This review highlights the recent clinical data in support of newer generation cytotoxic chemotherapies and systemic targeted agents in combination with radiation therapy.
Keywords: Radiation, Chemotherapy, Targeted therapy, Radiosensitization
Abstract
Combined modality therapy emerged from preclinical data showing that carefully chosen drugs could enhance the sensitivity of tumor cells to radiation while having nonoverlapping toxicities. Recent advances in molecular biology involving the identification of cellular receptors, enzymes, and pathways involved in tumor growth and immortality have resulted in the development of biologically targeted drugs. This review highlights the recent clinical data in support of newer generation cytotoxic chemotherapies and systemic targeted agents in combination with radiation therapy.
Introduction
Radiation therapy (RT) is used to control locally confined tumors with organ preservation. The ability of radiation alone to control soft tissue tumors is often limited by the tumor volume or the surrounding normal tissue tolerance to radiation. Cytotoxic chemotherapy drugs and biologic agents have been given before, during, or after RT in order to improve tumor responses. Neoadjuvant or induction chemotherapy can be employed to decrease micrometastases and decrease tumor size prior to RT with the hopes of improving tumor control with RT and/or decreasing the amount of normal tissue irradiated. Adjuvant chemotherapy is given after RT primarily to decrease systemic micrometastases. Concurrent chemoradiotherapy (chemo-RT) emerged from preclinical data showing that carefully chosen drugs could enhance the sensitivity of tumor cells to RT while having nonoverlapping toxicities. This spatial cooperation was the initial rationale for combining chemotherapy and RT, in which each agent had an independent mechanism of action at a different anatomic target. Since that time, concurrent chemoradiation has been used to enhance radioresponse locally within the primary tumor. Here, we review the recent clinical data in support of newer generation cytotoxic chemotherapies and systemic targeted agents in combination with RT.
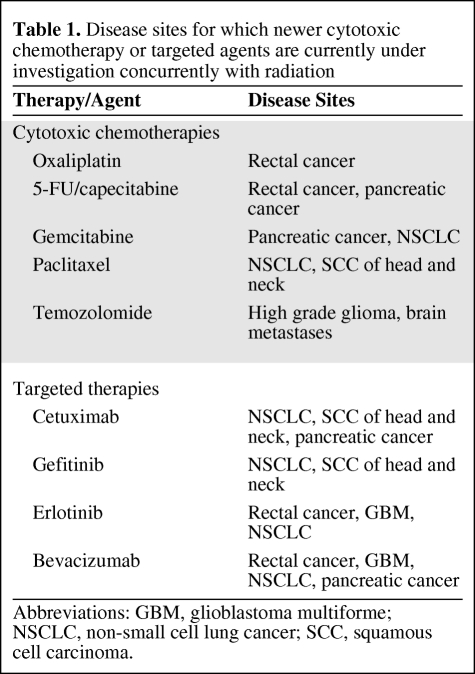
Combined modality therapy enhances RT or systemic therapies alone at both the tissue and cellular levels. At a tissue level, radiation can increase vascular permeability and allow greater drug delivery to the tumor as well as increase drug concentration by promoting drug retention within the tumor. At a cellular level, systemic therapies can enhance radiation sensitivity by inhibiting DNA repair mechanisms, enhancing oxygen radical formation to promote DNA double strand breaks, inhibiting progression through the cell cycle to lock cells in a radiosensitive phase, inducing apoptosis, and inhibiting cellular signaling cascades. Recent advances in molecular biology involving the identification of cellular receptors, enzymes, and pathways involved in tumor growth and immortality have resulted in the development of biologically targeted drugs. The use of targeted therapies in conjunction with RT promises to enhance the therapeutic ratio by increasing the efficacy of RT without significantly increasing treatment-related side effects. As individual tumor mutations and molecular markers are better understood, it may also be possible to improve patient selection by determining which patients will benefit from a given agent. (See Table 1.)
Table 1.
Disease sites for which newer cytotoxic chemotherapy or targeted agents are currently under investigation concurrently with radiation
Abbreviations: GBM, glioblastoma multiforme; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma.
It is appropriate to have an article on combined modality therapy in this issue, because Dr. Glatstein (or Eli, as we shall call him; he has never stood for the formality of being called Dr. Glatstein during the standard work day) felt that an important part of the reason he had been brought to the National Cancer Institute (NCI) was to “marry NCI chemotherapy with Stanford radiation therapy.” When the senior author started his residency in radiation oncology with Eli in 1984, “combined modality” for lymphomas, a major emphasis at the NCI, meant chemotherapy first, followed by RT. We had a particularly intensive study for advanced-stage follicular lymphoma that involved full-dose chemotherapy with a nine-drug myelosuppressive regimen followed by total nodal radiation. After all that chemotherapy, the patients' counts would drop after the dose delivered by simulation and would vaporize after the first week of treatment. It would take us months to get patients through this. In contrast, local toxicities (esophagitis and pneumonitis) dominated the treatment of limited-stage small cell lung cancer with concurrent aggressive chemotherapy and RT. Eli was among the early users of “adaptive therapy.” Treatment with chemoradiation would start on Monday, and we would resimulate patients every Friday in order to shrink the fields. (The total course was a spicy 40 Gy in 15 fractions with concurrent doxorubicin-containing chemotherapy!) Combined modality therapy was superior, but there was significant morbidity and even mortality from treatment.
Cytotoxic Chemotherapy
The Platins
Cisplatin is one of the most commonly used chemotherapy agents for radiosensitization. It forms both inter- and intrastrand DNA adducts that produce single-strand breaks when removed by DNA mismatch repair processes. These single-strand breaks can be converted to lethal double-strand breaks by radiation. Thus, mismatch repair defective cells are not radiosensitized by cisplatin and carboplatin [1]. Phase III trials have proven longer survival times for patients treated with concurrent cisplatin-based chemo-RT than for those treated with RT alone in cervical cancer, head and neck cancer, non-small cell lung cancer (NSCLC), and esophageal cancer.
Oxaliplatin is a third-generation platinum derivative that has been found to be a potent radiosensitizer [2]. It is a diaminocyclohexane that causes DNA adduct formation and prevents DNA synthesis [3]. Although oxaliplatin appears to cause DNA adduct formation like other platinum derivatives, oxaliplatin adducts are not repaired by the same DNA mismatch repair systems as other platinum adducts. Oxaliplatin, therefore, has been found to have activity in cisplatin- and carboplatin-resistant cells [4, 5].
Oxaliplatin results in longer survival times in metastatic colorectal cancer patients in combination with 5-fluorouracil (5-FU) than with 5-FU alone [6]. Oxaliplatin is under investigation in phase I/II studies in combination with RT and 5-FU or capecitabine for the neoadjuvant treatment of rectal cancer patients, with an 81% rate of R0 resection. The pathologic complete response rate has been in the range of 8%–26%, with rates of grade 3 or 4 diarrhea of 16%–38% [7–9]. Phase III studies need to be conducted prior to endorsing the routine use of oxaliplatin with concurrent RT.
5-FU and Capecitabine
5-FU is a pyrimidine analog of uracil and functions as an antimetabolite. Its radiosensitizing effects are a result of its ability to prevent DNA synthesis through inhibition of thymidylate synthase [10], rather than its RNA-dependent effects. The combination of 5-FU and RT was superior to RT alone in randomized trials for patients with head and neck and gastrointestinal (GI) cancers, including esophageal, gastric, pancreatic, and rectal cancers.
Capecitabine is an oral 5-FU prodrug; it is converted to 5-FU by the enzyme thymidine phosphorylase (TP). TP catalyzes the mutual transformation of the pyrimidine nucleosides thymidine and thymine in nucleic acid metabolism and also converts the FU-based drugs, including capecitabine, into active 5-FU. It is, therefore, a limiting factor of the mechanism of action for capecitabine. A proposed mechanism of selectivity is the preferential conversion in the liver, which is relatively resistant, and at the tumor site, because these tissues often have higher TP activity than normal tissue.
RT was reported to lead to better efficacy with capecitabine through tumor-associated induction of TP, especially within the radiation portal. However, this was not found to be consistently true in other preclinical studies of colon cancer cells [11], in tumor xenografts [12], or in a phase I dose-escalation study from the University of Alabama [13]. Although these results support the concurrent use of capecitabine and RT in pancreatic cancer, there appear to be additional genes (other than TP) associated with response to capecitabine alone and with RT.
Because of its ease of administration compared with continuous infusion 5-FU, attempts have been made to substitute capecitabine for infusional 5-FU in combination with RT in pancreatic cancer and rectal cancer patients [14, 15]. We are unaware of any head-to-head comparisons of infusional 5-FU versus capecitabine, but our overall impression is that these are probably approximately equivalent.
Gemcitabine
2′,2′-Difluoro-2′-deoxcytidine, or gemcitabine, requires intracellular phosphorylation in a rate-limiting step by deoxycytidine kinase to form its active metabolites difluorodeoxycytidine diphosphate (dFdCDP) and difluorodeoxycytidine triphosphate (dFdCTP). These metabolites of gemcitabine produce two distinct mechanisms of action contributing to gemcitabine's radiosensitizing and cytotoxic properties, respectively. DFdCDP is a direct inhibitor of ribonucleotide reductase and thus inhibits deoxynucleotide triphosphate synthesis whereas dFdCTP is incorporated into DNA, both leading to the inhibition of DNA synthesis. Early preclinical studies demonstrated that gemcitabine was an effective radiosensitizer in a variety of cell types [16, 17]. Subsequent studies investigating the mechanism(s) of radiosensitization suggested that dATP pool depletion (via ribonucleotide reductase inhibition) as well as redistribution of cells into the early S-phase of the cell cycle underlie gemcitabine-mediated radiosensitization [18]. Based on the observed preclinical radiosensitization by gemcitabine as well as the clinical efficacy of gemcitabine as a single agent, gemcitabine has been combined with RT in the clinic to treat many solid tumor types.
Gemcitabine has become the standard of care for metastatic pancreatic cancer [19]. We feel it has become a standard component for locally advanced disease. In this latter group, a recent trial phase III trial of 74 patients with unresectable pancreatic cancer randomized patients to either gemcitabine or gemcitabine and concurrent RT, showing a statistical survival advantage for patients in the chemo-RT arm (11 months versus 9.2 months; p < .04), but higher GI and hematologic toxicity rates in the chemo-RT arm [20]. A French phase III trial of induction chemo-RT followed by gemcitabine was compared with gemcitabine alone, showing the chemo-RT arm to be more toxic and less effective than gemcitabine alone. The 1-year overall survival rates were 32% versus 53% favoring gemcitabine alone [21]. In that trial, however, patients in the chemo-RT arm had significantly shorter survival than historical controls. This could be because concurrent, high-dose gemcitabine and RT is toxic when large radiation fields are used [22]. However, the use of conformal radiation to the primary tumor site without elective nodal radiation permits the safe administration of full systemic doses of gemcitabine or gemcitabine plus oxaliplatin [23, 24]. Although Eli has been noted to say, “Local control does not guarantee immortality,” local failure does occur in these patients [25] and can be the cause of death. This has motivated our recent attempts to dose escalate radiation using intensity-modulated RT with full-dose gemcitabine. Results are promising, with a median survival duration of 23 months [26].
In a phase III trial of adjuvant therapy for locally advanced resected pancreatic cancer, the addition of adjuvant gemcitabine after 5-FU and concurrent RT showed longer survival than with 5-FU and RT alone for patients with pancreatic head tumors [27]. Although a phase I/II study showed that concurrent full-dose gemcitabine and RT can be given safely as adjuvant treatment [28], it is not known whether this combination is superior to the more commonly used 5-FU or capecitabine plus RT combination.
Phase I/II studies have also evaluated the role of gemcitabine (alone or in combination with a platinum or paclitaxel) and concurrent RT in locally advanced NSCLC patients. Preliminary data have shown high rates of pulmonary toxicity, although gemcitabine, at a dose of 150 mg/m2, was tolerated in the setting of three-dimensional (3D) RT planning [29–33]. Caution is advised when gemcitabine is given with concurrent RT because of gemcitabine's enhanced radiosensitization effects in the lung and esophagus, and appropriate chemotherapy doses have not been well established.
Paclitaxel
The taxanes are mitotic spindle inhibitors that bind to the N-terminal amino acid in β-tubulin and stabilize tubulin polymers, thereby promoting microtubule assembly and inhibiting disaggregation [34]. This action causes cellular arrest in G2/M, the most radiosensitive phase of the cell cycle. Paclitaxel is the most commonly used taxane in combination with RT and appears to act by causing mitotic arrest and inducing apoptosis, and also may also induce reoxygenation within the tumor [35, 36].
Paclitaxel has been used in combination with carboplatin currently with RT in the setting of locally advanced NSCLC because it was thought to have a favorable toxicity profile. Initially, concurrent chemo-RT with cisplatin and etoposide was found, in trials, to be superior to sequential therapy in unresectable NSCLC patients [37]. Phase II data then showed that RT with low-dose weekly concurrent paclitaxel plus carboplatin and induction or paclitaxel plus carboplatin was feasible [38, 39]. Specifically, the Cancer and Leukemia Group B (CALGB) initially evaluated the combination of induction carboplatin and paclitaxel for two cycles followed by low-dose weekly concurrent chemotherapy with RT. They found a median survival time of 15.1 months, and the trial demonstrated the feasibility of this regimen [39]. A phase III trial, CALGB 39801, was then completed, in which all patients received low-dose weekly carboplatin and paclitaxel with concurrent RT to 66 Gy and were randomized to two cycles of induction chemotherapy. Both arms of that trial showed disappointing results, with a median survival time of 11–13 months, demonstrating that this was not an efficacious regimen [40]. The CALGB 30105 trial evaluated induction chemotherapy followed by concurrent carboplatin, paclitaxel, and RT or concurrent carboplatin, gemcitabine, and RT in stage IIIA/IIIB NSCLC patients. Both arms required 3D RT planning, and all patients received 74 Gy. They found that patients in the paclitaxel arm had a median overall survival time of 24 months whereas the gemcitabine arm was closed early secondary to a high rate of grade 4 or 5 pulmonary toxicity. The hypothesis generated by that trial is that the longer median survival time seen in the carboplatin–paclitaxel arm was a result of the higher RT dose [36].
To date, carboplatin and paclitaxel have not been compared with cisplatin-based chemo-RT, which continues to be considered the standard as definitive or neoadjuvant treatment in NSCLC patients.
In locally advanced and unresectable head and neck cancer, paclitaxel has been used concurrently with RT. The Eastern Cooperative Oncology Group (ECOG) E2399 study was a phase II trial of induction carboplatin plus paclitaxel followed by concurrent carboplatin, paclitaxel, and RT for patients with resectable stage III/IV squamous cell carcinoma of the larynx and oropharynx. That study showed an 81% organ preservation rate overall at 2 years, with a higher organ preservation rate in oropharynx patients, and the regimen was well tolerated [41]. Another phase II trial in unresectable head and neck cancer patients, including all sites, showed that RT with concurrent weekly carboplatin plus paclitaxel was well tolerated, but 42% of patients (n = 50) had some component of locoregional failure at 5 years, and the overall survival rate was 35% [42]. Chougule et al. [43] conducted a trial of concurrent carboplatin plus paclitaxel and RT, demonstrating a 91% response rate with a 26% locoregional failure rate in locally advanced head and neck cancer patients. This regimen, however, required that 31% of patients be hospitalized because of toxicity. Salama et al. [44] conducted a phase I/II trial at the University of Chicago using induction carboplatin and paclitaxel followed by concurrent carboplatin, paclitaxel, fluorouracil, and hydroxyurea and twice daily RT in stage III/IV head and neck cancer patients, showing a high locoregional control rate and overall survival rate of 91% and 62%, respectively, at 5 years. Ninety-one percent of patients experienced grade 3 or 4 acute toxicity during chemo-RT. As in locally advanced NSCLC patients, concurrent carboplatin and paclitaxel regimens with RT have not been prospectively compared with cisplatin-based chemo-RT in locally advanced head and neck cancer patients.
Temozolomide
Temozolomide is an orally administered alkylating agent that induces apoptosis by methylation of DNA at nucleophilic sites, causing the formation of O6-methylguanine. This DNA adduct induces futile cycling of the mismatch repair pathway, but the adduct can also be repaired by the enzyme O6-methylguanine-DNA methyltransferase (MGMT). Patients with high levels of MGMT methylation, which indicates that the gene has been silenced, have longer progression-free and overall survival times after treatment with alkylating agents [45].
In preclinical studies, temozolomide was found to have schedule-dependent activity against recurrent high-grade gliomas and advanced melanoma, to have oral bioavailability, and to penetrate the central nervous system [46, 47]. Its use in the concurrent setting with RT is largely based on early clinical data supporting a small advantage over current treatment at that time. A large phase II trial of temozolomide versus procarbazine in recurrent glioblastoma multiforme (GBM) patients demonstrated a significant 6-month progression-free and overall survival advantage for patients treated with temozolomide as a single agent, with similar toxicity [48]. Following this, a pilot phase II trial of concurrent temozolomide and RT followed by adjuvant temozolomide was then conducted, demonstrating a promising overall survival rate at 2 years of 31% [49]. Concurrent temozolomide and RT followed by temozolomide alone in GBM was then studied in a large phase III trial and found to result in longer survival versus RT alone [50], thereby establishing the standard of care.
Temozolomide with concurrent RT is currently being investigated for patients with high-risk, low-grade gliomas (Radiation Therapy Oncology Group [RTOG] 0424 trial). Additionally, temozolomide is being examined for use with concurrent RT in the setting of brain metastasis. A phase II single-institution trial of whole-brain RT and concurrent as well as adjuvant temozolomide in 27 patients with at least one brain metastasis from breast cancer or NSCLC revealed complete and partial response rates of 7% and 41%, respectively. The regimen was well tolerated [51]. RTOG 0320 is a phase III trial that is under way investigating whole-brain RT and stereotactic radiosurgery alone versus the same radiation with concurrent temozolomide or erlotinib.
Molecular Targeted Therapies
Epidermal Growth Factor Receptor–Directed Therapies
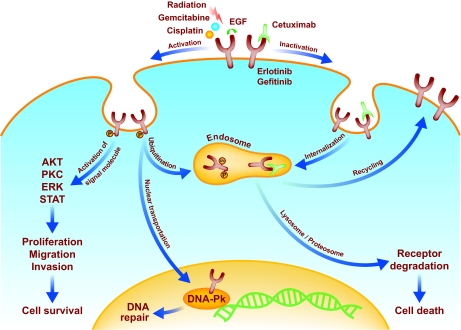
The human epidermal growth factor receptor (EGFR) family includes EGFR, human epidermal growth factor receptor 2 (HER-2), HER-3, and HER-4. (See Figure 1.) EGFR family members have been identified to play a critical role in development, progression, and resistance to chemo-RT in NSCLC, colorectal cancer, pancreatic cancer, squamous cell carcinoma of the head and neck, brain cancer, and breast cancer. Additionally, EGFR is either overexpressed or hyperphosphorylated in the majority of cancers of epithelial origin. Several clinical studies have suggested that EGFR expression is a powerful prognostic factor for response to both RT and chemo-RT. In a retrospective review, Kumar et al. [52] found that a high EGFR expression level predicts poor response to therapy and poor overall survival in head and neck cancer patients. Similarly, Pivot et al. [53] found that lower EGFR levels predicted better disease-free survival in patients with laryngeal and hypopharyngeal cancers treated with induction chemotherapy followed by RT. The idea that blocking these receptors produces selective response in tumors driven by these molecules has led to the development of several monoclonal antibodies (mAbs) and small molecules that inhibit receptor kinase activity [54].
Figure 1.
The effects of radiation, chemotherapy, and targeted agents on EGFR signaling. After stimulation by irradiation or certain chemotherapeutic agents, epidermal growth factor receptor (EGFR) can activate downstream signaling pathways that can promote cell survival or cell death. In addition to stimulating the pathways activated by epidermal growth factor (EGF), radiation can trigger the translocation of phosphorylated EGFR (pEGFR) into the nucleus. This process coincides with the transport of Ku70/80 and protein phosphatase 1 into the nucleus (not shown), which results in increases in DNA-dependent protein kinase (DNAPK) levels, the repair of DNA-strand breaks, and cell survival. Cetuximab blocks nuclear transport of pEGFR; it binds to EGFR and causes endosome internalization, ultimately causing receptor degradation and cell death. Gefitinib and erlotinib bind to the intracellular ATP binding site of EGFR, thereby inhibiting unregulated EGFR signaling. Gemcitabine causes the phosphorylation of EGFR. In this case, EGFR phosphorylation initially activates Akt promoting cell survival, but subsequently promotes the ubiquitination (Ub) of the receptor, which leads to its degradation along a proteosome or lysosome pathway. pEGFR degradation results in the downregulation of the survival signal pAkt, leading to apoptosis. Blocking EGFR degradation at various steps of this pathway reduces gemcitabine-mediated cytotoxicity. Whether an EGFR-activating insult leads to cell survival or cell death might ultimately be determined by the severity and duration of the stress.
Cetuximab
Cetuximab binds to the EGFR with approximately tenfold greater affinity than the mouse mAb C225 from which it is derived. Cetuximab not only blocks EGF-induced autophosphorylation of the EGFR but also induces its internalization and degradation, which may contribute to the outcome of treatment. It has also been reported that cetuximab inhibits nuclear translocation of EGFR, which may be particularly effective in combination with RT, because nuclear EGFR can induce DNA protein kinase activity, leading to efficient DNA repair. Therefore, inhibition of EGFR is considered an excellent target for radiosensitization. Still, many aspects of this therapy have yet to be elucidated. For example, only modest radiosensitization in cultured cells can be achieved via EGFR inhibitors, and even this requires prolonged inhibition of EGFR phosphorylation. However, xenograft data show much more promising results; the cause of this difference is not yet clearly understood. It has been postulated that, in addition to blocking direct effects on EGFR, cetuximab may have effects on cell cycle redistribution, angiogenesis, and immune reaction. This argument is supported by clinical findings suggesting that: (a) cetuximab can produce a response in tumors with negative EGFR staining by immunohistochemistry and (b) the strength of the EGFR staining does not predict response to EGFR inhibitors combined with chemotherapy. Although we cannot yet predict patient response based on the presence or absence of the target itself, recent findings suggest that patients carrying a KRAS mutation do not benefit from treatment with anti-EGFR agents.
A randomized trial in locally advanced head and neck cancer patients demonstrated that patients receiving the combination of RT and cetuximab had a statistically significant better locoregional disease response without significantly worse acute toxicities [55]. This is the first study to show that the combination of a molecularly targeted therapy with RT can result in better local control and survival than with RT alone. Based on this success, the addition of cetuximab to concurrent chemo-RT as definitive treatment in locally advanced head and neck cancer patients is currently being investigated in the RTOG 0522 study. In the postoperative setting, RTOG 0234 is a phase II trial now closed to accrual that evaluated cetuximab with concurrent chemo-RT for patients with head and neck cancer and high-risk pathologic features.
In NSCLC patients, preliminary phase II studies have shown that cetuximab can be safely administered with RT and that this does not appear to lead to higher rates of acute treatment toxicities. The SCRATCH trial, a phase II trial in stage III NSCLC patients treated with weekly cetuximab and thoracic RT to 64 Gy demonstrated acceptable toxicity for this regimen [56]. The Non-small cell lung cancer, Erbitux And Radiotherapy (NEAR) trial is an ongoing, phase II, feasibility trial of concurrent locoregional RT and cetuximab in stage III NSCLC patients [57].
Cetuximab in combination with gemcitabine and RT has been assessed in pancreatic cancer. A randomized phase II trial was conducted in inoperable patients receiving gemcitabine, cetuximab, and RT, who then were randomized to adjuvant gemcitabine or adjuvant gemcitabine plus cetuximab. No statistical difference was seen between arms; the 2-year overall survival rate was 20% [58]. This result is not surprising; the presence of mutated RAS in >90% of pancreatic cancer cases suggests that EGFR inhibition is unlikely to be successful [59].
Gefitinib and Erlotinib
Gefitinib and erlotinib are small molecule tyrosine kinase inhibitors used clinically. They inhibit unregulated EGFR signaling by binding to the intracellular ATP binding site, which prevents tyrosine kinase activity.
Recent data have demonstrated that schedule plays a critical role in the interaction between gefitinib and erlotinib with chemotherapy and with RT. When given prior to gemcitabine and cisplatin, gefitinib and erlotinib antagonize chemotherapy toxicity, whereas the small molecule tyrosine kinase inhibitors produce substantial synergy when given after cytotoxic chemotherapy [37, 60]. The molecular mechanism for this schedule dependence is now understood. Both gemcitabine and cisplatin cause initial EGFR phosphorylation, followed by EGFR degradation and cell death. Therefore, giving an EGFR inhibitor prior to chemotherapy blocks this initial phosphorylation, inhibits degradation, and increases survival. Indeed, this is likely the explanation for the negative results of the Iressa NSCLC Trial Assessing Combination Treatment (INTACT)-1 and INTACT-2 studies [61, 62], and a recent clinical trial that prospectively used chemotherapy followed by small molecule tyrosine kinase inhibition has produced promising results [63]. In contrast, administration of an EGFR inhibitor after chemotherapy-induced EGFR phosphorylation potentiates degradation and the subsequent cytotoxicity. A corollary of the hypothesis that EGFR degradation causes cytotoxicity is that a proteosome inhibitor such as bortezomib might antagonize chemo-RT by inhibiting EGFR degradation. In addition, we have recently shown that EGFR-driven log-phase and plateau-phase cells have a dramatically different response to an EGFR inhibitor combined with radiation. A brief (<2 hours) pretreatment with erlotinib can protect plateau-phase EGFR-expressing cells from radiation by inhibiting EGFR activation, thus preventing cells from being driven into a fatal S phase after radiation [64]. In contrast, long-term exposure (24 hours) of log-phase cells to EGFR inhibition ultimately arrests cells in late G1, which is a relatively sensitive phase of the cell cycle. These findings demonstrate the importance of schedule and cell cycle in the response to combination treatment with RT, chemotherapy, and EGFR inhibitors.
Gefitinib has been used in phase I/II trials with RT in NSCLC and head and neck cancer patients. In stage III NSCLC patients, a phase II trial of induction carboplatin, paclitaxel, and irinotecan followed by chemo-RT to 74 Gy with concurrent carboplatin, paclitaxel, and gefitinib (250 mg daily) showed that the regimen was well tolerated, but disappointing results of a 24% partial response rate and median OS time of 16 months were reported [65]. In stage III and IV head and neck cancer patients, a phase II trial using two cycles of neoadjuvant carboplatin and paclitaxel followed by concurrent RT, 5-FU, hydroxyurea, and gefitinib showed an estimated 2 year overall survival rate of 83% with a low rate of grade 4 toxicities [66].
Erlotinib has been combined with RT in the phase I/II setting in rectal cancer, glioma, and NSCLC patients, for which EGFR expression is commonly seen. Erlotinib was tried in a dose-escalation study of patients with locally advanced or recurrent rectal cancer with concurrent RT, demonstrating excellent local control but only a 5% pathologic complete response rate at a maximum-tolerated dose of 100 mg daily for 45 days [67]. In GBM patients, the North Central Cancer Treatment Group reported a phase I/II trial in which patients received concurrent RT, temozolomide, and erlotinib. The regimen was well tolerated, but the median survival time of 15.3 months did not show an advantage over historic controls in the European Organization for Research and Treatment of Cancer 26981 study [52].
It is noteworthy that despite the high frequency of mutant RAS in pancreatic cancer and lack of difference in response with cetuximab, erlotinib is the only molecularly targeted agent shown thus far to result in statistically longer survival in patients with advanced disease treated with gemcitabine [69].
Bevacizumab
Expression of angiogenic factors is required for tumor growth, and the most commonly studied factor is vascular endothelial growth factor (VEGF). Bevacizumab is a monoclonal antibody that binds to the VEGF receptor on the extracellular surface and is thought to normalize tumor vasculature, thereby potentially allowing enhanced oxygen and chemotherapy drug delivery locally. In rectal cancer, bevacizumab has been found to have antivascular effects, decreasing perfusion and intrastitial fluid pressure within the tumor [70]. In locally advanced rectal cancer, phase I and II trials have been conducted to evaluate the addition of bevacizumab to neoadjuvant therapy. When bevacizumab was added to neoadjuvant capecitabine and RT for locally advanced rectal cancer, the pathologic complete response rate was 32%, with a 72% sphincter preservation rate and no grade 3 GI or hematologic toxicities [71].
Bevacizumab is also under investigation with concurrent RT in the treatment of GBM patients. Preliminary phase II data with concurrent temozolomide and RT have shown that the regimen is well tolerated without a higher rate of acute toxicity [72].
Bevacizumab has also been evaluated with gemcitabine and RT in locally advanced pancreatic cancer patients. This regimen is now approached with caution, however, because of high toxicity manifest as surgical complications and ulceration or bleeding in the radiation field [73–75].
Despite some encouraging results for bevacizumab plus RT in small trials, there is no current recognized role for this therapy off protocol.
Future Directions
How can we best build on our knowledge of chemo-RT to design more effective combinations (with and without targeted therapies)? First, we feel that preclinical data may guide us in optimizing therapy and patient selection. For instance, preclinical studies show that it is possible for targeted therapies that block cell cycle progression (such as cetuximab) to antagonize some chemotherapies that depend on cell cycle progression (such as gemcitabine) [76]; it is possible that such effects may explain some of the negative results seen in adding targeted therapies to lung cancer treatment [77]. In another example, targeted therapies have typically been tested in unselected populations or, even worse, in populations in which preclinical data suggest that the patient population will be resistant to the therapy (erlotinib in pancreatic cancer, which commonly displays RAS mutations). It is possible that the addition of RT could alter signaling pathways so that these issues don't matter, but this is speculation.
A second possible focus for improvement would be to devote more attention to determining if the target is actually being hit. The senior author learned this directly from Eli during his residency, during which special efforts were made to obtain tumor and bone marrow biopsies to determine incorporation of the radiation sensitizers iododeoxy and bromodeoxyuridine. These studies are difficult, to be sure, but an understanding of how, for instance, cetuximab affects downstream signaling in tumors from even four to six patients might provide us with more understanding about how to optimize this agent in combination with RT or chemo-RT than a randomized phase II trial of 100 unselected patients without pharmacodynamic studies.
Third, we feel that these studies suggest a role for highly conformal RT to enable the use of chemotherapy and targeted therapies. We can't discuss this without bringing Eli into the picture. Eli has been unfairly characterized as “anti-technology”; in fact, he (and we) are opposed to the mindless application of technology that, sadly, often characterizes our field. Highly conformal therapy, which excludes clinically uninvolved regions, can permit full doses of chemotherapy and targeted therapies to be administered safely. Eli has been known to complain that radiation oncologists and medical oncologists are sometimes engaged in “a battle for the last megakaryocyte.” The increasing use of conformal RT and targeted therapies will, we hope, replace this battle with a collaboration over how to cure patients while treating acne and mild diarrhea.
Finally, we feel that these developments give the opportunity to expand the scope of practice of the radiation oncologist, and, here too, we celebrate Eli's lead. Eli always told his trainees that we are not “modality salesmen” but we need to be oncologists and physicians. Indeed, he would say that radiation oncologists are the only physicians who are consistently called on to treat both early-stage disease (like the surgeon) and metastatic disease (like the medical oncologist). During his medical oncology training, the senior author was struck by how much of his training was focused on ameliorating the toxicity of chemotherapy. The introduction of far less toxic targeted therapy permits the radiation oncologist to lead efforts to develop new radiation sensitizer strategies, as part of multidisciplinary care, without subjecting patients to severe toxicity.
Author Contributions
Conception/Design: Michelle L. Mierzwa, Theodore S. Lawrence
Manuscript writing: Michelle L. Mierzwa, Mukesh K. Nyati, Meredith A. Morgan, Theodore S. Lawrence
Final approval of manuscript: Michelle L. Mierzwa, Mukesh K. Nyati, Meredith A. Morgan, Theodore S. Lawrence
References
- 1.Raymond E, Faivre S, Woynarowski JM, et al. Oxaliplatin: Mechanism of action and antineoplastic activity. Semin Oncol. 1998;25(suppl 5):4–12. [PubMed] [Google Scholar]
- 2.Blackstock AW, Hess S, Chaney S, et al. Oxaliplatin: In vitro evidence of its radiation sensitizing activity—preclinical observations relevant to clinical trials. Int J Radiat Oncol Biol Phys. 1999;46:724. [Google Scholar]
- 3.Pendyala L, Kidani Y, Perez R, et al. Cytotoxicity, cellular accumulation and DNA binding of oxaliplatin isomers. Cancer Lett. 1995;97:177–184. doi: 10.1016/0304-3835(95)03974-2. [DOI] [PubMed] [Google Scholar]
- 4.Rixe O, Ortuzar W, Alvarez M, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: Spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol. 1996;52:1855–1865. doi: 10.1016/s0006-2952(97)81490-6. [DOI] [PubMed] [Google Scholar]
- 5.Dunn TA, Schmoll HJ, Grn̈wald V, et al. Comparative cytotoxicity of oxaliplatin and cisplatin in non-seminomatous germ cell cancer cell lines. Invest New Drugs. 1997;15:109–114. doi: 10.1023/a:1005800520747. [DOI] [PubMed] [Google Scholar]
- 6.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 7.Ryan DP, Niedzwiecki D, Hollis D, et al. Phase I/II study of preoperative oxaliplatin, fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer: Cancer and Leukemia Group B 89901. J Clin Oncol. 2006;24:2557–2562. doi: 10.1200/JCO.2006.05.6754. [DOI] [PubMed] [Google Scholar]
- 8.Hospers GA, Punt CJ, Tesselaar ME, et al. Preoperative chemoradiotherapy with capecitabine and oxaliplatin in locally advanced rectal cancer: A phase I-II multicenter study of the Dutch Colorectal Cancer Group. Ann Surg Oncol. 2007;14:2773–2779. doi: 10.1245/s10434-007-9396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roedel C, Arnold D, Hipp M, et al. Multicenter phase II trial of preoperative radiotherapy with concurrent and adjuvant capecitabine and oxaliplatin in locally advanced rectal cancer. J Clin Oncol. 2006;24(18 suppl):349. [Google Scholar]
- 10.Lawrence TS, Davis MA, Maybaum J. Dependence of 5-fluorouracil mediated radiosensitization on DNA-directed effects. Int J Radiat Oncol Biol Phys. 1996;70:273–280. doi: 10.1016/0360-3016(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence TS, Davis MA, Tang TY, et al. Fluorodeoxyuridine-mediated cytotoxicity and radiosensitization requires S phase progression. Int J Radiat Oncol Biol Phys. 1996;34:617–621. doi: 10.1080/095530096145003. [DOI] [PubMed] [Google Scholar]
- 12.Sawada N, Ishikawa T, Sekiguchi F, et al. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res. 1999;5:2948–2953. [PubMed] [Google Scholar]
- 13.Saif MW, Eloubeidi MA, Russo S, et al. Phase I study of capecitabine with concomitant radiotherapy for patients with locally advanced pancreatic cancer: Expression analysis of genes related to outcome. J Clin Oncol. 2005;23:8679–8687. doi: 10.1200/JCO.2005.02.0628. [DOI] [PubMed] [Google Scholar]
- 14.Schneider BJ, Ben-Josef E, McGinn CJ, et al. Capecitabine and radiation therapy preceded and followed by combination chemotherapy in advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:1325–1330. doi: 10.1016/j.ijrobp.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Dunst J, Reese T, Sutter T, et al. Phase I trial evaluating the concurrent combination of radiotherapy and capecitabine in rectal cancer. J Clin Oncol. 2002;20:3983–3991. doi: 10.1200/JCO.2002.02.049. [DOI] [PubMed] [Google Scholar]
- 16.Shewach DS, Hahn TM, Chang E, et al. Metabolism of 2′,2′-difluoro-2′-deoxycytidine and radiation sensitization of human colon carcinoma cells. Cancer Res. 1994;54:3218–3223. [PubMed] [Google Scholar]
- 17.Rockwell S, Grindley GB. Effect of 2′,2′-difluorodeoxycytidine on the viability and radiosensitivity of EMT6 cells in vitro. Oncol Res. 1992;4:151–155. [PubMed] [Google Scholar]
- 18.Lawrence TS, Chang EY, Hahn TM, et al. Radiosensitization of pancreatic cancer cells by 2′,2′-difluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–872. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 19.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 20.Loehrer PJ, Powell ME, Cardenes L, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized unresectable pancreatic cancer. J Clin Oncol. 2008;26(15 suppl):4506. [Google Scholar]
- 21.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 22.Wolff RA, Evans DB, Gravel DM, et al. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin Cancer Res. 2001;7:2246–2253. [PubMed] [Google Scholar]
- 23.Muler JH, McGinn CJ, Normolle D, et al. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol. 2004;22:238–243. doi: 10.1200/JCO.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 24.Desai SP, Ben-Josef E, Normolle DP, et al. Phase I study of oxaliplatin, full-dose gemcitabine, and concurrent radiation therapy in pancreatic cancer. J Clin Oncol. 2007;25:4587–4592. doi: 10.1200/JCO.2007.12.0592. [DOI] [PubMed] [Google Scholar]
- 25.Murphy JD, Adusumilli S, Griffith KA, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:801–808. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Josef E, Griffith K, Francis IR, et al. Phase I radiation dose-escalation trial of intensity modulated radiotherapy (IMRT) with concurrent fixed dose-rate gemcitabine (FDR-G) for unresectable pancreatic cancer. J Clin Oncol. 2009;27(15 suppl):4602. [Google Scholar]
- 27.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 28.Allen AM, Zalupski MM, Robertson JM, et al. Adjuvant therapy in pancreatic cancer: Phase I trial of radiation dose escalation with concurrent full-dose gemcitabine. Int J Radiat Oncol Biol Phys. 2004;59:1461–1467. doi: 10.1016/j.ijrobp.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Blanco R, Solé J, Montesinos J, et al. Induction chemotherapy with cisplatin and gemcitabine followed by concurrent chemoradiation with twice-weekly gemcitabine in unresectable stage III non-small cell lung cancer: Final results of a phase II study. Lung Cancer. 2008;62:62–71. doi: 10.1016/j.lungcan.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 31.Choy H, Jain AK, Moughan J, et al. RTOG 0017: A phase I trial of concurrent gemcitabine/carboplatin or gemcitabine/paclitaxel and radiation therapy (“ping-pong trial”) followed by adjuvant chemotherapy for patients with favorable prognosis inoperable stage IIIA/B non-small cell lung cancer. J Thorac Oncol. 2009;4:80–86. doi: 10.1097/JTO.0b013e318191503f. [DOI] [PubMed] [Google Scholar]
- 32.Arrieta O, Gallardo-Rincón D, Villarreal-Garza C, et al. High frequency of radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent radiotherapy and gemcitabine after induction gemcitabine and carboplatin. J Thorac Oncol. 2009;4:845–852. doi: 10.1097/JTO.0b013e3181a97e17. [DOI] [PubMed] [Google Scholar]
- 33.Zinner RG, Komaki R, Cox JD, et al. Dose escalation of gemcitabine is possible with concurrent chest three-dimensional rather than two-dimensional radiotherapy: A phase I trial in patients with stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73:119–127. doi: 10.1016/j.ijrobp.2008.03.069. [DOI] [PubMed] [Google Scholar]
- 34.Rao S, Krauss NE, Heerding JM, et al. 3′-(p-azidobenzamido)taxol photolabels the N-terminal 31 amino acids of beta-tubulin. J Biol Chem. 1994;269:3132–3134. [PubMed] [Google Scholar]
- 35.Manfredt JJ, Horwitz SB. Taxol: An antimitotic agent with a new mechanism of action. Pharmacol Ther. 1984;25:83–125. doi: 10.1016/0163-7258(84)90025-1. [DOI] [PubMed] [Google Scholar]
- 36.Milas L, Hunter NR, Mason KA, et al. Role of reoxygenation in induction of enhancement of tumor response by paclitaxel. Cancer Res. 1995;55:3564–3568. [PubMed] [Google Scholar]
- 37.Feng FY, Varambally S, Tomlins SA, et al. Role of epidermal growth factor receptor degradation in gemcitabine-mediated cytotoxicity. Oncogene. 2007;26:3431–3439. doi: 10.1038/sj.onc.1210129. [DOI] [PubMed] [Google Scholar]
- 38.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: A randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 39.Akerley BW, Herndon J, Turrisi AT, et al. Induction chemotherapy with paclitaxel and carboplatin followed by concurrent thoracic radiotherapy and weekly PC for patients with unresectable stage III non-small cell lung cancer: Preliminary analysis of a phase II trial by the CALGB [abstract 1915] Proc Am Soc Clin Oncol. 2000:19. [Google Scholar]
- 40.Vokes EE, Herndon J, Kelley MJ, et al. Induction chemotherapy followed by concomitant chemoradiotherapy (CT/XRT) versus CT/XRT alone for regionally advanced unresectable non-small cell lung cancer: Initial analysis of a randomized phase III trial. J Clin Oncol. 2004;22(14 suppl):7005. [Google Scholar]
- 41.Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: Results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol. 2007;25:3971–3977. doi: 10.1200/JCO.2007.10.8951. [DOI] [PubMed] [Google Scholar]
- 42.Agarwala SS, Cano E, Heron DE, et al. Long-term outcomes with concurrent carboplatin, paclitaxel and radiation therapy for locally advanced, inoperable head and neck cancer. Ann Oncol. 2007;18:1224–1229. doi: 10.1093/annonc/mdm088. [DOI] [PubMed] [Google Scholar]
- 43.Chougule PB, Akhtar MS, Rathore R, et al. Concurrent chemoradiotherapy with weekly paclitaxel and carboplatin for locally advanced head and neck caner: Long-term follow-up of a Brown University Oncology Group phase II study (HN-53) Head Neck. 2008;30:289–296. doi: 10.1002/hed.20700. [DOI] [PubMed] [Google Scholar]
- 44.Salama JK, Stenson KM, Kistner EO, et al. Induction chemotherapy and concurrent chemoradiotherapy for locoregionally advanced head and neck cancer: A multi-institutional phase II trial investigating three radiotherapy dose levels. Ann Oncol. 2008;19:1787–1794. doi: 10.1093/annonc/mdn364. [DOI] [PubMed] [Google Scholar]
- 45.Donson AM, Addo-Yobo SO, Handler MH, et al. MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma. Pediatr Blood Cancer. 2007;48:403–407. doi: 10.1002/pbc.20803. [DOI] [PubMed] [Google Scholar]
- 46.Friedman HS, Dolan ME, Pegg AE, et al. Activity of temozolomide in the treatment of central nervous system tumor xenografts. Cancer Res. 1995;55:2853–2857. [PubMed] [Google Scholar]
- 47.Wedge SR, Porteous JK, Newlands ES. Effect of single and multiple administration of an O6-benzylguanine/temozolomide combination: An evaluation in a human melanoma xenograft model. Cancer Chemother Pharmacol. 1997;40:266–272. doi: 10.1007/s002800050657. [DOI] [PubMed] [Google Scholar]
- 48.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 50.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 51.Addeo R, De Rosa C, Faiola V, et al. Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for nonsmall cell lung cancer and breast cancer patients with brain metastases. Cancer. 2008;113:2524–2531. doi: 10.1002/cncr.23859. [DOI] [PubMed] [Google Scholar]
- 52.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV titer, Bcl-xL and p53, sex and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pivot X, Magné N, Guardiola E, et al. Prognostic impact of the epidermal growth factor rector levels for patients with larynx and hypopharynx cancer. Oral Oncol. 2005;41:320–327. doi: 10.1016/j.oraloncology.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Nyati MK, Morgan MA, Feng FY, et al. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6:876–885. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 55.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Eng J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 56.Hughes S, Liong J, Miah A, et al. A brief report on the safety of induction chemotherapy followed by synchronous radiotherapy and cetuximab in stage III non-small cell lung cancer (NSCLC): SCRATCH study. J Thor Oncol. 2008;3:648–651. doi: 10.1097/JTO.0b013e3181757a60. [DOI] [PubMed] [Google Scholar]
- 57.Jensen AD, Münter MW, Bischoff H, et al. Treatment of non-small cell lung cancer with intensity-modulated radiation therapy in combination with cetuximab: The NEAR protocol ( NCT00115518) BMC Cancer. 2006;6:122. doi: 10.1186/1471-2407-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munter M, Timke C, Abdollahi A, et al. Final results of a phase II trial for patients with primary inoperable locally advanced pancreatic cancer combining intensity modulated radiotherapy (IMRT) with cetuximab and gemcitabine. J Clin Oncol. 2008;26(15 suppl):4613. [Google Scholar]
- 59.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 60.Chun PY, Feng FY, Scheurer AM, et al. Synergistic effects of gemcitabine and gefitinib in the treatment of head and neck carcinoma. Cancer Res. 2006;66:981–988. doi: 10.1158/0008-5472.CAN-05-2665. [DOI] [PubMed] [Google Scholar]
- 61.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 63.Langer CJ. The “lazarus response” in treatment-naive, poor performance status patients with non-small-cell lung cancer and epidermal growth factor receptor mutation. J Clin Oncol. 2009;27:1350–1354. doi: 10.1200/JCO.2008.20.4859. [DOI] [PubMed] [Google Scholar]
- 64.Ahsan A, Hiniker SM, Davis MA, et al. Role of cell cycle in epidermal growth factor receptor inhibitor-mediated radiosensitization. Cancer Res. 2009;69:5108–5114. doi: 10.1158/0008-5472.CAN-09-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stinchcombe TE, Morris DE, Lee CB, et al. Induction chemotherapy with carboplatin, irinotecan, and paclitaxel followed by high dose three-dimensional conformal thoracic radiotherapy with concurrent carboplatin, paclitaxel, and gefitinib in unresectable stage IIIA and stage IIIB non-small cell lung cancer. J Thorac Oncol. 2008;3:250–257. doi: 10.1097/JTO.0b013e3181653cf4. [DOI] [PubMed] [Google Scholar]
- 66.Ahmed SM, Cohen EE, Haraf DJ, et al. Updated results of a phase II trial integrating gefitinib into concurrent chemoradiation followed by G adjuvant therapy for locally advanced head and neck cancer. J Clin Oncol. 2007;25(18 suppl):6028. [Google Scholar]
- 67.Van Triest B, Kuenen B, Ghotra V, et al. Feasibility study of erlotinib plus radiotherapy in patients with locally advanced or recurrent rectal carcinoma [abstract 153] Int J Radiat Oncol Biol Phys. 2008;72(suppl 1):S69. [Google Scholar]
- 68.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 70.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine and radiotherapy for locally advanced rectal cancer. J Clin Oncol. 2008;26(15 suppl):4091. doi: 10.1016/j.ijrobp.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 72.Kirkpatrick JP, Desjardins A, Reardon DA, et al. Radiotherapy, temozolomide and bevacizumab followed by irinotecan, temozolomide and bevacizumab in newly diagnosed glioblastoma mutliforme: Preliminary results from an ongoing phase II trial [abstract 2089] Int J Radiat Oncol Biol Phys. 2008;72(1 suppl):S209. [Google Scholar]
- 73.Small W, Mulcahy M, Rademaker A, et al. A phase II trial of weekly gemcitabine and bevacizumab in combination with abdominal radiation therapy in patients with localized pancreatic cancer [abstract 13] Int J Radiat Oncol Biol Phys. 2008;72(1 suppl):S6. [Google Scholar]
- 74.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and bevacizumab-based chemoradiation for resectable pancreatic adenocarcinoma. J Clin Oncol. 2008;26(15 suppl):4630. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 75.Crane CH, Ellis LM, Abbruzzese JL, et al. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol. 2006;24:1145–1151. doi: 10.1200/JCO.2005.03.6780. [DOI] [PubMed] [Google Scholar]
- 76.Morgan MA, Parsels LA, Kollar LE, et al. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14:5142–5149. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]