Abstract
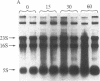
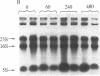
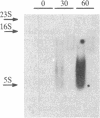
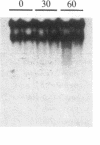
The isolation of a temperature-sensitive allele of RNase II (rnb) by in vitro mutagenesis has permitted the demonstration that RNase II and polynucleotide phosphorylase (PNPase) are required for cell viability and mRNA turnover in Escherichia coli. Double-mutant strains carrying the pnp-7 and rnb-500 alleles (PNPase deficient and RNase II thermolabile) ceased growing in Luria broth within 30 min after shift to the nonpermissive temperature. Cessation of growth was accompanied by an accumulation of mRNA fragments 100-1500 nucleotides long. In contrast, single-mutant and wild-type control strains grew normally at the nonpermissive temperature and did not accumulate mRNA. No significant changes in rRNA patterns were observed in any of the strains.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Donovan W. P., Kushner S. R. Amplification of ribonuclease II (rnb) activity in Escherichia coli K-12. Nucleic Acids Res. 1983 Jan 25;11(2):265–275. doi: 10.1093/nar/11.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra C. C., Prasher D., Kushner S. R. Physical and biochemical analysis of the cloned recB and recC genes of Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):21–27. doi: 10.1128/jb.157.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Har-El R., Silberstein A., Kuhn J., Tal M. Synthesis and degradation of lac mRNA in E. coli depleted of 30S ribosomal subunits. Mol Gen Genet. 1979 Jun 7;173(2):135–144. doi: 10.1007/BF00330303. [DOI] [PubMed] [Google Scholar]
- Kinscherf T. G., Apirion D. Polynucleotide phosphorylase can participate in decay of mRNA in Escherichia coli in the absence of ribonuclease II. Mol Gen Genet. 1975 Sep 8;139(4):357–362. doi: 10.1007/BF00267975. [DOI] [PubMed] [Google Scholar]
- Kivity-Vogel T., Elson D. A correlation between ribonuclease II and the in vivo inactivation of messenger RNA in E. coli. Biochem Biophys Res Commun. 1968 Nov 8;33(3):412–417. doi: 10.1016/0006-291x(68)90587-1. [DOI] [PubMed] [Google Scholar]
- Kivity-Vogel T., Elson D. On the metabolic inactivation of messenger RNA in Escherichia coli: ribonuclease I and polynucleotide phosphorylase. Biochim Biophys Acta. 1967 Mar 29;138(1):66–75. doi: 10.1016/0005-2787(67)90586-2. [DOI] [PubMed] [Google Scholar]
- Krishna R. V., Rosen L., Apirion D. Increased inactivation and degradation of messenger RNA in an Escherichia coli strain containing a thermolabile polynucleotide phosphorylase. Nat New Biol. 1973 Mar 7;242(114):18–20. doi: 10.1038/newbio242018a0. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Ono M., Endo H., Hori K., Nakamura K., Hirota Y., Ohnishi Y. Gene affecting longevity of messenger RNA: a mutant of Escherichia coli with altered mRNA stability. Mol Gen Genet. 1977 Sep 9;154(3):279–285. doi: 10.1007/BF00571283. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lennette E. T., Gorelic L., Apirion D. An Escherichia coli mutant with increased messenger ribonuclease activity. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3140–3144. doi: 10.1073/pnas.68.12.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev N., Folsom V., Schlessinger D. Escherichia coli mutants deficient in exoribonucleases. Biochem Biophys Res Commun. 1976 Jun 7;70(3):920–924. doi: 10.1016/0006-291x(76)90679-3. [DOI] [PubMed] [Google Scholar]
- Ono M., Kuwano M. Genetic analysis of mutations affecting ribonuclease II in Escherichia coli. Mol Gen Genet. 1977 May 20;153(1):1–4. doi: 10.1007/BF01035989. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Characterization of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol. 1969 Mar;97(3):1437–1443. doi: 10.1128/jb.97.3.1437-1443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Isolation and mapping of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol. 1969 Mar;97(3):1431–1436. doi: 10.1128/jb.97.3.1431-1436.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SPAHR P. F. PURIFICATION AND PROPERTIES OF RIBONUCLEASE II FROM ESCHERICHIA COLI. J Biol Chem. 1964 Nov;239:3716–3726. [PubMed] [Google Scholar]
- Staudenbauer W. L. REPLICAtion of small plasmids in extracts of Escherichia coli: requirement for both DNA polymerases I and II. Mol Gen Genet. 1976 Dec 8;149(2):151–158. doi: 10.1007/BF00332883. [DOI] [PubMed] [Google Scholar]
- Weatherford S. C., Rosen L., Gorelic L., Apirion D. Escherichia coli strains with thermolabile ribonuclease II activity. J Biol Chem. 1972 Sep 10;247(17):5404–5408. [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]