The article examines epidermal growth factor receptor inhibitor–induced papulopustular eruptions and their relation to staphylococcus coagulase–positive inflammation, and identifies an early phase and a late phase to this side effect.
Keywords: Staphylococcus coagulase positive, Epidermal growth factor receptor inhibitors, Erlotinib, Cetuximab
Abstract
Objective.
Cutaneous eruptions, mainly papulopustular, are the most common associated side effects of epidermal growth factor receptor inhibitors (EGFRIs).
This study investigated the possible role of bacterial infection in EGFRI-induced eruptions and its relation to clinical morphology.
Patients and Methods.
The study group consisted of all 29 patients referred for dermatologic evaluation of side effects of cetuximab or erlotinib from March 2008 to November 2009. Specimens were taken for bacterial culture from pustules in patients with grade >1 papulopustular rash and from periungual secretions in patients with paronychia.
Results.
Twenty-four of 29 patients had a papulopustular reaction; five of 29 had paronychia/xerosis. Of the papulopustular eruption patients, time to rash appearance yielded two distinct groups: early-phase, median 8 days after drug initiation, located mainly on the face (n = 17) and late-phase, median ∼200 days after drug initiation, located mainly on the trunk (n = 7). Bacterial culture grew Staphylococcus aureus (SA) in seven of 13 early-phase patients tested and in all late-phase patients. Treatment consisted of topical steroids with or without topical/systemic antibiotics. All patients had a clear improvement in their cutaneous symptoms within a few days. Dose reduction or temporary discontinuation of the EGFRI was necessary in only four of 29 patients.
Conclusions.
As described in the literature, EGFRI-induced papulopustular eruption may appear early and probably is an inflammatory process with or without SA secondary infection. The papulopustular eruption may also appear as a late phase, described here for the first time, which is an infectious process with all patients being SA+.
The >50% overall incidence of SA infection in our study highlights the need for routine bacterial cultures from EGFRI-induced eruption.
Introduction
Epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein that plays a role in the biological cascade that modulates cell proliferation, survival, adhesion, migration, differentiation, and other functions [1–3]. In cancer cells, it enhances many of the processes responsible for tumor growth and progression [1]. Accordingly, researchers have found that epidermal growth factor receptor inhibitors (EGFRIs) serve as effective therapy for various kinds of malignancies. Small molecule EGFRIs, such as gefitinib (Iressa®; AstraZeneca Pharmaceuticals, Wilmington, DE) and erlotinib (Tarceva®; Genentech, Inc., South San Francisco, CA; OSI Pharmaceuticals Inc., Melville, NY; F. Hoffmann-La Roche Ltd, Basel, Switzerland), selectively inhibit the tyrosine kinase (TK) activity of the intercellular domain of EGFR, thereby arresting autophosphorylation and subsequent activation of signaling cascades [2, 3]. Monoclonal antibodies (mAbs), such as cetuximab (Erbitux®; Bristol-Myers Squibb, Princeton, NJ) and panitumumab (Vectibix®; Amgen Inc., Thousand Oaks, CA), bind to the extracellular domain of EGFR, blocking its activation and signal transduction [4].
In adults the EGFRs are expressed in the skin, primarily on proliferating undifferentiated keratinocytes located within the basal layers of the epidermis, keratinocytes of sebocytes, and the outer root sheath of the hair follicle [5].
The main EGFRI-related toxicity is cutaneous, with the most common being a papulopustular eruption [6–13]. It is currently assumed that this eruption is an inflammatory reaction, with most cases described in the literature revealing sterile lesions when bacterial cultures are taken [6, 7].
The aim of the present study was to investigate the possible role of bacterial infection in EGFRI-induced eruptions and its relationship to clinical morphology.
Methods
The study group consisted of all patients referred from the Institute of Oncology, Davidoff Center, to the Department of Dermatology for evaluation of cutaneous side effects of cetuximab or erlotinib treatment from March 2008 to November 2009.
Only patients who were naïve to any kind of prior topical or systemic treatment for their skin eruption were included.
The cetuximab-treated patients had advanced-stage colon or head and neck carcinoma (squamous cell carcinoma [SCC]). The drug was given by i.v. infusion with an initial loading dose of 400 mg/m2 followed by 250 mg/m2 once a week or 500 mg/m2 every 2 weeks. The erlotinib-treated patients had metastatic non-small cell lung cancer or pancreatic cancer. Doses were in the range of 100–150 mg/day.
Skin toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC), version 3 [6], as follows: grade 1, minor asymptomatic macular or papulopustular erythematous eruption in an acneiform distribution; grade 2, resembles grade 1 but symptomatic (itching and pruritus), with more prominent lesions; grade 3, eruption extending beyond the acneiform distribution, in the head and neck, chest, or back or associated with confluent lesions; grade 4, ulcerating dermatitis.
Pruritus was also graded according to the NCI-CTC version 3 as grade 1, 2, or 3 [6]. Paronychia and xerosis were graded as mild, moderate, or severe.
Specimens were taken for bacterial culture from all patients with papulopustular rash of grade >1. They were obtained after cleaning the cutaneous pustules with alcoxidine solution, which contains 70% alcohol, and pinching them with a thin sterile needle. Because pus was discharged from pustules to the outer surface, bacterial cultures were taken from the upper part of the pus itself in order to avoid contamination. Twelve patients suffering from papulopustular eruption had nasal cultures taken as well.
In patients with moderate or severe paronychia, cultures were taken from secretions around the nail.
Response to treatment was considered complete when all lesions had disappeared and partial when at least 50% of lesions had disappeared, compared with baseline.
A single dermatologist evaluated all patients (I.A.-L.).
This was a prospective cohort, retrospectively summarized. The assessment and treatment approaches for the skin toxicity were standard of care at the Dermatology Department; therefore, no consent was needed from each individual. The protocol of the study was approved by the institutional review board.
Results
Thirty-four patients with cutaneous side effects of EGFRIs were referred for evaluation and treatment during the study period. Five were excluded from the study because they were already receiving treatment for cutaneous side effects at the time of referral. The remaining 29 patients included 15 men and 14 women with a mean age of 67 years (range, 50–92 years). Table 1 summarizes their clinical data. Twelve patients were treated with cetuximab (nine for colorectal cancer and three for SCC of the head and neck), and 17 were treated with erlotinib (16 for lung cancer and one for pancreatic cancer).
Table 1.
Clinical data of patients treated with cetuximab or erlotinib, suffering from dermatological side effects
Abbreviations: CNT, culture not taken; EP, early phase; LP, late phase; NSCLC, non-small cell lung cancer; SA, Staphylococcus aureus; SCC, squamous cell carcinoma.
Twenty-four patients had a papulopustular reaction (also described as an acneiform rash), of whom nine received cetuximab and 15 received erlotinib. Evaluation by time of onset of the rash yielded two distinct groups (Table 2). Seventeen patients (nine treated with erlotinib and eight treated with cetuximab) had an early-phase (EP) response, which started within the first 2 weeks of EGFRI treatment (median, 8 days; range, 4–14 days) and peaked in severity during the first 1–3 weeks. The other seven patients (six treated with erlotinib and one treated with cetuximab) had a late-phase (LP) papulopustular eruption, which started at a median of 200 days (range, 150–300) after initiation of EGFRI treatment.
Table 2.
Differences noted between early- and late-phase papulopustular eruptions
Abbreviations: EGFRI, epidermal growth factor receptor inhibitor; SA, Staphylococcus aureus.
The EP papulopustular eruption was located mainly on the face, usually in the seborrheic areas (forehead, nose, nasolabial folds, chin, and scalp), with or without upper trunk involvement. The lesions appeared as erythematous papules, follicular pustules, and, in some cases, yellow crusts (Figs. 1A, 1B, 2A, 2B). No comedos or cysts were present. Of the 17 patients with EP papulopustular eruption, in 13 patients the severity was grade >1 and bacterial cultures were performed. Seven cultures (55%) grew Staphylococcus coagulase–positive, all methicillin-sensitive Staphylococcus aureus (SA); one culture grew Enterobacter and one culture grew Citerobacter diversus; four cultures were sterile.
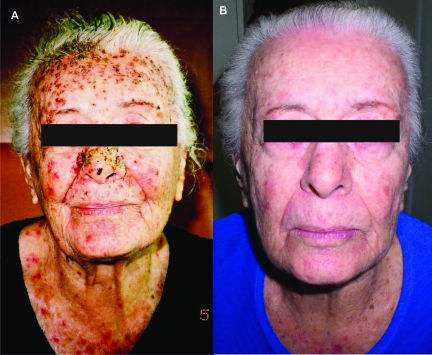
Figure 1.
Early-phase papulopustular eruption on the face, National Cancer Institute Common Toxicity Criteria grade 2–3 severity. (A) Before treatment. (B) One week after topical treatment.
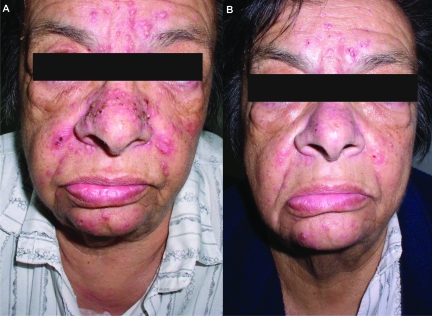
Figure 2.
Early-phase papulopustular eruption on the face, National Cancer Institute Common Toxicity Criteria grade 1 severity. (A) Before treatment. (B) One week after topical treatment.
The seven patients in the LP group (Fig. 3A, 3B) developed a papulopustular rash akin to the classic EP rash, but somewhat different. Four of the seven patients claimed they also had an EP rash during the 2–4 weeks after drug initiation. All patients reported that this EP eruption resolved completely a few months before the appearance of the LP rash.
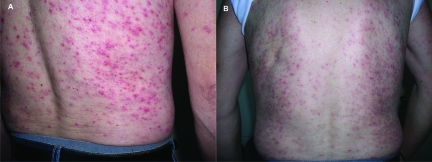
Figure 3.
Late-phase papulopustular eruption on the back, National Cancer Institute Common Toxicity Criteria grade 3 severity. (A) Before treatment. (B) Five days after treatment with oral ofloxacin.
The LP eruption was located on the chest, abdomen, back, buttocks, and thighs, and not on the face like the EP papulopustular eruption. Furthermore, the erythematous papules and follicular pustules were more pruritic—four patients with LP eruption had grade 3 severity pruritus and three patients had grade 2 pruritus, whereas, of the EP group, 14 patients had pruritus with grade 1–2 severity and only three patients had grade 3 pruritus. Bacterial cultures of specimens taken from all seven patients with LP rash (100%) grew SA, all methicillin-sensitive.
Nasal cultures taken from 12 of the 24 patients with papulopustular eruption grew SA from the nostril mucosa in nine (75%) of these cultures; in eight of these cases, the cutaneous cultures were also SA+. Among the remaining three patients, the nasal culture was positive for Citerobacter diversus in one and sterile in two.
All patients had a normal neutrophil value on CBC.
The treatment strategy for patients with papulopustular eruptions included antibacterial agents combined with class I steroids for the face and class II–III steroids for the trunk. Most were treated with prednisolone (0.5%) and chloramphenicol (3%) ointment, combined with clindamycin (1%) solution for the pustular lesions, for 2 weeks, after which the dose was gradually tapered down. Nine patients were also given systemic antibiotics, mostly because of the severity of their rash. Of those, four patients received initial empiric treatment with tetracycline, which was changed to ofloxacin in two cases because of SA resistance found on bacterial cultures. The other five patients were treated with ofloxacin, cephaxin, or clindamycin. In only four patients, the EGFRI dosage was reduced or temporarily halted because of cutaneous side effects. The condition of the patients treated only topically improved partially or completely within a mean of 5 days (Figs. 1A, 1B, 2A, 2B). The patients treated with oral antibiotics had a more severe rash and showed major though gradual improvement within 1–2 weeks.
Two patients, both treated with erlotinib, acquired severe multifocal eruptions consisting of very painful, disabling furuncles, carbuncles, and large abscesses, mainly on the upper and lower limbs and the buttocks. Both had lymphadenopathy around drainage areas of the eruption; one also had an elevated temperature of 38.5°C. Aside from these two patients, none of the others had systemic signs of infection. Both were positive for SA. The lesions began as a severe papulopustular eruption and then progressed. In one patient, the eruption appeared a few days after EGFRI initiation, and in the other it appeared 150 days after. In both, treatment with erlotinib was either halted or reduced, and systemic antibiotics were administered. An almost complete disappearance of the eruption was noted within 2 weeks.
Four patients, two treated with cetuximab and two treated with erlotinib, were referred because of disturbing paronychia. The paronychia started at a median of 150 days after treatment initiation (range, 120–180) and involved mainly the great toes, with nailfold swelling, erythema, and thick discharge. Bacterial cultures, taken from three patients with moderate–severe findings, grew SA.
One patient treated with cetuximab was referred because of severe xerosis, which appeared several months after initiating treatment, accompanied by deep fissures, up to 1.5 cm long, located on the palms and chest.
Discussion
According to the current literature, dermatologic toxicities are the most common adverse events associated with EGFRIs. They are more often associated with mAbs (found in approximately 85% of the patients treated with cetuximab) than with TK inhibitors (found in 75% of those given erlotinib). The rash appears to be dose dependent, and in most patients is mild to moderate (grades 1–2) [6, 7].
The most commonly reported cutaneous toxicity is the papulopustular eruption located predominantly on the seborrheic areas of the face, shoulders, and trunk. The eruption peaks in severity during the first 1–2 weeks of therapy and ends within a few weeks with the appearance of erythematotelangiectasias [6–10]. To our knowledge, this is the first study to also describe a later phase of papulopustular eruption, months after EGFRI initiation. Other less commonly reported side effects are xerosis, paronychia, alterations in hair growth (alopecia of the scalp, trichomegaly, and hypertichosis of the face), and telangiectasia [6].
In the present series, the EP reaction to EGFRIs was compatible with the classic EGFRI-induced papulopustular rash reported in the literature. The lesions resembled acne vulgaris, but, in contrast to acne, most lesions were larger, with erythematous papules and pustules, and lacked the basic lesion of typical acne vulgaris, the comedos. These findings suggest a different etiology for EGFRI-induced eruptions than for acne vulgaris. Seven of the 13 patients with the EP papulopustular eruption were SA+, which could indicate a secondary infection.
A significant observation was the LP papulopustular eruption identified in seven patients, six of whom were treated with erlotinib. The LP rash, although resembling the EP papulopustular eruption, should be thought of as a distinct cutaneous side effect of EGFRIs (Table 2). In addition to the timing of appearance, it had several distinct features: cutaneous location on the chest, back, buttocks, and thighs and not on the face; lesions were also erythematous papules and pustules, but were larger and more pruritic, and exclusively SA+. Taken together, and especially because all culture grew SA, these may imply a different, possibly infectious bacterial, pathogenesis underlying LP EGFRI-induced papulopustular eruption. This is as opposed to the EP rash, which is considered mainly an inflammatory process sometimes associated with secondary bacterial infection. The noninfectious, possibly immunological, etiology of the EP response is supported by the correlation of the severity of the rash with the response of the tumor to treatment [6–9].
Although the well-known inflammatory process in the epidermis caused by EGFRIs [14–16] may favor bacterial overgrowth, earlier studies reported that microbiological stains and bacterial cultures are usually sterile [6–13, 17]. However, in the present study, of the 20 patients who had papulopustular lesions of grade >1 severity, 14 (55% of the EP patients and 100% of the LP patients) showed SA overgrowth on bacterial cultures. This difference in bacterial culture results between the literature and our report could be explained by the fact that the vast majority of patients examined and reported on in the literature had EP papulopustular eruption, thought to be immunologic and less infectious. In our report, only half of the EP group harbored SA infection. Also, most reported patients did not have systematic skin cultures [7]. The distinction between bacterial colonization, in which SA presents on tissue surfaces that are in contact with the external environment, and bacterial infection, in which the bacterium invades the tissue, is crucial. We assume the positive bacterial results in this work represent a true infectious process, because all cultures were taken from the inner content of a pustule, after the outer surface was cleaned.
Two of our patients had also acquired large SA abscesses. Both were receiving erlotinib, and both had previously had a papuloerythematous eruption. This finding also highlights the need for routine cultures taken from lesions in this setting.
We also found nasal carriage of SA in nine of the 12 patients from whom nose cultures were taken, eight of whom had SA in the skin lesions as well. It thus would be interesting to further explore the relationship between nasal and anal carriage of SA in a larger cohort, as well as the severity and location of the eruption, and whether nasal sterilization could prevent EGFRI bacterial skin-related toxicity.
The specific treatment algorithms for rashes caused by EGFRI vary in different medical centers. Nonetheless, some basic principles may apply [6, 18, 19]. In our patients with grade 1–2 papulopustular eruptions, treatment with topical class I steroids for the face and class II or III for the trunk, combined with topical antibiotics, proved very effective, leading to an improvement in the eruption within a few days. Patients with grade 3 eruptions were treated with systemic antibiotics as well. Earlier studies, which reported sterile cultures, attributed the good results under treatment with oral semisynthetic tetracyclines (i.e., doxycycline or minocycline) to the drugs anti-inflammatory action via matrix metalloproteinase inhibition [7, 17]. However, given that we found SA positivity, it is probable that the antibacterial effect of tetracyclines also plays an important role in treating these patients.
Our study was limited by the small number of patients, a questionable referral selection bias, and the lack of independent observers, because only one dermatologist graded the lesions. Nevertheless, considering the thorough dermatological evaluation performed in each case and the clinical implications of the novel late infectious pattern of skin toxicity described here, we believe this issue warrants further research.
Furthermore, a problem shared by the present study and earlier ones is the reliance on the NCI-CTC (version 3.0) to grade rash severity. The terms used in the guideline to describe skin toxicity are vague and overly general, and its relevance to EGFRI-associated rashes, which are often asymptomatic or mildly symptomatic, though at the same time aesthetically severe, needs to be investigated. The NCI CTC sometimes fail to capture the functional impairment associated with the cosmetic appearance of the rash [19].
In conclusion, EGFRI-induced papulopustular eruptions could appear early or late after initiating treatment. The EP reaction, which is described in the literature, is probably an inflammatory process, and the occasional bacterial infection seems to be secondary. The LP papulopustular rash, described for the first time here, may be a direct result of SA infection.
Our work also demonstrates that >50% of cases of EGFRI-induced skin eruptions are SA+. These data suggest that routine bacterial culture should be performed prior to any treatment for EGFRI-induced skin lesions in order to determine the need for an antibacterial agent. Additional studies in a larger cohort of patients are needed to corroborate these findings.
Author Contributions
Conception/Design: Iris Amitay-Laish, Michael David
Provision of study material or patients: Salomon M. Stemmer
Collection and/or assembly of data: Iris Amitay-Laish, Salomon M. Stemmer, Michael David
Data analysis and interpretation: Iris Amitay-Laish, Salomon M. Stemmer, Michael David
Manuscript writing: Iris Amitay-Laish, Salomon M. Stemmer
Final approval of manuscript: Iris Amitay-Laish, Salomon M. Stemmer, Michael David
Other: Iris Amitay-Laish
References
- 1.Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 2.Albanell J, Rojo F, Averbuch S, et al. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: Histopathologic and molecular consequences of receptor inhibition. J Clin Oncol. 2002;20:110–124. doi: 10.1200/JCO.2002.20.1.110. [DOI] [PubMed] [Google Scholar]
- 3.Malik SN, Siu LL, Rowinsky EK, et al. Pharmacodynamic evaluation of the epidermal growth factor receptor inhibitor OSI-774 in human epidermis of cancer patients. Clin Cancer Res. 2003;9:2478–2486. [PubMed] [Google Scholar]
- 4.Baselga J. The EGFR as a target for anticancer therapy—focus on cetuximab. Eur J Cancer. 2001;37(suppl 4):s16–s22. doi: 10.1016/s0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 5.Nanney LB, Magid M, Stoscheck CM, et al. Comparison of epidermal growth factor binding and receptor distribution in normal human epidermis and epidermal appendages. J Invest Dermatol. 1984;83:385–393. doi: 10.1111/1523-1747.ep12264708. [DOI] [PubMed] [Google Scholar]
- 6.Lynch TJ, Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: An evolving paradigm in clinical management. The Oncologist. 2007;12:610–621. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- 7.Roé E, García Muret MP, Marcuello E, et al. Description and management of cutaneous side effects during cetuximab or erlotinib treatments: A prospective study of 30 patients. J Am Acad Dermatol. 2006;55:429–437. doi: 10.1016/j.jaad.2006.04.062. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 9.Racca P, Fanchini L, Caliendo V, et al. Efficacy and skin toxicity management with cetuximab in metastatic colorectal cancer: Outcomes from an oncologist/dermatologic cooperation. Clin Colorectal Cancer. 2008;7:48–54. doi: 10.3816/CCC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd FA, Rodriques Pereira JR, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 11.Bruno R, Mass RD, Jones C, et al. Preliminary population pharmacokinetics (PPK) and exposure safety (E-S) relationships of erlotinib HCL in patients with metastatic breast cancer. Proc Am Soc Clin Oncol. 2003;22:205. [Google Scholar]
- 12.Sipples R. Common side effects of anti-EGFR therapy: Acneiform rash. Semin Oncol Nurs. 2006;22(suppl 1):28–34. doi: 10.1016/j.soncn.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Nanney LB, Stoscheck CM, King LE, Jr, et al. Immunolocalization of epidermal growth factor receptors in normal developing human skin. J Invest Dermatol. 1990;94:742–748. doi: 10.1111/1523-1747.ep12874601. [DOI] [PubMed] [Google Scholar]
- 14.Fox LP. Pathology and management of dermatologic toxicities associated with anti-EGFR therapy. Oncology (Williston Park) 2006;20(suppl 2):26–34. [PubMed] [Google Scholar]
- 15.Peus D, Hamacher L, Pittelkow MR. EGF-receptor tyrosine kinase inhibition induced keratinocyte growth arrest and terminal differentiation. J Invest Dermatol. 1997;109:751–756. doi: 10.1111/1523-1747.ep12340759. [DOI] [PubMed] [Google Scholar]
- 16.Lacouture ME, Lai SE. The PRIDE (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to Epidermal growth factor receptor inhibitors) syndrome. Br J Dermatol. 2006;155:852–854. doi: 10.1111/j.1365-2133.2006.07452.x. [DOI] [PubMed] [Google Scholar]
- 17.Gridelli C, Maione P, Amoroso D, et al. Clinical significance and treatment of skin rash from erlotinib in non-small cell lung cancer patients: Results of an experts panel meeting. Crit Rev Oncol Haematol. 2008;66:155–162. doi: 10.1016/j.critrevonc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Lacouture ME, Basti S, Patel J, et al. The SERIES clinic: An interdisciplinary approach to the management of toxicities to EGFR inhibitors. J Support Oncol. 2006;4:236–238. [PubMed] [Google Scholar]
- 19.Duvic M. EGFR inhibitor-associated acneiform folliculitis: Assessment and management. Am J Clin Dermatol. 2008;9:285–294. doi: 10.2165/00128071-200809050-00002. [DOI] [PubMed] [Google Scholar]