The scientific review of the application leading to the approval of Teysuno™ (S-1) for the treatment of advanced gastric cancer when given in combination with cisplatin in the European Union is presented.
Keywords: Tegafur/gimeracil/oteracil, S-1, Gastric cancer, EMA, European Medicines Agency
Abstract
The product Teysuno™ (S-1) contains tegafur, a prodrug of 5-fluorouracil (5-FU), and two modulators of 5-FU metabolism, gimeracil and oteracil.
The main clinical study in this application was a randomized controlled study comparing S-1 plus cisplatin with 5-FU plus cisplatin. In this study, median overall survival times of 8.6 months and 7.9 months for S-1 plus cisplatin and 5-FU plus cisplatin, respectively, were observed (hazard ratio, 0.92; 95% confidence interval, 0.80–1.05). The Committee for Medicinal Products for Human Use of the European Medicines Agency concluded that S-1 in combination with cisplatin (75 mg/m2) was noninferior to 5-FU plus cisplatin (100 mg/m2) in patients with advanced gastric cancer and adopted a positive opinion recommending the marketing authorization for this product for the treatment of advanced gastric cancer when given in combination with cisplatin. The recommended dose of S-1 is 25 mg/m2 (expressed as tegafur content) twice a day, for 21 consecutive days followed by 7 days rest (one treatment cycle), in combination with 75 mg/m2 cisplatin i.v. administered on day 1. This treatment cycle is repeated every 4 weeks.
The most common side effects reported in the pivotal study were anemia, neutropenia, vomiting, diarrhea, abdominal pain, weight decrease, anorexia, and fatigue.
The objective of this paper is to summarize the scientific review of the application leading to approval in the EU. The full scientific assessment report and the summary of product characteristics are available on the European Medicines Agency website (http://www.ema.europa.eu).
Background
The most common clinical presentations of advanced gastric cancer include locally advanced unresectable or metastatic gastric cancer at the time of diagnosis and recurrent gastric cancer after resection. Cisplatin in combination with fluoropyrimidines and anthracyclines has been used as the standard treatment for advanced gastric cancer [1]. In 2006, a meta-analysis from randomized studies showed that the best survival results were obtained from patients treated with three-drug regimens containing a fluoropyrimidine, an anthracycline, and cisplatin [2]. Other combinations, such as epirubicin, oxaliplatin, and capecitabine and docetaxel, cisplatin, and 5-fluorouracil (5-FU), have claimed efficacy similar to or better than that of epirubicin, cisplatin, and 5-FU [3, 4].
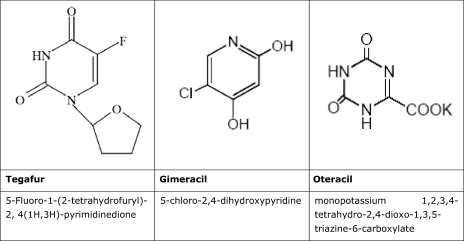
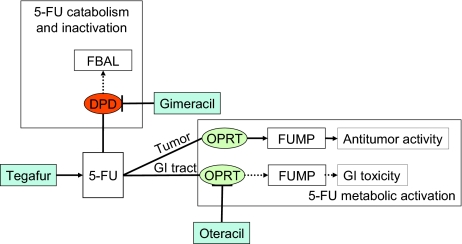
Teysuno™ (S-1) is an oral fixed-dose combination of three active substances—tegafur, gimeracil, and oteracil [5]. After absorption, tegafur is converted into 5-FU. Gimeracil is a dihydropyrimidine dehydrogenase (DPD) inhibitor that prevents degradation of 5-FU. Oteracil, an orotate phosphoribosyltransferase (OPRT) inhibitor, is intended to decrease the activity of 5-FU in the gut in order to minimize toxicity to the normal gastrointestinal (GI) mucosa (Figs. 1 and 2).
Figure 1.
Structural formula and chemical name of tegafur, gimeracil, and oteracil.
Figure 2.
Schematic illustration of 5-FU metabolism and pharmacodynamic rationale for S-1. There are two competing routes of metabolism of 5-FU. Anabolic metabolism results in the active metabolites allowing the fluoropyrimidines to exert their therapeutic effect, whereas the dominant catabolic metabolism leads to inactive metabolites and the elimination of the drug. Anabolism of 5-FU to FUMP is catalyzed by OPRT. FUMP is subsequently metabolized to a series of products including FdUMP, which is ultimately the cytotoxic metabolite. Oteracil is intended to inhibit anabolism of 5-FU to FUMP by OPRT. In preclinical models, oteracil distributed into normal GI tract tissues to a larger extent than tumor cells and was associated with less 5-FU toxicity to GI tract tissues. Catabolism of 5-FU to 5,6 dihydro-5-FU, FUPA, and FBAL is principally mediated by the enzyme DPD. Gimeracil is intended to inhibit the catabolism and subsequent inactivation of 5-FU by reversibly inhibiting DPD, so that higher plasma concentrations of 5-FU could be achieved with the administration of a lower dose of tegafur.
Abbreviations: 5-FU, 5-fluorouracil; DPD, dihydropyrimidine dehydrogenase; FBAL, α-fluoro-β-alanine; FdUMP, 5-fluoro-2′-deoxyuridine monophosphate; FUMP, 5-fluorouridine-5′-monophosphate; FUPA, α fluoro β ureidopropionate; GI, gastrointestinal; OPRT, orotate phosphoribosyl transferase.
The applicant company, Taiho Pharma Europe Ltd (Birmingham, U.K.), submitted, on October 28, 2009, an application for marketing authorization for S-1 given in combination with cisplatin for the treatment of advanced gastric cancer. The scientific review was conducted by the Committee for Medicinal Products for Human Use (CHMP). The CHMP recommended granting marketing authorization for S-1 based on a positive benefit–risk balance. On March 14, 2011, the European Commission issued a marketing authorization.
Nonclinical Aspects
Following oral administration, the prodrug tegafur is gradually converted into 5-FU in vivo, mainly by cytochrome P450 2A6 enzyme (CYP2A6) activity in the liver. 5-FU is activated within cells by phosphorylation of its active metabolite, 5-fluoro-2′-deoxyuridine-monophosphate (FdUMP). 5-FU is metabolized in the liver and, to a lesser degree in other tissues, via DPD. FdUMP and reduced folate are bound to thymidylate synthase, leading to formation of a ternary complex that inhibits DNA synthesis. In addition, 5-fluorouridine-triphosphate is incorporated into RNA causing disruption of RNA function.
In vitro, gimeracil selectively inhibited the target enzyme DPD at 50% inhibitory concentration (IC50) of 95 nM and oteracil selectively inhibited the target enzyme OPRT with an IC50 value of 4.2 μM, whereas the metabolites of oteracil did not significantly inhibit the activity of enzymes involved in 5-FU metabolism. The optimum ratio of tegafur:gimeracil:oteracil was determined in mice and rats to be 1:0.4:1. The effect of tegafur and gimeracil was greatest when both substances were administered simultaneously. In mice bearing various murine tumor types, S-1 was observed to be consistently more potent in inhibiting tumor growth or increasing life span than tegafur alone, 5-FU, or tegafur-uracil (UFT). The antitumor activity of S-1 given as a single daily or twice-daily divided dose was evaluated in a rat tumor model. Antitumor activity of S-1 given as a twice daily (b.i.d.) divided dose was similar to once-daily administration. However, the b.i.d. divided dose was associated with less hematologic toxicity and faster recovery of leukocytes, thus supporting the b.i.d. regimen.
Rats treated with S-1 showed higher levels of active 5-FU metabolites in tumor tissue than in GI tract tissue. In contrast, levels of active 5-FU metabolites were similar in tumor tissue and GI tract tissue in rats treated with UFT. In nontumor-bearing rats and cynomolgus monkeys, the addition of oteracil to tegafur and gimeracil (FCD) resulted in a lower incidence of FCD-induced diarrhea. According to the company, the lower rate of GI toxicity was considered to be most likely related to oteracil-induced suppression of 5-FU phosphorylation. However, in nontumor bearing rats, GI toxicity was significantly recovered only when 2 M instead of 1 M oteracil was added, suggesting that S-1 in its present molar range might not reduce FCD-induced GI toxicity. On the other hand, in a rat model of primary colorectal cancer, the addition of oteracil resulted in a significantly lower incidence of FCD-induced GI toxicity without affecting FCD-induced antitumor activity.
In repeat-dose toxicity studies in rats, dogs, and monkeys, S-1 was associated with toxicities typically associated with the administration of an anticancer cytotoxic drug, such as anemia, a decrease in immune and digestive system functions, disruption of spermatogenesis, and atrophy in male and female reproductive organs. In vivo, during repeat-dose toxicology studies in the dog, S-1 and FCD induced melanosis in the sclera, conjunctiva, skin, and lymph nodes. Repeat dosing of S-1 was associated with skin and eye toxicity in the rat and dog. The tegafur component of S-1 appeared to be responsible for the melanin deposition and eye toxicity.
In reproductive toxicity studies in the rat, administration of S-1 at any time after conception resulted in a range of fetal abnormalities. Therefore, there is a high risk for developmental toxicity at clinical doses, primarily because of tegafur cytotoxicity. In lactating rats, considerable amounts of S-1 and its metabolites were found in milk. Based on these findings, S-1 is contraindicated during pregnancy and lactation.
Studies in juvenile animals were not provided. This was considered acceptable by the CHMP, because S-1 is not recommended for children aged <18 years. S-1 was clastogenic in vitro and was weakly clastogenic in vivo.
Pharmacokinetics, Potential for Interaction with Other Medicinal Products, and Other Forms of Interaction
In human subjects, S-1 components are readily absorbed after oral administration. However, if S-1 was administered with food, the area under the concentration–time curve from time zero to infinity was approximately 71%, 25%, and 15% lower for oteracil, gimeracil, and 5-FU, respectively, compared with fasting conditions. Therefore, it is recommended to take S-1 at least 1 hour before or after a meal.
The main metabolic pathway for tegafur involves conversion to 5-FU via CYP2A6 in the liver. Coadministration of a CYP2A6 inhibitor and S-1 should be avoided because there is a possibility that the effectiveness of S-1 could be decreased. Other potential interactions to highlight are the irreversible inhibition of the liver enzyme DPD by sorivudine, or its chemically related analogs such as brivudine, which can result in a significant increase in 5-FU exposure and lead to a higher incidence of clinically significant fluoropyrimidine-related toxicities with potentially fatal outcomes; nitromidazoles, including metronidazole and misonidazole, which may reduce the clearance of 5-FU; and polyglutamated methotrexate, which inhibits thymidylate synthase and dihydrofolate reductase, potentially increasing the cytotoxicity of 5-FU. For clozapine, because of possible additive pharmacodynamic effects (myelotoxicity), caution is advised because coadministration may increase the risk for and severity of hematologic toxicity from S-1. The coadministration of cimetidine may decrease clearance, and thus increase plasma levels of 5-FU. Coadministration of S-1 and coumarin anticoagulation therapy may increase the risk for bleeding. Fluoropyrimidines may increase the phenytoin plasma concentration when administered concomitantly. Frequent monitoring of phenytoin blood/plasma levels is advised when S-1 and phenytoin are administered concomitantly. Allopurinol may decrease S-1 antitumor activity as a result of suppression of phosphorylation of 5-FU. Therefore, concurrent administration with S-1 should be avoided.
Gimeracil was not found to be metabolized in vitro in human liver fractions, which is in line with the large proportion of gimeracil excreted unchanged in urine. Oteracil is metabolized mainly by phosphoribosyl pyrophosphate to cyanuric acid. In the renal impairment study, exposure to 5-FU was affected by renal impairment. This is likely to be explained by increased plasma levels of gimeracil, which is, for a large part, excreted renally.
No data are available on the concomitant use of folinic acid with S-1 in combination with cisplatin. Caution is advised because folinic acid is known to enhance the activity of 5-FU.
A population pharmacokinetic analysis in 315 patients was performed to assess the influence of various factors, including gender, age, food, ethnicity (white versus Asian), renal function, and hepatic function on S-1 components and metabolites. Renal function, as measured by creatinine clearance (CrCl), was the primary factor that influenced gimeracil and 5-FU exposure. As renal function decreased, there was an increase in 5-FU steady-state exposure. Therefore, it is important to determine CrCl before the start of treatment on day 1 for each cycle. S-1 and cisplatin dose modification should be applied according to CrCl values: patients with moderate renal impairment (CrCl, 30–49 mL/minute) at the start of a cycle of treatment should start treatment with S-1 at one reduced dose level and cisplatin treatment at a 50% dose reduction from the previous cycle. Patients with a CrCl ≤30 mL/minute should be withheld treatment with S-1 and cisplatin until the resumption criterion (≥30 mL/minute) is met and then be started on treatment with S-1 at one reduced dose level and cisplatin treatment at a 50% dose reduction from the previous cycle.
Studies in Japanese patients have suggested an effect of CYP2A6*4 polymorphism on S-1 pharmacokinetics. Japanese patients with the CYP2A6*4/*4 genotype treated with S-1 appear to have significantly lower 5-FU levels. The CYP2A6*4 allele is uncommon in the white population. No dose advice for this subpopulation is provided.
Clinical Efficacy
S-1301/FLAGS Study (ClinicalTrials.gov identifier, NCT00400179)
The S-1301/FLAGS (First-Line Advanced Gastric Cancer Study) study was a phase III trial (open-label, multicenter, randomized, parallel group, active controlled) comparing S-1 plus cisplatin with 5-FU plus cisplatin in a non-Asian patient population with advanced gastric cancer who were untreated with chemotherapy for advanced disease [6].
The study enrolled male and female patients >18 years of age with histologically confirmed, unresectable, locally advanced (stage IV) or metastatic gastric cancer, including adenocarcinoma of the gastroesophageal junction, and who had no prior cytotoxic chemotherapy for advanced gastric cancer. Patients must have been at least 4 weeks postradiotherapy and at least 3 weeks since major surgery, with an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, and had to meet minimum laboratory test requirements.
The study was conducted in 147 sites in 24 countries in Eastern/Western Europe, North America, Latin America, Australia, and South Africa.
S-1 (25 mg/m2) was administered b.i.d. for 21 consecutive days followed by a 7-day recovery period combined with 75 mg/m2 cisplatin i.v. administered on day 1, and repeated every 28 days. S-1 was taken orally 1 hour before or after a meal with a glass of water (approximately 100 mL). Cisplatin was administered as a 1- to 3-hour infusion on day 1 following the morning dose of S-1. The dose selection for the S-1 plus cisplatin arm was based on the results of the S-1101 phase I study in which a 25-mg/m2 dose of S-1 in combination with 75 mg/m2 cisplatin was well tolerated with no dose-limiting toxicities [7]. Patients in the control arm received 1,000 mg/m2 per 24 hours of 5-FU administered by continuous i.v. infusion over 120 hours (days 1–5) in combination with 100 mg/m2 cisplatin i.v. on day 1, both repeated every 28 days. Cisplatin treatment in both study arms was limited to six cycles.
The primary endpoint was overall survival. Stratification at randomization was based on the extent of disease (locally advanced, one metastatic site, or two or more metastatic sites), any prior adjuvant therapy, measurable versus nonmeasurable disease, and center. The full analysis set consisted of all patients who were dosed, with study drug assignment designated according to initial randomization.
In total, 1,053 patients were randomized between May 18, 2005 and March 7, 2007, and 1,029 patients (S-1 plus cisplatin, n = 521; 5-FU plus cisplatin, n = 508) received at least one dose of study drug. Baseline characteristics were similar in the two groups and reflect the population of patients with advanced gastric cancer. The majority of patients were male (70.8%) and white (86.0%). The mean age was 59 years (range, 18–85 years) and 14.2% were ≥70 years of age. The ECOG performance status score was 0 in 41.4% of patients. All patients had histologically confirmed adenocarcinoma: 83.1% in the stomach and 16.9% in the gastroesophageal junction. The most frequent pathology was poorly differentiated adenocarcinoma, in 38.8% of patients. The overall incidence of diffuse type histology (poorly differentiated adenocarcinoma, signet-ring cell carcinoma, or mucinous adenocarcinoma) was 57.3%. Metastatic disease was present in 95.7% of patients.
The S1301/FLAGS study was designed and conducted as a superiority study. However, the company switched the hypothesis from superiority to noninferiority after the primary analysis failed to show superiority of S-1 plus cisplatin over 5-FU plus cisplatin. The switching from superiority to noninferiority was performed without prespecification of a noninferiority margin. This was considered a major methodological flaw. To justify the choice of delta, the company presented the results of a meta-analysis showing that combination chemotherapy versus monotherapy was associated with a hazard ratio (HR) for overall survival of 0.83 [2], and proposed a noninferiority margin of 1.10 based, among other things, on literature comparing combination chemotherapy (e.g., 5-FU plus cisplatin) with either best supportive care or monotherapy alone [3, 8–10] with noninferiority margins in the range of 1.08–1.25.
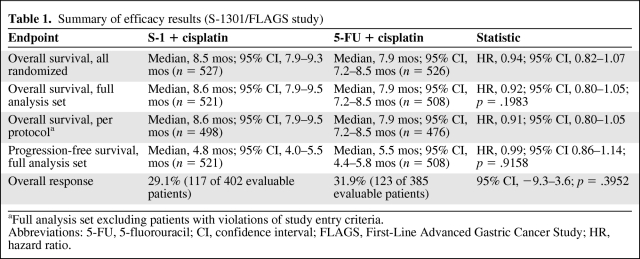
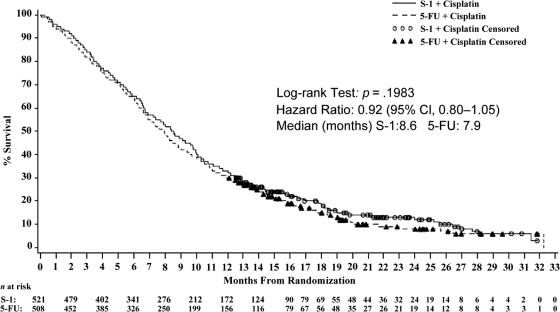
The results for the primary endpoint, overall survival, are shown in Table 1 and Figure 3. Median overall survival times were 8.6 months and 7.9 months for S-1 plus cisplatin and 5-FU plus cisplatin, respectively (HR, 0.92; 95% confidence interval [CI], 0.80–1.05). Although the choice of delta was justified retrospectively, the CHMP considered that, based on convincing efficacy results, in particular the narrow CI, and overall consistent results in terms of secondary endpoints, the switch to noninferiority was adequately justified, in line with current CHMP guidelines [11].
Table 1.
Summary of efficacy results (S-1301/FLAGS study)
aFull analysis set excluding patients with violations of study entry criteria.
Abbreviations: 5-FU, 5-fluorouracil; CI, confidence interval; FLAGS, First-Line Advanced Gastric Cancer Study; HR, hazard ratio.
Figure 3.
Survival (full analysis set population)—study S1301/FLAGS.
Abbreviations: CI, confidence interval; 5-FU, 5-fluorouracil.
Clinical Safety
Overall, the adverse events associated with S-1 were consistent with known adverse events for fluoropyrimidines. They included stomatitis, mucositis, and other GI toxicity (i.e., diarrhea and dehydration). S-1 plus cisplatin was also associated with treatment-related bone marrow suppression, including neutropenia, leukopenia, thrombocytopenia, anemia, and pancytopenia.
The most common treatment-related ocular disorders associated with S-1 in combination with cisplatin were lacrimal disorders (8.8%), including increased lacrimation, dry eye, and dacryostenosis.
Although DPD inhibition by gimeracil was expected to result in a lower incidence of palmar–plantar erythrodysesthesia (PPE) as a complication of 5-FU administration, the PPE incidence was higher in the S-1 plus cisplatin arm (all grades of PPE were observed in 5.4% and 2.6% of patients in the S-1 plus cisplatin and 5-FU plus cisplatin arms, respectively).
The most common severe adverse reactions (grade ≥3 with a frequency ≥10%) with S-1 plus cisplatin were neutropenia, anemia, and fatigue. The majority of grade ≥3 adverse events resolved during the treatment period or after treatment discontinuation.
The overall incidence of grade >3 adverse events was higher in patients aged >70 years in both treatment groups. Leukopenia, neutropenia, thrombocytopenia, diarrhea, asthenia, disease progression, dehydration hypokalemia, and hyponatremia were more frequent in older patients in the S-1 plus cisplatin group (≥5% difference in incidence) than in younger patients. Similar differences were observed in the 5-FU plus cisplatin group.
The S-1 plus cisplatin arm had lower incidences of renal toxicity, consistent with the lower dose of cisplatin that was used in this regimen. This was also the case for ototoxicity, peripheral neuropathy, and alopecia.
The incidences of serious adverse events (SAEs) and treatment-related SAEs were 49.3% versus 48.8% and 20.5% versus 29.7% for S-1 plus cisplatin versus 5-FU plus cisplatin, respectively. The most commonly reported treatment-related SAEs were myelosuppression (anemia, neutropenia, thrombocytopenia, febrile neutropenia), stomatitis, nausea, vomiting, and dehydration. Significant differences in treatment-related SAEs between treatment groups were observed for neutropenia (1.5% versus 6.1%), febrile neutropenia (1.5% versus 6.1%), and stomatitis (0.6% versus 4.5%) (for S-1 plus cisplatin versus 5-FU plus cisplatin, respectively).
The incidences of death as a result of toxicity from the study medication were 2.5% for S-1 plus cisplatin and 4.9% for 5-FU plus cisplatin. These were frequently caused by myelosuppression and its consequences (0.8% and 2.8% for S-1 plus cisplatin and 5-FU plus cisplatin, respectively). Times to myelosuppression-related treatment discontinuation or death were similar in the two groups.
S-1 has not been studied in gastric cancer patients with microsatellite instability (MSI). The association between 5-FU sensitivity and MSI in patients with gastric cancer is unclear. The company committed to investigate the effect of tumor MSI on the efficacy and safety of S-1 in the tissue samples from the S1301/FLAGS pharmacogenomics substudy.
Pharmacovigilance
The company submitted a pharmacovigilance plan and a risk minimization plan for the product in order to mitigate the safety risks associated with the treatment. Important identified risks were bone marrow suppression, GI symptoms, GI perforation/hemorrhage, PPE, lacrimal disorders, renal toxicity, hearing impairment, peripheral neuropathy, cardiovascular events, hepatic toxicity, disseminated intravascular coagulation, interstitial lung disease, leukoencephalopathy, Stevens-Johnson syndrome/toxic epidermal necrolysis, and acute pancreatitis. The proposed risk minimization activities include dose recommendations and dose modification, supportive treatment, and early ophthalmologic consultation in the event of any persistent or vision-reducing ocular symptoms such as lacrimation or corneal symptoms.
There are currently no data on the pharmacokinetics in patients with severe renal impairment (CrCl <30 mL/minute). Other important missing information includes data in patients with a cardiac disorder, clinical safety and efficacy of an S-1–containing triplet regimen, and S-1 treatment in gastric cancer patients with MSI. All missing information will be monitored and updated in the periodic safety update report submitted to the European Medicines Agency (EMA).
Overall Conclusions, Risk–Benefit Assessment, and Recommendation
The CHMP concluded that the combination of S-1 plus cisplatin (75 mg/m2) was noninferior to 5-FU plus cisplatin (100 mg/m2) with respect to overall survival. The adverse events reported for S-1 plus cisplatin in the target population were consistent with the known adverse events of fluoropyrimidines in patients with advanced gastric cancer. Overall, the benefits and risks of S-1 plus cisplatin were considered similar to those of parenteral 5-FU plus cisplatin in the patient population and the CHMP considered the benefit–risk balance to be positive.
In their application, the company claimed that the overall adverse event profile of S-1 plus cisplatin versus 5-FU plus cisplatin was in favor of the S-1 combination. However, this claim could not be established because the dose of cisplatin was different in the two groups and the myelosuppression and GI toxicity were inconsistent across studies.
The product S-1, compared with the standard 5-FU continuous i.v. infusion treatment, has the added benefit of being an oral product for which the daily dosing does not require a central venous catheter and hospital admission. Indeed, oral fluoropyrimidines have nowadays largely replaced continuous infusion 5-FU in the treatment of advanced gastric cancer because of better tolerability and convenience, and even some data suggesting possible superior efficacy.
Based on the data submitted for this application, the CHMP concluded that the noninferior efficacy and safety of S-1 plus cisplatin have been established only compared with the 5-FU plus cisplatin schedule used in the pivotal study, and only in the advanced gastric cancer indication. The efficacy and safety of S-1 plus cisplatin have not been established for other dosing regimens or combinations, including a triplet regimen for advanced gastric cancer, or monotherapy or combination treatment for other indications. Thus, based on the current application, S-1 cannot be considered as a general alternative to parenteral 5-FU or 5-FU prodrugs. The company committed to conducting further clinical trials to investigate an S-1–containing triplet regimen. The EMA will review new information about S-1 on a regular basis. The most current information on this medicinal product can be found on the EMA website (http://www.ema.europa.eu).
Acknowledgments
The scientific assessment summarized in this report is based on important contributions from the rapporteur and corapporteur assessment teams, CHMP members, and additional experts following the application for a marketing authorization from the company. We thank Silvy Da Rocha Dias for reviewing the manuscript.
This publication is a summary of the European Public Assessment Report, the summary of product characteristics, and other product information as published on the EMA website (http://www.ema.europa.eu). For the most current information on this marketing authorization, please refer to the EMA website. The authors of this paper remain solely responsible for the opinions expressed in this publication.
Author Contributions
Data analysis and interpretation: Barbara van Zwieten-Boot, Gonzalo Calvo Rojas, Hadewych ter Hofstede, Rocio Garcia-Carbonero, Jorge Camarero, Eric Abadie
Manuscript writing: Petra Matt, Francesco Pignatti
References
- 1.Jackson C, Cunningham D, Oliveira J ESMO Guidelines Working Group. Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(suppl 4):34–36. doi: 10.1093/annonc/mdp122. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 5.Schöffski P. The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs. 2004;15:85–106. doi: 10.1097/00001813-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: The FLAGS trial. J Clin Oncol. 2010;28:1547–1553. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol. 2005;23:6957–6965. doi: 10.1200/JCO.2005.01.917. [DOI] [PubMed] [Google Scholar]
- 8.Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: A randomised phase 3 study. Lancet Oncol. 2009;10:1063–1069. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 9.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 10.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency. CHMP Points to Consider on Switching Between Superiority and Non-Inferiority (CPMP/EWP/482/99) [accessed July 4, 2011]. Available at http://www.ema.europa.eu/pdfs/human/ewp/048299en.pdf.