The literature supporting the prognostic and predictive ability of the 21-gene assay in hormone receptor–positive, node-negative and node-positive breast cancer patients in chemotherapy- and endocrine-treated and untreated populations is reviewed.
Keywords: Recurrence score, Prognostic test, Breast cancer, Adjuvant chemotherapy
Abstract
Purpose.
A major challenge in treating early-stage hormone receptor (HR)+ breast cancer is selecting women who, after initial surgery, do not require chemotherapy. Better prognostic and predictive tests are needed. The 21-gene assay is the only widely commercially available gene signature that can be performed on formalin-fixed paraffin-embedded tissue.
Methods.
We conducted a review of the literature supporting the prognostic and predictive ability of the 21-gene assay in HR+ node-negative and node-positive breast cancer patients in chemotherapy-/endocrine-treated and untreated populations.
We considered: (a) How accurate is the recurrence score (RS) as a prognostic factor for distant recurrence? (b) How accurate is the RS as a predictive factor for benefit from systemic therapy? (c) How does the RS compare with other prognostic/predictive factors such as tumor size, tumor grade, patient age, and integrated decision aids such as Adjuvant! Online? (d) How do patients and physicians view the 21-gene assay? (e) What are the cost implications of the 21-gene assay?
Results.
The 21-gene assay: (a) provided accurate risk information; (b) predicted response to cyclophosphamide, methotrexate, and 5-fluorouracil and to cyclophosphamide, doxorubicin, and 5-fluorouracil chemotherapy; (c) added additional information to traditional biomarkers; (d) was viewed positively by both physicians and patients; and (e) fell within the cost-effectiveness values in North America.
Conclusion.
This assay may be offered to patients with node-negative HR+ breast cancer to assist in adjuvant treatment decisions. Data are accumulating to support the use of the 21-gene assay in HR+ node-positive patients.
Introduction
A major challenge in the treatment of early-stage hormone-receptor (HR)+ breast cancer is the selection of women who, after initial surgery, do not require chemotherapy. There are a number of international guidelines for the adjuvant treatment of node-negative HR+ breast cancer patients based on standard clinicopathological features [1–3]. There is also the software program Adjuvant! Online (AOL), which projects outcomes at 10 years based on clinicopathological features and therapy [4, 5].
There are three commercially available prognostic tests based on gene expression technology. The 21-gene assay (OncotypeDX; Genomic Health, Inc., Redwood City, CA) and the H/I assay (AvariaDx, Carlsbad, CA) use real-time reverse transcriptase polymerase chain reaction (RT-PCR) to quantify mRNA in formalin-fixed, paraffin-embedded tissue. MammaPrint (Agendia BV, Amsterdam, The Netherlands) uses DNA microarray technology (labeled patient mRNA hybridized to DNA sequences from known genes on a customized microarray chip) to identify mRNA in fresh or frozen tumor tissue. The analytical validity and other aspects of each test have been reviewed elsewhere [6–10].
The subject of this review is the widely commercially available 21-gene assay, an RT-PCR assay that measures the gene expression of 16 cancer-related and five control genes in paraffin-embedded tissue. Based on the level of expression of each gene, a recurrence score (RS) is calculated with a 95% confidence interval. An RS <18 is considered low risk, whereas an RS ≥18 and <31 is considered intermediate risk and an RS ≥31 is considered high risk for distant recurrence at 10 years [11, 12].
We specifically wanted to address the clinical utility of the 21-gene assay as a diagnostic test for prognosis and prediction of response to therapy. We conducted a review of the literature in order to examine the following questions.
How accurate is the RS as a prognostic factor for distant recurrence?
How accurate is the RS as a predictive factor for therapeutic benefit to systemic therapy?
How does the RS compare with other prognostic/predictive factors such as tumor size, tumor grade, patient age, and integrated decision aids such as AOL?
How do patients and physicians view the 21-gene assay in clinical practice?
What are the cost implications of the 21-gene assay?
Methods
Data Sources and Searches
MEDLINE (from 1996) and EMBASE (from 1980) were searched using the medical subject heading “Gene expression profiling” and the following text terms: 21-gene assay, recurrence score, RT-PCR assay, OncotypeDX, breast neoplasm. Further relevant studies were identified by hand searching the references from original and review articles. Abstracts published in the proceedings of annual meetings of the American Society of Clinical Oncology (ASCO) and the San Antonio Breast Cancer Symposium were reviewed.
We calculated the test properties for some studies. The pretest probability for a distant recurrence was extracted from trial outcome data. If a clinical trial reported the proportion of patients without a distant recurrence at 10 years as 85%, it was estimated that all patients meeting the trial entry criteria had a risk for distant recurrence of approximately 15%. In practice however, clinicopathological parameters and decision aids can refine risk estimates. The usefulness of the RS was determined by the accuracy with which it could distinguish patients who developed a distant recurrence from those who did not. The accuracy measure we used was the likelihood ratio (LR), that is, how likely is a high RS among patients who develop a distant recurrence and how likely is a high RS among patients who do not develop a distant recurrence? The ratio of these two likelihoods, the LR, is used in this review [13].
Study Selection
All validation studies examining the prognostic accuracy of the RS were reviewed in addition to studies assessing prediction. Studies including abstracts that allowed calculation of the test properties were selected. We included articles that examined populations other than the population for which the test was intended, for example, node-positive patients, patients receiving neoadjuvant chemotherapy, and patients assessed using other outcome measures, such as locoregional failure, breast cancer death, and disease-free survival (DFS), which should be considered when interpreting the study results.
How Accurate Is the RS as a Prognostic Factor for Distant Recurrence?
Tamoxifen-Treated Node-Negative Patients
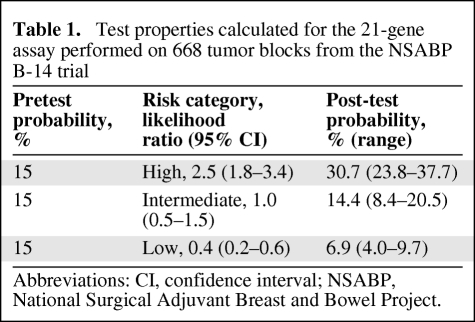
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 trial randomized 2,617 node-negative HR+ women in order to examine the benefit of tamoxifen versus placebo [14]. The 21-gene assay was performed on 668 paraffin-embedded tumor blocks from the tamoxifen arm of the trial. The proportion of patients in the low-risk group who were free from distant recurrence at 10 years (93.2%) was significantly greater than the proportion in the high-risk category (69.5%) (p < .001) [15].
A high RS was 2.5× (LR, 2.5; 95% confidence interval [CI], 1.8–3.4) as likely to occur in a patient who developed a distant recurrence at 10 years compared to a patient who did not. A low RS was 2.5× (LR, 0.4; 95% CI, 0.2.–0.6) as likely to occur in a patient who did not develop a distant recurrence at 10 years compared to a patient who did. The LR for the intermediate group (LR, 1.0; 95% CI, 0.5–1.5) was 1, which suggests that the test provided little additional information. However, it does indicate that these patients are not falling into the low- or high-risk category but somewhere in between.
The pretest probability for the cohort for the development of distant recurrence at 10 years was 15%. The post-test probabilities for high-, intermediate-, and low-risk categories were 30.7%, 14.4%, and 6.9%, respectively, demonstrating the ability of the test to categorize tumors according to the risk for distant recurrence (Table 1).
Table 1.
Test properties calculated for the 21-gene assay performed on 668 tumor blocks from the NSABP B-14 trial
Abbreviations: CI, confidence interval; NSABP, National Surgical Adjuvant Breast and Bowel Project.
The sensitivity of the RS was 76.9% (95% CI, 75.1%–80.3%), indicating that about 77% of patients who developed a distant recurrence had a high/intermediate RS. The specificity was 55.4% (95% CI, 54.1%–56.8%), indicating that 55% of patients who did not have a recurrence had a low RS.
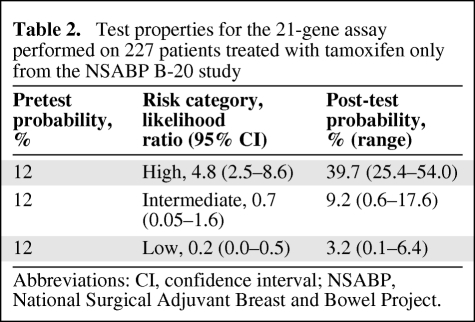
The NSABP B-20 trial examined the benefit of concurrent tamoxifen and chemotherapy compared with tamoxifen alone in node-negative estrogen receptor (ER)+ breast cancer patients [16]. Tumor specimens from the tamoxifen-only arm were used as a training set in the development of the assay, and therefore the test properties and prognostic ability were superior to what would be expected in an independent cohort [17]. A high RS in the tamoxifen-only group was almost five times (LR, 4.8; 95% CI, 2.5–8.6) as likely to occur in a patient who developed a distant recurrence at 10 years compared to a patient who did not. A low RS was five times (LR, 0.2; 95% CI, 0.0–0.5) as likely to occur in a patient who did not develop a distant recurrence at 10 years compared to a patient who did (Table 2). There was an overall pretest probability of 12%; however, the post-test probabilities were 40%, 9%, and 3% for the high-, intermediate-, and low-risk categories, respectively (Table 2). The sensitivity was 84% (95% CI, 79%–98%) and specificity was 65% (95% CI, 62.8%–68.3%).
Table 2.
Test properties for the 21-gene assay performed on 227 patients treated with tamoxifen only from the NSABP B-20 study
Abbreviations: CI, confidence interval; NSABP, National Surgical Adjuvant Breast and Bowel Project.
A nested case–control study was conducted by Habel et al. [18] to determine the degree to which the RS could predict the risk for breast cancer–specific mortality among HR+ node-negative patients. A case was a patient whose first event was death from breast cancer. At each case's death, up to three controls were randomly selected from the patients alive and under follow-up, matched for age, race, tamoxifen treatment, year of diagnosis, and treating hospital. The relative risks for the RS were calculated with regard to an increment of 50 units. For the 55 cases and 150 controls who had been treated with tamoxifen, the risk for death was positively associated with the RS (relative risk [RR], 7.6; 95% CI, 2.6–21.9).
The association between the RS and locoregional recurrence was studied in 895 tamoxifen-treated patients from the NSABP B-14 and B-20 trials, 355 placebo-treated patients (from the NSABP B-14 trial), and 424 chemotherapy plus tamoxifen–treated patients (from the NSABP B-20 trial). Locoregional recurrence was significantly associated with the RS in tamoxifen-treated patients (p = < .00001), with locoregional recurrence rates of 4.3%, 7.2%, and 15.8% in the low, intermediate, and high RS groups, respectively. The RS was significantly associated with locoregional recurrence in placebo-treated patients (p = .022) and in tamoxifen plus chemotherapy–treated patients (p = .028) [19].
Untreated Node-Negative Patients
In a study by Esteva et al. [20] in 149 node-negative patients who did not receive systemic therapy and had a minimum follow-up of 5 years, there was no significant difference observed in the 10-year distant recurrence-free survival (DRFS) rate among RS groups. However, both HR+ and HR− patients were included in the final analysis.
The RS was examined in 355 patients from the placebo arm of the NSABP B-14 trial [21]. The DRFS rates at 10 years were 85.9%, 62.2%, and 68.7% for the low, intermediate, and high RS groups, respectively. Habel et al. [18] also included HR+ patients not treated with tamoxifen. The risk for breast cancer death was associated with the RS (RR, 4.1; 95% CI, 2.1–8.1), but not as strongly as for the tamoxifen-treated patients, which likely relates to the inclusion of genes in the RS that are weakly prognostic but highly predictive of tamoxifen benefit.
Node-Positive Disease Treated with Tamoxifen
The Southwest Oncology Group (SWOG) 8814 study randomized 1,477 postmenopausal women with HR+ node-positive breast cancer to tamoxifen alone or anthracycline-based chemotherapy—cyclophosphamide, doxorubicin, and 5-fluorouracil (CAF)—plus tamoxifen. The 21-gene assay was performed on 40% of the trial population (148 tamoxifen-treated patients and 219 chemotherapy plus tamoxifen–treated patients). For the patients treated with tamoxifen alone, the 10-year DFS rates were 60%, 49%, and 43% for the low, intermediate, and high RS categories (p = .017). The continuous RS was prognostic in the first 5 years (HR, 5.55; 95% CI, 2.32–3.28; p = .0002 for a 50-point difference), but not beyond 5 years (HR, 0.86; 95% CI, 0.27–2.74; p = .80) [22].
Node-Negative and Node-Positive Patients Treated with Anastrozole or Tamoxifen
The RS was examined in 1,231 postmenopausal, chemotherapy-untreated women randomized to either anastrozole or tamoxifen in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial. There were 872 women with node-negative disease (432 tamoxifen-treated patients and 440 anastrozole-treated patients) and 306 women with node-positive disease (152 tamoxifen-treated patients and 154 anastrozole-treated patients). The treatment arms were combined and the 9-year distant recurrence rates for women with node-negative disease were 4%, 12%, and 25% for the low, intermediate, and high RS groups, respectively (p < .001), and for those with node-positive disease, the 9-year distant recurrence rates were 17%, 28%, and 49% (p < .001) for the low, intermediate, and high RS groups, respectively [23].
Node-Negative or Node-Positive Disease Treated with Chemotherapy Followed by Hormonal Therapy
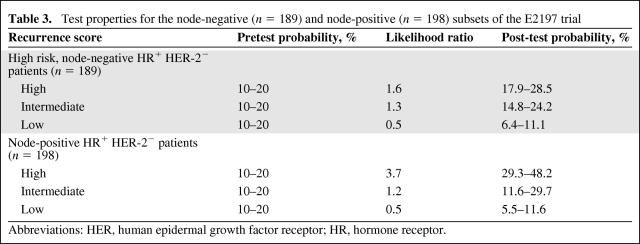
The E2197 trial included 2,885 evaluable patients with zero to three positive nodes treated with four cycles of doxorubicin and cyclophosphamide or four cycles of docetaxel and cyclophosphamide followed by hormonal therapy for 5 years. There was no difference in the DFS rate between treatment arms at 76 months [24]. The RS was examined in 776 patients from that trial [25]. Test properties for the node-negative (n = 189) and node-positive (n = 198), human epidermal growth factor receptor (HER)2− HR+ cases are shown in Table 3. Because the 5-year reported DFS rate for this group was 90% and the follow-up for this study was 6.3 years, a pretest probability of recurrence was estimated at 10%–20%. The low RS group (node negative and node positive) had an excellent outcome, with a post-test probability of 5.5%–11% depending on the pretest probability used.
Table 3.
Test properties for the node-negative (n = 189) and node-positive (n = 198) subsets of the E2197 trial
Abbreviations: HER, human epidermal growth factor receptor; HR, hormone receptor.
How Accurate Is the RS as a Factor Predictive of Therapeutic Benefit?
Adjuvant Chemotherapy Benefit in Node-Negative Patients Receiving Tamoxifen
The RS was assessed in 651 patients from the NSABP B-20 trial to determine if it could predict the magnitude of benefit from cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) [17]. Patients in the tamoxifen-only arm with a high RS had a DRFS rate of 60.5% (95% CI, 46.2%–74.8%). Chemotherapy in addition to tamoxifen led to a higher DRFS rate of 88.1% (95% CI, 82.0%–94.2%). In contrast, patients treated with tamoxifen only who had a low RS had a DRFS rate of 96.8% (95% CI, 83.3%–92.3%) and the addition of CMF did not change this significantly (95.6%; 95% CI, 92.7%–98.6%).
Adjuvant Chemotherapy in Node-Positive Patients Receiving Tamoxifen
As previously discussed, Albain et al. [22] studied the RS in a subset of patients from the SWOG 8814 study to determine if there were node-positive patients who did not benefit from chemotherapy. The high RS group appeared to benefit more from CAF (HR, 0.59; 95% CI, 0.35–1.01) than from tamoxifen alone. The addition of chemotherapy in the high RS group resulted in a statistically significant higher DFS rate—43% (95% CI, 28%–57%) versus 55% (95% CI, 40%–67%). There was a trend toward a lack of CAF benefit in the low RS group (HR, 1.02; 95% CI, 0.54–1.93); however, for both the low and intermediate RS groups (HR, 0.72; 95% 0.39–1.31) the CIs were wide and benefit cannot be ruled out entirely in these groups.
Prediction of Response to Neoadjuvant Chemotherapy
Gianni et al. [26] studied the gene expression profiles of pretreatment core biopsies of 89 patients with locally advanced breast cancer who received neoadjuvant paclitaxel and doxorubicin. The RS was positively associated with the likelihood of a pathological complete response (p = .005) [26].
Prediction of Response to Tamoxifen in Node-Negative Patients
Paik et al. [21] examined the ability of the RS to predict tamoxifen benefit, comparing the DRFS by RS group in the placebo and tamoxifen-only arms of the NSABP B-14 study. There were 645 evaluable specimens (355 placebo treated and 290 tamoxifen treated). Patients in the intermediate- and low-risk groups benefited from tamoxifen, but those in the high risk group derived little benefit (Table 4).
Table 4.
Predictive ability of the 21-gene assay using specimens from the placebo (n = 355) and tamoxifen-treated (n = 290) arms of the NSABP B-14 study
Abbreviations: CI, confidence interval; NSABP, National Surgical Adjuvant Breast and Bowel Project; RS, recurrence score.
How Does the 21-Gene Assay Compare with Other Prognostic/Predictive Factors?
AOL and the 21-Gene Assay
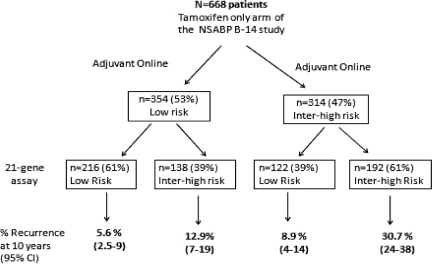
The 21-gene assay generates an estimate of the risk for distant recurrence (metastatic disease). In contrast, risk estimates generated by AOL include all causes of recurrence (local, regional, contralateral, and distant recurrence). As a result, recurrence risks are generally higher with AOL than with the 21-gene assay [27]. Bryant reclassified 668 patients from the tamoxifen-only arm of the NSABP B-14 study using AOL (Fig. 1) [28]. Patients classified by both tests as low risk had a 10-year distant recurrence rate of 5.6% (95% CI, 2.5%–9%). If AOL classified a patient as intermediate/high risk and the 21-gene assay reclassified them as low risk, the 10-year recurrence rate was 8.9% (95% CI, 4%–14%). The CIs here are wide and suggest that there may be patients who would benefit from additional therapy. If both tests classified a patient as high risk, the 10-year recurrence rate was 30.7% (95% CI, 24%–38%). If AOL classified a patient as low risk, but the 21-gene assay indicated intermediate to high risk, the 10-year recurrence rate was 12.9% (95% CI, 7%–19%).
Figure 1.
Risk for breast cancer recurrence as determined by Adjuvant! Online and the modified risk for breast cancer recurrence using the 21-gene assay. Shown is a determination of the risk for breast cancer recurrence by Adjuvant! Online for 668 patients from the tamoxifen-only arm of the NSABP B-14 study and reclassification by OncotypeDX and recurrence at 10 years.
Abbreviations: CI, confidence interval; NSABP, National Surgical Adjuvant Breast and Bowel Project.
Based on data from Bryant J. Toward a more rational selection of tailored adjuvant therapy. Data from the National Surgical Adjuvant Breast and Bowel Project. Presented at the Primary Therapy of Early Breast Cancer – 9th International Conference, St Gallen, Switzerland, January 26–28, 2005.
The prognostic utility of the RS compared with an algorithm similar to AOL but modified for outcome at 5 years and referred to as the Integrator was examined using 465 patients from the Eastern Cooperative Oncology Group E2197 trial [29]. There was poor concordance between predictions made by the RS and by the Integrator using either risk group or risk percentile classification for comparison and using both local and centrally determined tumor grade. In a proportional hazards regression model that included only the RS and the Integrator risk percentiles, only the RS remained statistically significant, and the RS by Integrator interaction term was significant, indicating that the RS was independent of Integrator risk. There was a fourfold higher RR for recurrence for a patient classified as low risk by the Integrator, but with a high RS. In addition, for patients with a low RS and classified as high risk by the Integrator, the RR was 3.15-fold higher. Both tests provided information that was independent of and in addition to the other. Data from the ATAC trial showed that the RS and AOL were independent predictors of distant recurrence and relapse, but the correlation was weak between the tests (r = 0.234) [23].
Multivariate Analyses Examining the RS in Models with Clinicopathological Factors
In multivariate models from the NSABP B-14 trial including the RS, age at surgery, tumor size, tumor grade (moderate and high), HER-2 amplification, and ER, the RS and high tumor grade were significant predictors of distant recurrence [15]. In the E2197 subset, the RS was a significant predictor of recurrence for node-positive and node-negative patients and for the HER-2− subset [29]. In Cox proportional hazards models for recurrence, when the linear RS, centrally determined tumor grade, HER-2 expression level, tumor size, age, and number of positive nodes were examined, only two to three positive nodes, young age, and grade remained significant predictors, and there was a trend toward significance for the RS (HR for a 50-point difference in RS, 2.12; 95% CI, 0.97–4.65; p = .06, linear trend test). However, the RS was a significant predictor when locally determined grade was used (HR, 3.13; 95% CI, 1.60–6.14; p = .0009). In a model with only HER-2− patients, the RS was not predictive, regardless of whether the tumor grade was determined locally or centrally. In the Habel et al. [18] study, in models with the RS, tumor size, and tumor grade, only the RS and tumor size retained statistical significance as predictors of breast cancer mortality. In patients treated without tamoxifen, tumor grade, tumor size, and the RS were all significant predictors of breast cancer mortality when included in one model [18]. In 872 postmenopausal women with node-negative disease who received no chemotherapy and were randomized to anastrozole or tamoxifen, a model adjusted for age and treatment and including centrally determined tumor grade found tumor size (p < .001) and the RS (p < .001) to be significant predictors of recurrence [23].
Preliminary data suggest that combining routine clinical and centrally tested immunohistochemical (IHC) markers into a single composite prognostic risk profile might be more powerful than any single marker [30–32]. Using multivariate proportional hazards regression, the transATAC group built a model based on IHC staining for ER, progesterone receptor, HER-2, and Ki67 (IHC4). The RS and IHC4 contributed equivalent information to routine clinical data (age, tumor grade, tumor size, and treatment). Concordance between the IHC4 and RS was high (ρ = 0.70) [30].
How Do Patients and Physicians View the 21-Gene Assay in Clinical Practice?
In a prospective multicenter study, 89 patients were assessed prior to and after the 21-gene assay [33]. Patient satisfaction was high. There was a significant reduction in conflict over treatment decisions, reduction in anxiety scores, greater patient satisfaction, and increased confidence with their choice of adjuvant therapy after taking the test. About 76% of medical oncologists involved in their care also found that the assay increased their confidence in treatment recommendations [34].
A number of studies reported significant changes in the recommendation for adjuvant treatment with the use of the RS [35–38]. Ben-Baruch et al. [36] found that knowledge of the RS led to 58% of patients initially offered chemotherapy being treated with hormonal treatment only, whereas 12% of patients initially offered hormonal treatment were treated with chemotherapy. Erb et al. [37] found a reduction in chemotherapy administration to node-negative HR+ breast cancer patients from 55% to 25% after the RS was introduced. Interviews conducted on 163 stage I/II breast cancer patients who had completed adjuvant chemotherapy found that health literacy affected retention of information about the RS but not the desire for information regarding the test [39].
What Are the Cost Implications of the 21-Gene Assay?
Hornberger et al. [40] conducted a cost–utility analysis using the RS in patients classified as having a low or high risk for distant recurrence based on National Comprehensive Cancer Network (NCCN) (http://www.nccn.org) risk criteria. Two scenarios were considered, involving patients with node-negative ER+ early breast cancer expected to receive 5 years of hormonal therapy; that is, patients classified as either (a) high risk (tumor size >1 cm or, for smaller tumors, if associated with high risk features) or (b) low risk by the NCCN risk criteria. The RS was then used to reclassify these patients independently based on results from the NSABP B-14 trial data. The assumption was that all patients assigned as intermediate/high risk by the RS would undergo chemotherapy and all patients assigned as low risk by the RS would not receive chemotherapy. Both taxane-containing and nontaxane-containing regimens were considered. Cost estimation included the cost of the drug, infusion, patient time, use of colony-stimulating factors (CSFs), and management of chemotherapy-related side effects. The analysis considered survival, quality of life, and costs from a societal perspective. At baseline values, the RS applied to 100 potential patients predicted a quality-adjusted survival time that was 8.6 years longer with overall costs that were lower by US$202,828 (survival time 1.02 months longer and cost saving of $2,028.20 per patient).
Lyman et al. [41] incorporated the extended validation results for predictive accuracy into an economic model to guide the use of adjuvant systemic therapy in patients with node-negative HR+ early-stage breast cancer. Three adjuvant treatment strategies were compared: (a) treat all patients with chemotherapy followed by tamoxifen, (b) treat all patients with tamoxifen alone, and (c) treat patients by RS-guided therapy, with low-risk patients receiving tamoxifen only and intermediate- and high-risk patients receiving chemotherapy plus tamoxifen. RS-guided therapy was found to be associated with an individual life expectancy that was 2.2 years longer than with tamoxifen alone, and was associated with a life expectancy similar to that seen with the chemotherapy plus tamoxifen strategy. An estimated net cost savings of $2,256 per patient with RS-guided therapy was seen, compared with chemotherapy plus tamoxifen, with an incremental cost-effectiveness ratio of $1,944 per life-year saved, compared with tamoxifen alone. Cost estimation included five commonly used adjuvant chemotherapy regimens (doxorubicin and cyclophosphamide [AC] × 4; dose-dense [dd] AC × 4 with CSF; AC plus paclitaxel [AC-T] × 8; dd AC-T × 8 with CSF, docetaxel, doxorubicin, and cyclophosphamide [TAC] × 6 with CSF). The estimated cost saving was likely an underestimation because only drug cost was included in the analysis, and no indirect costs associated with chemotherapy were considered.
A recently published cost-effectiveness analysis compared RS-guided treatment with treatment guided by either the NCCN guideline or St. Gallen recommendation in the context of Japan's health care system. It concluded that RS-guided treatment was cost-effective, quoting incremental cost-effectiveness ratios of US$26,065 per quality-adjusted life year (QALY) compared with NCCN-guided treatment and US$10,774 per QALY compared with St. Gallen–guided treatment. Both were well under the suggested social willingness to pay for 1 life-year gained from an innovative medical intervention in Japan of US$52,174 per QALY [42].
Discussion
There are no prospective data evaluating the 21-gene assay. Most studies examining the prognostic and predictive accuracy of the test have used retrospective subsets from clinical trials. Results from node-negative tamoxifen-treated patients in the NSABP B-14 and B-20 trials and a case cohort study provide convincing evidence of the prognostic ability of the RS in node-negative tamoxifen-treated patients. Recent data from the SWOG 8814 and ATAC trials have demonstrated that the RS can provide prognostic information for node-positive tamoxifen-treated and aromatase inhibitor–treated patients. The RS was prognostic for the chemotherapy-treated node-negative and node-positive subsets in the E2197 study.
Patients with node-negative HR+ tumors with a high RS derived more benefit from CMF chemotherapy and hormones than similar patients with a low RS. Whether the intermediate RS group derives benefit from chemotherapy added to hormones is being studied in a multinational prospective trial. The objective of the Trial Assigning Individualized Options for Treatment (TAILORx) is to determine whether adjuvant hormonal therapy (i.e., the experimental arm) is noninferior to adjuvant chemohormonal therapy (the standard arm) for patients with an intermediate RS. The RS cutoffs were adjusted for this trial in order to allow patients with an estimated risk for distant recurrence >10% to be randomized to chemotherapy or not in the intermediate arm.
Recent data indicate that patients with HR+ node-positive tumors with a high RS derive more benefit from CAF chemotherapy and hormones than similar patients with an intermediate or low RS. Whether the RS predicts benefit from contemporary chemotherapeutic agents such as taxanes is uncertain. However, a higher rate of pathological complete response in patients treated with neoadjuvant taxane-based therapy suggests that this is likely [26]. For premenopausal women, a limitation of the studies is that the predictive value of the RS has not been tested for women given the combination of ovarian suppression plus tamoxifen.
In multivariate models with standard clinicopathological markers, the RS remained or trended toward being a significant predictor of recurrence [13, 16]. The RS provided information beyond that provided by AOL, although both provide independent prognostic information, and they should be used together [26, 27]. Preliminary data showed that quantitatively similar prognostic information was provided by an IHC composite score and the RS [30]. There was significant user satisfaction from both the patient and physician perspectives. Decisions based on RS-guided therapy were associated with a longer quality-adjusted survival time and superior cost-effectiveness. However, these studies made broad assumptions about treatment decisions that may not reflect clinical reality.
The 21-gene assay has been endorsed by ASCO for use as a tumor marker [43], and the NCCN Breast Cancer Panel considers it an option for patients with ER+ node-negative breast cancer as an aid to decision making regarding adjuvant chemotherapy [1]. The Evaluation of Genomic Applications Working Group considered current data insufficient to draw strong conclusions regarding the clinical utility of the assay for guiding treatment decisions for patients with early invasive breast cancer [44].
Conclusion
The 21-gene assay may be considered in patients with node-negative HR+ breast cancer to assist in adjuvant treatment decisions in conjunction with traditional clinicopathological markers. Patients with a low RS may be adequately treated with hormonal therapy alone. Those with a high RS are likely to benefit from chemotherapy in addition to hormones. The TAILORx study should assist in directing treatment decisions for the intermediate RS group. Data are accumulating to support the use of this test in the HR+ node-positive population.
Author Contributions
Conception/Design: Catherine M. Kelly
Collection and/or assembly of data: Catherine M. Kelly
Data analysis and interpretation: Catherine M. Kelly, Ellen Warner, Daphne T. Tsoi, Sunil Verma, Kathleen I. Pritchard
Manuscript writing: Catherine M. Kelly, Ellen Warner, Daphne T. Tsoi, Sunil Verma, Kathleen I. Pritchard
Final approval of manuscript: Catherine M. Kelly, Ellen Warner, Daphne T. Tsoi, Sunil Verma, Kathleen I. Pritchard
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™. Breast Cancer. V.I. 2010. [accessed January 19, 2010]. Available at http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
- 2.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health. Adjuvant Therapy for Breast Cancer. NIH Consens Statement. 2000;17:1–35. [PubMed] [Google Scholar]
- 4.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 5.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 6.Marchionni L, Wilson RF, Wolff AC, et al. Systematic review: Gene expression profiling assays in early-stage breast cancer. Ann Intern Med. 2008;148:358–369. doi: 10.7326/0003-4819-148-5-200803040-00208. [DOI] [PubMed] [Google Scholar]
- 7.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 8.Desmedt C, Ruiz-García E, André F. Gene expression predictors in breast cancer: Current status, limitations and perspectives. Eur J Cancer. 2008;44:2714–2720. doi: 10.1016/j.ejca.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Ross JS, Hatzis C, Symmans WF, et al. Commercialized multigene predictors of clinical outcome for breast cancer. The Oncologist. 2008;13:477–493. doi: 10.1634/theoncologist.2007-0248. [DOI] [PubMed] [Google Scholar]
- 10.Dowsett M, Dunbier AK. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res. 2008;14:8019–8026. doi: 10.1158/1078-0432.CCR-08-0974. [DOI] [PubMed] [Google Scholar]
- 11.Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. The Oncologist. 2007;12:631–635. doi: 10.1634/theoncologist.12-6-631. [DOI] [PubMed] [Google Scholar]
- 12.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26:721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 15.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Redmond C. Systemic therapy in node-negative patients: Updated findings from NSABP clinical trials. National Surgical Adjuvant Breast and Bowel Project. J Natl Cancer Inst Monogr. 1992;(11):105–116. [PubMed] [Google Scholar]
- 17.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 18.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamounas E, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: Results from NSABP B-14 and NSABP B-20. J Clin Oncol. doi: 10.1200/JCO.2009.23.7610. 2010 Jan 11 [Epub ahead of print]. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteva FJ, Sahin AA, Cristofanilli M, et al. Prognostic role of a multigene reverse transcriptase-PCR assay in patients with node-negative breast cancer not receiving adjuvant systemic therapy. Clin Cancer Res. 2005;11:3315–3319. doi: 10.1158/1078-0432.CCR-04-1707. [DOI] [PubMed] [Google Scholar]
- 21.Paik S, Shak S, Tang G, et al. Expression of the 21-genes of the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer. J Clin Oncol. 2005;23(16 suppl):510. [Google Scholar]
- 22.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowsett M, Cuzick J, Wales C, et al. Risk of recurrence using OncotypeDX in postmenopausal primary breast cancer patients treated with anastrozole or tamoxifen: A TransATAC study. Presented at the 31st San Antonio Breast Cancer Symposium; December 10–14, 2008; San Antonio, TX. [Google Scholar]
- 24.Goldstein L, O'Neill A, Sparano J, et al. E2197: Phase III AT (doxorubicin/docetaxel) vs. AC (doxorubicin/cyclophosphamide) in the adjuvant treatment of node-positive and high risk node-negative breast cancer. J Clin Oncol. 2005;23(16 suppl):512. [Google Scholar]
- 25.Goldstein L, Gray R, Childs BH, et al. Prognostic utility of the 21-gene assay in hormone receptor positive operable breast cancer and 0–3 positive axillary nodes treated with adjuvant chemohormonal therapy: An analysis of Intergroup Trial E2197. J Clin Oncol. 2007;25(18 suppl):526. [Google Scholar]
- 26.Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23:7265–7277. doi: 10.1200/JCO.2005.02.0818. [DOI] [PubMed] [Google Scholar]
- 27.Adjuvant! Online. [accessed December 1, 2009]. Available at http://www.adjuvantonline.com.
- 28.Bryant J. Toward a more rational selection of tailored adjuvant therapy. Data from the National Surgical Adjuvant Breast and Bowel Project. Presented at the Primary Therapy of Early Breast Cancer – 9th International Conference; January 26–28, 2005; St. Gallen, Switzerland. [Google Scholar]
- 29.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26:4063–4071. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuzick J, Dowsett M, Wale C, et al. Prognostic value of a combined ER, PgR, Ki67, HER2 immunohistochemical (IHC4) score and comparison with the GHI recurrence score—results from TransATAC. Presented at the 32nd CTRC-AACR Annual San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, Texas. [Google Scholar]
- 31.Bartlett J, Brookes C, Robson T. The TEAM trial pathology study identifies potential prognostic and predictive biomarker models for postmenopausal patients treated with endocrine therapy. Presented at the 32nd CTRC-AACR Annual San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, Texas. [Google Scholar]
- 32.Viale G, Regan MM, Dell'Orto P, et al. Central review of ER, PgR and HER2 in BIG 1–98 evaluating letrozole vs. letrozole followed by tamoxifen vs. tamoxifen followed by letrozole as adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive breast cancer. Presented at the 32nd CTRC-AACR Annual San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, Texas. [Google Scholar]
- 33.Mumby P, Lo S, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on patient satisfaction, anxiety and decisional conflict for adjuvant breast cancer treatment selection. Breast Cancer Res Treat. 2007;106(1 suppl):10. [Google Scholar]
- 34.Lo SS, Norton P, Mumby PB, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2007;25(18 suppl):577. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 35.Liang H, Brufsky AM, Lembersky BB, et al. A retrospective analysis of the impact of OncotypeDX low recurrence score results on treatment decisions in a single academic breast cancer center. Breast Cancer Res Treat. 2007;106(1 suppl):2061. [Google Scholar]
- 36.Ben-Baruch N, Hammerman A, Klang S, et al. A prospective study of the impact of the 21-gene assay recurrence score on treatment decisions in N−, ER+ early breast cancer patients. J Clin Oncol. 2007;25(18 suppl):11008. [Google Scholar]
- 37.Erb C, Fox KR, Patel M, et al. Evaluation of practice patterns in the treatment of node-negative, hormone receptor-positive breast cancer patients with the use of the OncotypeDX assay at the University of Pennsylvania. Breast Cancer Res Treat. 2007;106(1 suppl):3082. [Google Scholar]
- 38.Oratz R, Paul D, Cohn AL, et al. Impact of OncotypeDX on decision making in breast cancer clinical practice. J Oncol Pract. 2007;3:182–186. doi: 10.1200/JOP.0742001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lillie SE, Brewer NT, O'Neill SC, et al. Retention and use of breast cancer recurrence risk information from genomic tests: The role of health literacy. Cancer Epidemiol Biomarkers Prev. 2007;16:249–255. doi: 10.1158/1055-9965.EPI-06-0525. [DOI] [PubMed] [Google Scholar]
- 40.Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11:313–324. [PubMed] [Google Scholar]
- 41.Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: An economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109:1011–1018. doi: 10.1002/cncr.22506. [DOI] [PubMed] [Google Scholar]
- 42.Kondo M, Hoshi SL, Ishiguro H, et al. Economic evaluation of 21-gene reverse transcriptase-polymerase chain reaction assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer in Japan. Breast Cancer Res Treat. 2008;112:175–187. doi: 10.1007/s10549-007-9842-y. [DOI] [PubMed] [Google Scholar]
- 43.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 44.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: Can tumor gene expression profiling improve outcomes in patients with breast cancer? Genet Med. 2009;11:66–73. doi: 10.1097/GIM.0b013e3181928f56. [DOI] [PMC free article] [PubMed] [Google Scholar]