The cause of death in prostate cancer patients is examined using the Swedish Family-Cancer Database. Prostate cancer patients were found to have a higher risk for dying from various causes other than prostate cancer, including external causes and heart failure.
Keywords: Prostate cancer, Cause of death, Comorbidity, Regression analysis
Abstract
Background.
A recent rise in the incidence of prostate cancer and a more favorable outcome have increased the proportions of other causes of death in affected men. Extending the survival of prostate cancer patients thus requires knowledge of all causes of death.
Methods.
Data on the population, cancers, and causes of death were gathered from the nationwide Swedish Family-Cancer Database. A Cox regression model, comparing prostate cancer patients with all other men, was applied. Hazard ratios (HR) were calculated both for the underlying cause and for dying with a specific cause listed among multiple causes of death.
Findings.
Among 686,500 observed deaths, 62,500 were prostate cancer patients. For underlying causes other than prostate cancer, the highest cause-specific HRs were found for external causes (HR, 1.24; 95% confidence interval [CI], 1.16–1.31), diseases of the pulmonary circulation (HR, 1.22; 95% CI, 1.09–1.37), and heart failure (HR, 1.18; 95% CI, 1.11–1.24). For specific multiple causes, the highest HRs were found for anemia (HR, 2.28; 95% CI, 2.14–2.42), diseases of the pulmonary circulation (HR, 1.61; 95% CI, 1.55–1.68), and urinary system disease (HR, 1.90; 95% CI, 1.84–1.96).
Interpretations.
Prostate cancer patients have a higher risk for dying from various causes other than prostate cancer, including external causes and heart failure. Mechanisms have been proposed linking these elevated risks to both cancer and treatment. More attention should be paid to comorbidities in men with prostate cancer. The present study fulfills a gap in the knowledge of death causes in prostate cancer patients.
Introduction
Approximately half of the men who receive a diagnosis of prostate cancer (PC) die from PC itself [1]. This proportion can be expected to decrease, considering the recent increase in the incidence of PC and the fact that mortality from PC has stabilized or even started to decline [2]. More efficient treatment and earlier diagnosis resulting from the introduction of prostate-specific antigen tests are thought to explain these epidemiologic changes [2, 3]. As a consequence, fewer patients are dying from PC whereas other conditions are playing a greater role. Improvements in survival of PC patients will increasingly require consideration of comorbidities.
Although we are not aware of any studies extensively covering causes of death in PC patients, some studies have reported higher risks for cardiovascular disease [1, 4] and suicide [4, 5] following a PC diagnosis. PC patients with heart failure have been shown to have a higher risk for death than PC patients without heart failure [6]. Also, PC is less frequently mentioned as the underlying cause of death in older patients [7] or patients with lower stage disease [6]. In the present study, we used the nationwide Swedish Family-Cancer Database to analyze causes of death in PC patients compared with all other men in the database. The aim of our study was to investigate whether a diagnosis of PC was associated with a greater risk for dying from any specific condition. We analyzed both the underlying cause and multiple causes of death, and furthermore made a distinction depending on the number of death causes. The findings may motivate a greater attention by physicians to their PC patients regarding other diseases.
Materials and Methods
The 2006 update of the Swedish Family-Cancer Database includes all individuals born after 1931 who are residing in Sweden, together with their biological parents, totaling around 11.8 million individuals [8]. The first version of the database was created in 1996 by combining the Swedish Cancer Registry and the Swedish Multigenerational Register. The database has been regularly updated and now includes more than one million cancer cases diagnosed up to the end of 2006 [8]. The database includes information about cancers, socioeconomic data, and death causes. Death causes (up to 10 multiple causes plus the one underlying cause) are coded according to different versions of the International Classification of Diseases (ICD-7 to ICD-10), depending on the year of death. The underlying cause of death is, as defined by the World Health Organization, “the disease or injury which initiated the train of morbid events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury,” whereas the listed multiple causes are complications and other contributing causes [9].
The chosen study time was 1987–2006, because during this time all death causes were coded according to either the ICD-9 (used in 1987–1996) or the ICD-10 (used since 1997), which are easy to compare. All men born before 1957 residing in Sweden were defined as the study population, totaling 2.27 million men, of which 117,000 were PC patients. PC patients were defined as those who were diagnosed with PC (ICD-7 code 177) as the first invasive cancer. The rest of the men were part of the nonprostate group, which was the reference group. The underlying cause and multiple causes of death were allocated into several distinguishable disease categories (see below). Hazard ratios (HRs) for death from a specific cause for PC patients compared with men without PC were calculated using a Cox regression model with age as the underlying time scale. All calculations were made with SAS software (PROC PHREG; SAS Version 9.2; SAS Institute, Cary, NC). Subjects entered the study at the time of immigration, presence at census, or January 1987, whichever occurred most recently. Men initially in the reference group were assigned to the PC group at the time of their PC diagnosis. Censoring events were death from a cause other than the cause of interest, emigration, absence at census, or December 2006. First, HRs were calculated for the underlying cause of death, wherein the event of interest was having a specific condition as the underlying cause. Then, analyses were made on multiple causes of death, wherein the event of interest was having a specific cause listed among multiple causes. Finally, a distinction was made depending on the number of death causes. Here, the event of interest was having a specific death cause listed among multiple causes, as well as having a certain number of death causes: two, three, and four or more. Thus, it could be determined whether some death causes were more frequent in either PC patients with few death causes or PC patients with many contributing conditions causing death. The underlying cause was always included in the calculations on multiple causes. The socioeconomic index and the geographical region of residence were included as covariates, with individuals grouped according to last known entry in a census.
Definitions of Disease Categories
ICD codes used in the disease categories were the following (ICD-10; ICD-9): myocardial infarction (I21-I22; 410), other coronary heart disease (I20, I23-I25; 411–414), cerebrovascular accident (I60-I69; 430–438), arterial disease (I70-I79; 440–448), heart failure (I50; 428), pneumonia (J10-J18; 480–487), chronic lower respiratory disease (J40-J49; 490–496), external causes (S00-T98, V01-Y98; 800–900, e800-e999), complications of diagnostic or surgical procedures (Y60-Y84; e870-e879), complications of therapeutic drug or vaccine usage (Y40-Y59; e930-e949), suicide (X60-X84; e950-e959), traffic accident (V01-V99; e800-e848), falls (W00-W19; e880-e888), other heart disease (I30-I49, I52; 420–427), gastrointestinal disease (K00-K93; 520–579), dementia (F00-F03, G30; 290, 331.0), diabetes (E10-E14; 250), complications of heart disease (I51; 429), urinary system disease (N00-N39; 580–599), symptoms (R00-R99; 780–799), pulmonary circulation (I26-I28, J81; 415–417, 514), nervous system disease (G00-G99, except G30; 320–359, except 331.0), hypertensive disease (I10-I19; 401–405), other bacterial disease (A30-A49; 030–041), psychic disease (F04-F99; 291–319), anemia (D50-D64; 280–285), tumors other than prostate cancer (C00-D48, excluding C61, C77-C80, and C97; 140–239, excluding 185, 196–199), and prostate cancer (C61; 185).
Results
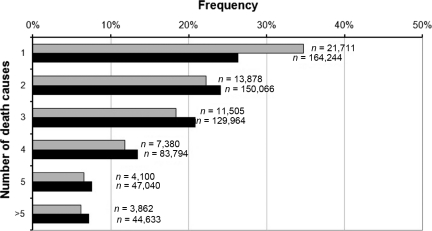
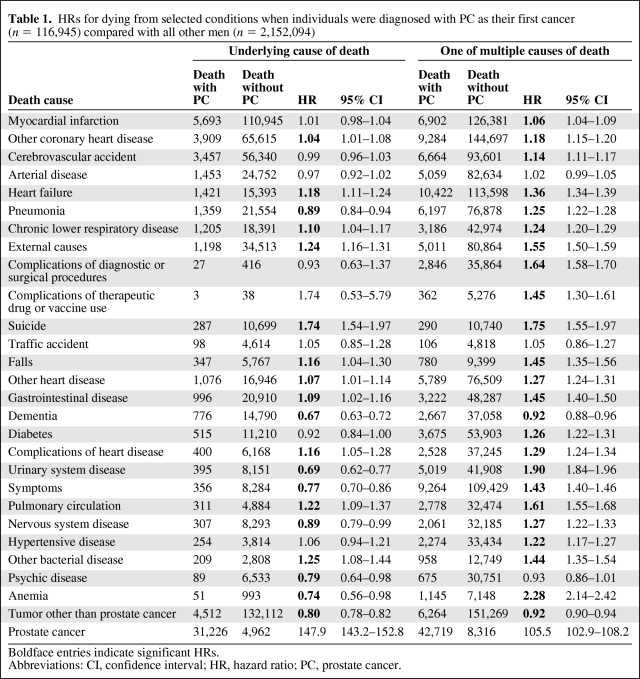
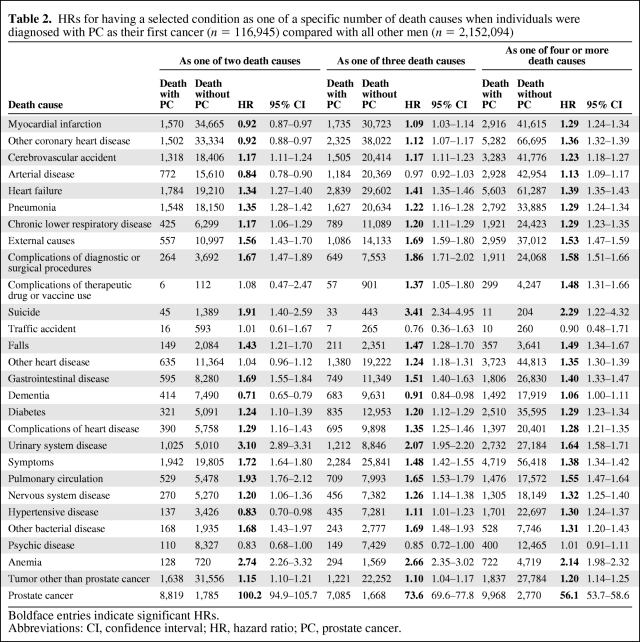
The total number of deaths was 686,500. Of these, 62,500 were PC patients. PC patients had fewer death causes than all other men (Fig. 1). In the initial calculations, underlying causes were investigated (Table 1). Apart from PC, which was the underlying cause in 50% of decedents in the PC group, the most common underlying causes in PC patients were various cardiovascular diseases, other tumors, and respiratory diseases. The highest HRs were found for external causes (1.24), pulmonary circulation (1.22), and heart failure (1.18). Anemia, dementia, and urinary system disease featured low HRs as underlying causes. Separate HRs (not shown) were computed for two follow-up times: <5 years and >5 years following diagnosis. Differences between these follow-up times were minor. Nevertheless, the HR for heart failure was higher >5 years after diagnosis of PC (HR, 1.27; 95% confidence interval [CI], 1.18–1.36) than <5 years after diagnosis (HR, 1.07; 95% CI, 0.99–1.16), whereas the HR for pulmonary circulation was lower—1.13 (95% CI, 0.95–1.35) versus 1.30; (95% CI, 1.11–1.51). In analyses on multiple causes of death, the number of death causes was not considered (Table 1). The highest HRs were attributed to anemia (2.28), urinary system disease (1.90), and pulmonary circulation (1.61). In further analyses on multiple causes, the number of death causes was accounted for (Table 2). Urinary tract disease (3.10), anemia (2.74), and pulmonary circulation (1.93) showed the highest HRs when they were one of the two listed death causes.
Figure 1.
Number of death causes in deceased men with (gray) and without (black) prostate cancer.
Table 1.
HRs for dying from selected conditions when individuals were diagnosed with PC as their first cancer (n = 116,945) compared with all other men (n = 2,152,094)
Boldface entries indicate significant HRs.
Abbreviations: CI, confidence interval; HR, hazard ratio; PC, prostate cancer.
Table 2.
HRs for having a selected condition as one of a specific number of death causes when individuals were diagnosed with PC as their first cancer (n = 116,945) compared with all other men (n = 2,152,094)
Boldface entries indicate significant HRs.
Abbreviations: CI, confidence interval; HR, hazard ratio; PC, prostate cancer.
Discussion
We investigated causes of death in PC patients, with a group of men without PC as the comparison group. Having access to a large database, we used a Cox regression model with death from a specific cause as the event of interest. We found that PC patients had a higher risk for dying from external causes, heart failure, and diseases of the pulmonary circulation. Moreover, we discovered PC patients to have a greater risk for dying with anemia and urinary system disease among the listed multiple causes. The highest HRs for these two causes were seen among decedents with only two listed death causes. There were also some lower risks, most notably a substantially lower risk for dying from dementia. A previous Swedish study found that neoplastic conditions were infrequently mentioned as the underlying cause of death in dementia patients, compared with patients without dementia [10]. Our results might be a result of fewer cancer diagnoses in dementia patients. We also suspect a possible overrepresentation of PC as the underlying cause, especially in deaths occurring shortly after diagnosis and in older men. This possible overrepresentation of PC was our motivation for also comparing death cause–specific risks <5 years and >5 years after diagnosis. More benign conditions, such as anemia, are possibly more seldom chosen as the underlying cause of death if the physician issuing the death certificate is aware of a previous PC diagnosis. This could explain the lower risks.
Our study compared 27 non-PC death causes among both the underlying cause and multiple causes of death. The reliability of the underlying cause is probably higher than that of multiple causes, but these may be used complementary to each other. Multiple causes of death are important contributors to mortality and they show the spectrum of comorbidities. Also, because half of the dead PC patients had PC listed as the underlying cause, excluding multiple causes would yield less information. To date, most studies on mortality are restricted to using the underlying cause only, a shortcoming that was pointed out earlier [10]. Our study also differs from earlier studies in the use of cause-specific HRs. In contrast, some other studies have analyzed death causes reported on the death certificates of PC patients, compared with men without PC [1, 7]. The influence of comorbidities on survival [6] and the odds of death [7] has also been investigated.
In the interpretation of our study, the limitation that our method does not take causality into consideration has to be taken into account. Thus, we cannot conclude whether PC (or its treatment) increases the risk for a comorbidity, or vice versa. Moreover, our results rely on the validity of death certificates. Studies on the reliability of Swedish death certificates have found that reports of a malignant neoplasm or ischemic heart disease as the underlying cause are reliable [12]. In comparison, certificates with chronic obstructive pulmonary disease or nonischemic heart disease as the underlying cause are not as accurate. Therefore, the reliability of our results on more common death causes is most likely higher than the reliability of our results on less common causes. One study specifically examined the reliability of death certificates in PC patients, concluding that the reliability is high, especially in younger men with localized disease [13]. In Sweden, the physician who issues the death certificate may vary, depending on where the death occurred. In 1997–2003, approximately 42%–45% of Swedish men aged >65 years died in hospitals [14]. Even though very limited data are available on death locations of Swedish PC patients, one can assume that the majority of terminally ill patients are nursed in hospitals or hospices.
In the following paragraphs we discuss disease-specific mechanisms as possible explanations for our findings.
Cardiovascular Diseases
Heart failure showed the highest excess risk among cardiovascular diseases in PC patients. Possible mechanisms linking cancer to cardiac disease have been proposed, although not specifically addressing PC patients. Chemotherapy is widely known to have general cardiotoxic effects. Although no link has been shown between cardiotoxicity and radiation therapy in PC patients, it may be cardiotoxic in other cancers. A link between androgen deprivation therapy (ADT), the primary noncurative therapy for PC, and cardiovascular disease has been shown; previous studies have found that ADT-treated PC patients had a greater risk for being diagnosed with several types of cardiac disease [15]. Possible reasons for this higher risk are related to the metabolic effects of ADT: increased body fat composition, weight gain, insulin resistance, and alterations in blood lipid values [16–18]. However, the effect of ADT on cardiovascular mortality is unclear.
Anemia
Although we found that PC patients had a lower risk for dying from anemia as the underlying cause, they had a substantially higher risk for dying with it as one cause among multiple causes, especially as one of two death causes. Some mechanisms linking PC to anemia have been suggested. Treatment with ADT may cause anemia [19, 20], because testosterone is known to increase erythropoiesis [20]. Another mechanism is through bone metastasis, termed leukoerythroblastic anemia [21].
Diseases of the Pulmonary Circulation
Pulmonary embolism, often caused by a venous thromboembolus, is the major death cause in this category. Van Hemelrijck and coworkers found a higher incidence of pulmonary embolism and venous thromboembolism in PC patients receiving treatment, especially those treated with ADT, during the first 6 months after the diagnosis of PC [22]. Similarly, a substantial proportion of PC patients undergoing prostatectomy (a frequently used curative therapy for PC) have a thrombotic event shortly after the surgery [23]. Thrombotic events are well-known complications of various medical procedures, most notably after surgery in cancer patients [24, 25]. Thrombotic events may even be the first manifestations of malignancy [26]. A previous Swedish study concluded that cancer diagnoses, PC included, are frequent within 6 months after a diagnosis of deep venous embolism. Some cancer-related mechanisms have been proposed explaining the thrombotic tendency in cancer patients, sometimes referred to as Trousseau's syndrome [27]. Possible mechanisms include protease-induced activation of coagulation factors and upregulation of procoagulative enzymes by tumor hypoxia and oncogenes.
Diseases of the Urinary System
PC patients had a lower risk for dying from urinary system disease as the underlying cause, but at a higher risk for dying with it among multiple causes. End-stage renal disease patients on dialysis have a greater risk for cancer, including PC [28], and the stage of PC is also higher in these patients than in patients not on dialysis [29]. Urinary tract infections are also included in this category; men undergoing prostatectomy have a higher risk for these infections [30]. We believe that urinary tract infections and renal failure could explain our results.
External Causes
This category includes various events, such as fractures, accidents, suicide, and iatrogenic causes. Many studies have shown a decrease in bone mineral density in PC patients treated with pharmacological ADT or orchiectomy [18, 31]. The mechanism is thought to be a shortage of estrogen, which is produced through aromatization of circulating testosterone [32]. Low testosterone levels increase bone frailty, and therefore increase the risk for fractures. Another explanation for the greater prevalence of fractures in PC patients could be bone metastasis, which also causes bone frailty [22]. We also found high HRs for suicide, which is supported by a recent study by Fall and coworkers [4]. They found that the risk for suicide in PC patients was highest during the first weeks after diagnosis, suggesting that diagnosis-associated emotional stress plays a greater role than treatment- or cancer-related mechanisms. Furthermore, Bill-Axelson and coworkers described a higher suicide risk in men with advanced or metastatic PC, but not in those with localized disease [5].
In conclusion, the present study indicates that PC patients have a higher risk for dying from various causes. Most notably, heart failure and external causes were listed as the underlying cause of death, whereas urinary tract disease, diseases of the pulmonary circulation, and anemia were more frequently listed among multiple causes of death. Mechanisms have been proposed linking these conditions to both PC and its treatment. Success in fighting PC and improving the quality of life of patients requires more focus on comorbidities.
Acknowledgments
Supported by the Swedish Council for Working Life and Social Research, the Swedish Cancer Society, and Deutsche Krebshilfe. The database used was created by linking registers maintained at Statistics Sweden and the Swedish Cancer Registry.
Author Contributions
Conception/Design: Hauke Thomsen, Kari Hemminki
Collection and/or assembly of data: Jan Sundquist
Data analysis and interpretation: Hauke Thomsen, Matias Riihimäki, Andreas Brandt, Kari Hemminki
Manuscript writing: Hauke Thomsen, Matias Riihimäki
Final approval of manuscript: Hauke Thomsen, Andreas Brandt, Kari Hemminki, Jan Sundquist
References
- 1.Satariano WA, Ragland KE, Van Den Eeden SK. Cause of death in men diagnosed with prostate carcinoma. Cancer. 1998;83:1180–1188. doi: 10.1002/(sici)1097-0142(19980915)83:6<1180::aid-cncr18>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Kvåle R, Auvinen A, Adami HO, et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst. 2007;99:1881–1887. doi: 10.1093/jnci/djm249. [DOI] [PubMed] [Google Scholar]
- 3.Hemminki K, Rawal R, Bermejo JL. Prostate cancer screening, changing age-specific incidence trends and implications on familial risk. Int J Cancer. 2005;113:312–315. doi: 10.1002/ijc.20568. [DOI] [PubMed] [Google Scholar]
- 4.Fall K, Fang F, Mucci LA, et al. Immediate risk for cardiovascular events and suicide following a prostate cancer diagnosis: Prospective cohort study. PLoS Med. 2009;6:e1000197. doi: 10.1371/journal.pmed.1000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bill-Axelson A, Garmo H, Lambe M, et al. Suicide risk in men with prostate-specific antigen–detected early prostate cancer: A nationwide population-based cohort study from PCBaSe Sweden. Eur Urol. 2010;57:390–395. doi: 10.1016/j.eururo.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Ketchandij M, Kuo YF, Shahinian VB, et al. Cause of death in older men after the diagnosis of prostate cancer. J Am Geriatr Soc. 2009;57:24–30. doi: 10.1111/j.1532-5415.2008.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newschaffer CJ, Otani K, McDonald MK, et al. Causes of death in elderly prostate cancer patients and in a comparison nonprostate cancer cohort. J Natl Cancer Inst. 2000;92:613–621. doi: 10.1093/jnci/92.8.613. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki K, Ji J, Brandt A, et al. The Swedish Family-Cancer Database 2009: Prospects for histology-specific and immigrant studies. Int J Cancer. 2010;126:2259–2267. doi: 10.1002/ijc.24795. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organisation. International Statistical Classification of Diseases and Related Health Problems. [accessed April 2, 2010];Tenth Revision. (Second Edition). Volume 2 Available at http://www.who.int/classifications/icd/ICD-10_2nd_ed_volume2.pdf. [Google Scholar]
- 10.Brunnström HR, Englund EM. Cause of death in patients with dementia disorders. Eur J Neurol. 2009;16:488–492. doi: 10.1111/j.1468-1331.2008.02503.x. [DOI] [PubMed] [Google Scholar]
- 11.Redelings MD, Sorvillo F, Simon P. A comparison of underlying cause and multiple causes of death: US vital statistics, 2000–2001. Epidemiology. 2006;17:100–103. doi: 10.1097/01.ede.0000187177.96138.c6. [DOI] [PubMed] [Google Scholar]
- 12.Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: Implications for mortality statistics. Int J Epidemiol. 2000;29:495–502. [PubMed] [Google Scholar]
- 13.Fall K, Strömberg F, Rosell J, et al. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42:352–357. doi: 10.1080/00365590802078583. [DOI] [PubMed] [Google Scholar]
- 14.Socialstyrelsen [Swedish National Board of Health and Welfare] Var Dör de äldre—på Sjukhus, Särskilt Boende Eller Hemma? - En Registerstudie. [Where Do Elderly People Die—In a Hospital, Care Home, or Home?—A Registry Study] In Swedish. [accessed March 17, 2010]. Available at http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/10098/2005–123-30_200512331.pdf.
- 15.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 16.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 17.Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93:2042–2049. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 18.Basaria S, Lieb J, 2nd, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779–786. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 19.Saylor PJ, Keating NL, Smith MR. Prostate cancer survivorship: Prevention and treatment of the adverse effects of androgen deprivation therapy. J Gen Intern Med. 2009;24(suppl 2):S389–S394. doi: 10.1007/s11606-009-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrucci L, Maggio M, Bandinelli S, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166:1380–1388. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 22.Van Hemelrijck M, Adolfsson J, Garmo H, et al. Risk of thromboembolic diseases in men with prostate cancer: Results from the population-based PCBaSe Sweden. Lancet Oncol. 2010;11:450–458. doi: 10.1016/S1470-2045(10)70038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beyer J, Wessela S, Hakenberg OW, et al. Incidence, risk profile and morphological pattern of venous thromboembolism after prostate cancer surgery. J Thromb Haemost. 2009;7:597–604. doi: 10.1111/j.1538-7836.2009.03275.x. [DOI] [PubMed] [Google Scholar]
- 24.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(suppl 1):I9–I16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 25.Kakkar AK. Prevention of venous thromboembolism in the cancer surgical patient. J Clin Oncol. 2009;27:4881–4884. doi: 10.1200/JCO.2009.23.2009. [DOI] [PubMed] [Google Scholar]
- 26.Nordström M, Lindblad B, Anderson H, et al. Deep venous thrombosis and occult malignancy: An epidemiological study. BMJ. 1994;308:891–894. doi: 10.1136/bmj.308.6933.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varki A. Trousseau's syndrome: Multiple definitions and multiple mechanisms. Blood. 2007;110:1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet. 1999;354:93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 29.Taneja S, Mandayam S, Kayani ZZ, et al. Comparison of stage at diagnosis of cancer in patients who are on dialysis versus the general population. Clin J Am Soc Nephrol. 2007;2:1008–1013. doi: 10.2215/CJN.00310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartsch GC, Kuefer R, Braun C, et al. Nosocomial bacteriuria in patients with indwelling catheter after radical retropubic prostatectomy for prostate cancer. Urol Int. 2008;81:389–393. doi: 10.1159/000167834. [DOI] [PubMed] [Google Scholar]
- 31.Melton LJ, 3rd, Alothman KI, Khosla S, et al. Fracture risk following bilateral orchiectomy. J Urol. 2003;169:1747–1750. doi: 10.1097/01.ju.0000059281.67667.97. [DOI] [PubMed] [Google Scholar]
- 32.Gennari L, Nuti R, Bilezikian JP. Aromatase activity and bone homeostasis in men. J Clin Endocrinol Metab. 2004;89:5898–5907. doi: 10.1210/jc.2004-1717. [DOI] [PubMed] [Google Scholar]