar]
- 15.Webb JA, Thomsen HS, Morcos SK. Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol. 2005;15:1234–1240. doi: 10.1007/s00330-004-2583-y. [DOI] [PubMed] [Google Scholar]
- 16.Schackmuth EM, Harlow CL, Norton LW. Milk fistula: A complication after core breast biopsy. AJR Am J Roentgenol. 1993;161:961–962. doi: 10.2214/ajr.161.5.8273635. [DOI] [PubMed] [Google Scholar]
- 17.Dominici LS, Kuerer HM, Babiera G, et al. Wound complications from surgery in pregnancy-associated breast cancer (PABC) Breast Dis. 2010 Jun 1; doi: 10.3233/BD-2009-0289. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy: A registry study of 5405 cases. J Obstet Gynecol. 1989;161:1178–1185. doi: 10.1016/0002-9378(89)90659-5. [DOI] [PubMed] [Google Scholar]
- 19.Duncan PG, Pope WD, Cohen M, et al. Fetal risk of anesthesia and surgery during pregnancy. Anesthesiology. 1986;64:790–794. doi: 10.1097/00000542-198606000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Cardonick E, Dougherty R, Grana G, et al. Breast cancer during pregnancy: Maternal and fetal outcomes. Cancer J. 2010;16:76–82. doi: 10.1097/PPO.0b013e3181ce46f9. [DOI] [PubMed] [Google Scholar]
- 21.Keleher A, Wendt R, 3rd, Delpassand E, et al. The safety of lymphatic mapping in pregnant breast cancer patients using Tc-99m sulfur colloid. Breast J. 2004;10:492–495. doi: 10.1111/j.1075-122X.2004.21503.x. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery LL, Thorne AC, Van Zee KJ, et al. Isosulfan blue dye reactions during sentinel lymph node mapping for breast cancer. Anesth Analg. 2002;95:385–388. doi: 10.1097/00000539-200208000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Raut CP, Hunt KK, Akins JS, et al. Incidence of anaphylactoid reactions to isosulfan blue dye during breast carcinoma lymphatic mapping in patients treated with preoperative prophylaxis: Results of a surgical prospective clinical practice protocol. Cancer. 2005;104:692–699. doi: 10.1002/cncr.21226. [DOI] [PubMed] [Google Scholar]
- 24.Doll DC, Ringenberg QS, Yarbro JW. Antineoplastic agents and pregnancy. Semin Oncol. 1989;16:337–346. [PubMed] [Google Scholar]
- 25.Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5:283–291. doi: 10.1016/S1470-2045(04)01466-4. [DOI] [PubMed] [Google Scholar]
- 26.Hahn KM, Johnson PH, Gordon N, et al. Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer. 2006;107:1219–1226. doi: 10.1002/cncr.22081. [DOI] [PubMed] [Google Scholar]
- 27.Berry DL, Theriault RL, Holmes FA, et al. Management of breast cancer during pregnancy using a standardized protocol. J Clin Oncol. 1999;17:855–861. doi: 10.1200/JCO.1999.17.3.855. [DOI] [PubMed] [Google Scholar]
- 28.Giacalone P, Laffargue F, Bénos P. Chemotherapy for breast carcinoma during pregnancy A French national survey. Cancer. 1999;86:2266–2272. [PubMed] [Google Scholar]
- 29.Van Calsteren K, Heyns L, De Smet F, et al. Cancer during pregnancy: An analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J Clin Oncol. 2010;28:683–689. doi: 10.1200/JCO.2009.23.2801. [DOI] [PubMed] [Google Scholar]
- 30.Mir O, Berveiller P, Rouzier R, et al. Chemotherapy for breast cancer during pregnancy: Is epirubicin safe? Ann Oncol. 2008;19:1814–1815. doi: 10.1093/annonc/mdn553. [DOI] [PubMed] [Google Scholar]
- 31.Ring AE, Smith IE, Jones A, et al. Chemotherapy for breast cancer during pregnancy: An 18-year experience from five London teaching hospitals. J Clin Oncol. 2005;23:4192–4197. doi: 10.1200/JCO.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Mir O, Berveiller P, Goffinet F, et al. Taxanes for breast cancer during pregnancy: A systematic review. Ann Oncol. 2010;21:425–426. doi: 10.1093/annonc/mdp517. [DOI] [PubMed] [Google Scholar]
- 33.Anderson GD. Pregnancy-induced changes in pharmacokinetics: A mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 34.Lycette JL, Dul CL, Munar M, et al. Effect of pregnancy on the pharmacokinetics of paclitaxel: A case report. Clin Breast Cancer. 2006;7:342–344. doi: 10.3816/CBC.2006.n.049. [DOI] [PubMed] [Google Scholar]
- 35.Beale JM, Tuohy J, McDowell SJ. Herceptin (trastuzumab) therapy in a twin pregnancy with associated oligohydramnios. Am J Obstet Gynecol. 2009;201:e13–e14. doi: 10.1016/j.ajog.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Shrim A, Garcia-Bournissen F, Maxwell C, et al. Trastuzumab treatment for breast cancer during pregnancy. Can Fam Physician. 2008;54:31–32. [PMC free article] [PubMed] [Google Scholar]
- 37.Goodyer MJ, Ismail JR, O'Reilly SP, et al. Safety of trastuzumab (Herceptin®) during pregnancy: Two case reports. Cases J. 2009;2:9329. doi: 10.1186/1757-1626-2-9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanale MA, Uyei AR, Theriault RL, et al. Treatment of metastatic breast cancer with trastuzumab and vinorelbine during pregnancy. Clin Breast Cancer. 2005;6:354–356. doi: 10.3816/CBC.2005.n.040. [DOI] [PubMed] [Google Scholar]
- 39.Watson WJ. Herceptin (trastuzumab) therapy during pregnancy: Association with reversible anhydramnios. Obstet Gynecol. 2005;105:642–643. doi: 10.1097/01.AOG.0000141570.31218.2b. [DOI] [PubMed] [Google Scholar]
- 40.Waterston AM, Graham J. Effect of adjuvant trastuzumab on pregnancy. J Clin Oncol. 2006;24:321–322. doi: 10.1200/JCO.2005.04.6607. [DOI] [PubMed] [Google Scholar]
- 41.Bader AA, Schlembach D, Tamussino KF, et al. Anhydramnios associated with administration of trastuzumab and paclitaxel for metastatic breast cancer during pregnancy. Lancet Oncol. 2007;8:79–81. doi: 10.1016/S1470-2045(06)71014-2. [DOI] [PubMed] [Google Scholar]
- 42.Sekar R, Stone PR. Trastuzumab use for metastatic breast cancer in pregnancy. Obstet Gynecol. 2007;110:507–510. doi: 10.1097/01.AOG.0000267133.65430.44. [DOI] [PubMed] [Google Scholar]
- 43.Witzel ID, Ml̈ler V, Harps E, et al. Trastuzumab in pregnancy associated with poor fetal outcome. Ann Oncol. 2008;19:191–192. doi: 10.1093/annonc/mdm542. [DOI] [PubMed] [Google Scholar]
- 44.Pant S, Landon MB, Blumenfeld M, et al. Treatment of breast cancer with trastuzumab during pregnancy. J Clin Oncol. 2008;26:1567–1569. doi: 10.1200/JCO.2008.16.0309. [DOI] [PubMed] [Google Scholar]
- 45.Kelly H, Graham M, Humes E, et al. Delivery of a healthy baby after first-trimester maternal exposure to lapatinib. Clin Breast Cancer. 2006;7:339–341. doi: 10.3816/CBC.2006.n.048. [DOI] [PubMed] [Google Scholar]
- 46.Clark S. Prophylactic tamoxifen. Lancet. 1993;342:168. [Google Scholar]
- 47.Cullins SL, Pridjian G, Sutherland CM. Goldenhar's syndrome associated with tamoxifen given to the mother during gestation. JAMA. 1994;271:1905–1906. [PubMed] [Google Scholar]
- 48.Cunha GR, Taguchi O, Namikawa R, et al. Teratogenic effects of clomiphene, tamoxifen, and diethylstilbestrol on the developing human female genital tract. Hum Pathol. 1987;18:1132–1143. doi: 10.1016/s0046-8177(87)80381-7. [DOI] [PubMed] [Google Scholar]
- 49.Isaacs RJ, Hunter W, Clark K. Tamoxifen as systemic treatment of advanced breast cancer during pregnancy—case report and literature review. Gynecol Oncol. 2001;80:405–408. doi: 10.1006/gyno.2000.6080. [DOI] [PubMed] [Google Scholar]
- 50.Tewari K, Bonebrake RG, Asrat T, et al. Ambiguous genitalia in infant exposed to tamoxifen in utero. Lancet. 1997;350:183. doi: 10.1016/S0140-6736(97)24029-8. [DOI] [PubMed] [Google Scholar]
- 51.Bilgin K, Yaramis A, Haspolat K, et al. A randomized trial of granulocyte-macrophage colony-stimulating factor in neonates with sepsis and neutropenia. Pediatrics. 2001;107:36–41. doi: 10.1542/peds.107.1.36. [DOI] [PubMed] [Google Scholar]
- 52.Schibler KR, Osborne KA, Leung LY, et al. A randomized, placebo-controlled trial of granulocyte colony-stimulating factor administration to newborn infants with neutropenia and clinical signs of early-onset sepsis. Pediatrics. 1998;102:6–13. doi: 10.1542/peds.102.1.6. [DOI] [PubMed] [Google Scholar]
- 53.Briggs G, Freeman R, Yaffee S. A reference guide to fetal and neonatal risk: Drugs in pregnancy and lactation. Philadelphia: Lippincott Williams & Wilkins. 2008;447:719–720. [Google Scholar]
- 54.Ribeiro G, Jones DA, Jones M. Carcinoma of the breast associated with pregnancy. Br J Surg. 1986;73:607–609. doi: 10.1002/bjs.1800730805. [DOI] [PubMed] [Google Scholar]
- 55.Tretli S, Kvalheim G, Thoresen S, et al. Survival of breast cancer patients diagnosed during pregnancy or lactation. Br J Cancer. 1988;58:382–384. doi: 10.1038/bjc.1988.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonnier P, Romain S, Dilhuydy JM, et al. Influence of pregnancy on the outcome of breast cancer: A case-control study. Société Française de Senologie et de Pathologie Mammaire Study Group. Int J Cancer. 1997;72:720–727. doi: 10.1002/(sici)1097-0215(19970904)72:5<720::aid-ijc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 57.Beadle BM, Woodward WA, Middleton LP, et al. The impact of pregnancy on breast cancer outcomes in women ≤35 years. Cancer. 2009;115:1174–1184. doi: 10.1002/cncr.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ezzat A, Raja A, Berry J, et al. Impact of pregnancy on non-metastatic breast cancer: A case control study. Clin Oncol. 1996;8:367–370. doi: 10.1016/s0936-6555(96)80081-1. [DOI] [PubMed] [Google Scholar]
- 59.Zemlickis D, Lishner M, Degendorfer P, et al. Maternal and fetal outcome after breast cancer in pregnancy. Am J Obstet Gynecol. 1992;166:781–787. doi: 10.1016/0002-9378(92)91334-7. [DOI] [PubMed] [Google Scholar]
- 60.Lethaby AE, O'Neill MA, Mason BH, et al. Overall survival from breast cancer in women pregnant or lactating at or after diagnosis. Auckland Breast Cancer Study Group. Int J Cancer. 1996;67:751–755. doi: 10.1002/(SICI)1097-0215(19960917)67:6<751::AID-IJC1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 61.Murphy C, Mallam D, Stein S, et al. Pathologic features and outcomes of pregnancy-associated breast cancer (PABC): A case control study. J Clin Oncol. 2010;28(15 suppl):1589. [Google Scholar]
- 62.Queisser-Luft A, Stolz G, Wiesel A, et al. Malformations in newborn: Results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990–1998) Arch Gynecol Obstet. 2002;266:163–167. doi: 10.1007/s00404-001-0265-4. [DOI] [PubMed] [Google Scholar]
- 63.Avilés A, Neri N. Hematologic malignancies and pregnancy: A final report of 84 children who received chemotherapy in utero. Clin Lymphoma. 2001;2:173–177. doi: 10.3816/clm.2001.n.023. [DOI] [PubMed] [Google Scholar]
- 64.Avilés A, Neri N, Nambo MJ. Long-term evaluation of cardiac function in children who received anthracyclines during pregnancy. Ann Oncol. 2006;17:286–288. doi: 10.1093/annonc/mdj053. [DOI] [PubMed] [Google Scholar]
- 65.Reynoso EE, Shepherd FA, Messner HA, et al. Acute leukemia during pregnancy: The Toronto Leukemia Study Group experience with long-term follow-up of children exposed in utero to chemotherapeutic agents. J Clin Oncol. 1987;5:1098–1106. doi: 10.1200/JCO.1987.5.7.1098. [DOI] [PubMed] [Google Scholar]
- 66.Zoccarato G, Romagnolo C, Ghiotto C, et al. High frequency of premature births in concomitant pregnancy and cancer [abstract e12020] J Clin Oncol. 2010;28:e12020. [Google Scholar]
- 67.Nugent P, O'Connell TX. Breast cancer and pregnancy. Arch Surg. 1985;120:1221–1224. doi: 10.1001/archsurg.1985.01390350007001. [DOI] [PubMed] [Google Scholar]
- 68.Clark RM, Chua T. Breast cancer and pregnancy: The ultimate challenge. Clin Oncol (R Coll Radiol) 1989;1:11–18. doi: 10.1016/s0936-6555(89)80004-4. [DOI] [PubMed] [Google Scholar]
- 69.Deemarsky L, Neishtadt E. Breast cancer and pregnancy. Breast. 1981;7:17. [Google Scholar]
- 70.Durodola JI. Administration of cyclophosphamide during late pregnancy and early lactation: A case report. J Natl Med Assoc. 1979;71:165–166. [PMC free article] [PubMed] [Google Scholar]
- 71.Antoniou AC, Shenton A, Maher ER, et al. Parity and breast cancer risk among BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2006;8:R72. doi: 10.1186/bcr1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrieu N, Goldgar DE, Easton DF, et al. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS) J Natl Cancer Inst. 2006;98:535–544. doi: 10.1093/jnci/djj132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tryggvadottir L, Olafsdottir EJ, Gudlaugsdottir S, et al. BRCA2 mutation carriers, reproductive factors and breast cancer risk. Breast Cancer Res. 2003;5:R121–R125. doi: 10.1186/bcr619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jernström H, Lerman C, Ghadirian P, et al. Pregnancy and risk of early breast cancer in carriers of BRCA1 and BRCA2. Lancet. 1999;354:1846–1850. doi: 10.1016/s0140-6736(99)04336-6. [DOI] [PubMed] [Google Scholar]
- 75.Cullinane CA, Lubinski J, Neuhausen SL, et al. Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int J Cancer. 2005;117:988–991. doi: 10.1002/ijc.21273. [DOI] [PubMed] [Google Scholar]
- 76.Kotsopoulos J, Lubinski J, Lynch HT, et al. Age at first birth and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2007;105:221–228. doi: 10.1007/s10549-006-9441-3. [DOI] [PubMed] [Google Scholar]
- 77.Tryggvadóttir L, Tulinius H, Eyfjord JE, et al. Breast cancer risk factors and age at diagnosis: An Icelandic cohort study. Int J Cancer. 2002;98:604–608. doi: 10.1002/ijc.10217. [DOI] [PubMed] [Google Scholar]
- 78.Jernström H, Lubinski J, Lynch HT, et al. Breast-feeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2004;96:1094–1098. doi: 10.1093/jnci/djh211. [DOI] [PubMed] [Google Scholar]