This study aims to address the current knowledge gap in the diagnosis and treatment of epithelioid sarcoma (ES) and unclassified sarcoma with epithelioid features (USEF) by: (a) characterizing and comparing the clinical behavior and outcome of ES and USEF patients treated at a single institution, (b) evaluating the expression of a panel of differentiation and other tumor-related molecular markers in human ES and USEF specimens, and (c) developing an ES experimental model for future studies to enhance our understanding of ES molecular determinants while developing more effective therapeutic strategies.
Keywords: Epithelioid sarcoma, Molecular markers, INI-1, CA-125, Cell lines, Animal model
Learning Objectives
After completing this course, the reader will be able to:
Characterize and compare the clinical behavior and outcome of patients with epithelioid sarcoma and unclassified sarcoma with epithelioid features.
Identify differentiation and other tumor-related molecular markers in human ES and USEF specimens described in this study.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Epithelioid sarcoma (ES) and unclassified sarcoma with epithelioid features (USEF) are clinically and therapeutically unresolved. We compared ES and USEF patients' clinical behavior, treatment, outcome, and molecular marker expression. Furthermore, preclinical ES study models were developed to enable comprehensive benchside investigations.
Patients and Methods.
A database of ES and USEF patients (n = 116) treated since 1992 was created. A clinically annotated ES–USEF tissue microarray (TMA) was assayed for tumor-related markers. Newly established human and commercially available ES cell lines were characterized and tested in vivo.
Results.
ES and USEF patients presenting with localized disease exhibited 22% and 25% local recurrence rates, 35% and 19% nodal metastasis rates, and 41% and 53% distant metastasis rates (median follow-up, 54 months and 39 months, respectively). The 5- and 10-year disease-specific survival rates were 88% and 43% and 52% and 42% (ES and USEF, respectively). TMA immunohistochemistry identified integrase interactor (INI)-1 loss, cancer antigen 125, and p53 nuclear expression as significantly more common in ES than USEF cases. Both cell lines preserved ES morphological and biochemical characteristics in vitro and in vivo; loss of INI-1 was shown to occur in both lines.
Conclusions.
Enhanced knowledge of ES and USEF clinical behavior, marker expression, and molecular determinants, extended via experimental models, will hopefully accelerate development of urgently needed effective targeted therapies for ES and USEF.
Introduction
Epithelioid sarcoma (ES) is uniquely composed of cells exhibiting both mesenchymal and epithelial differentiation markers (e.g., vimentin and cytokeratin, respectively); the cell of origin is unknown [1, 2]. First described in 1961 by Lakowski [3] as “sarcoma aponeuroticum” and renamed “epithelioid sarcoma” by Enzinger in 1970 [1], these tumors are rare, comprising <1% of cases of soft tissue sarcoma (STS) [1, 3, 4]. Two distinct histopathological ES subtypes have been defined: the conventional classic distal type and the more recently identified proximal or axial type [5, 6]. Histologically, the classic type is characterized by nodular arrangements of epithelioid tumor cells surrounding an area of central necrosis, resulting in a granuloma-like appearance, whereas the latter exhibits larger epithelioid cells, with vesicular nuclei and prominent nucleoli, copious, eccentric cytoplasm, marked cytologic atypia, and frequent rhabdoid features [1, 5]. The differential diagnosis is broad; consequently, establishing a diagnosis of ES heavily depends on pathologist expertise, histomorphology, and comprehensive immunohistochemical (IHC) analyses [7–9]. Commonly, the final ES diagnosis is made by excluding other potential benign and malignant conditions [10]. We have noted a subset of unclassified sarcoma with epithelioid features (USEF) that are not consistent with the diagnosis of ES. A diagnosis of exclusion, USEF likely represents a heterogeneous cohort of unclassifiable malignancies.
As with other orphan tumors, clinical knowledge of ES stems from published case reports, small patient cohorts, and population-based registries [11–15]; these suggest a propensity for local recurrence and distant metastasis [13, 14]. In contrast to more common sarcoma subtypes, lymphatic spread is quite common in ES [13–16]. Five-year survival rates of 60%–75% have been reported; proximal ES outcomes are less favorable [5, 6]. To our knowledge, studies specifically evaluating USEF clinical behavior and outcome have not been published.
We therefore sought to address this current knowledge gap by: (a) characterizing and comparing the clinical behavior and outcome of ES and USEF patients treated at a single institution, (b) evaluating the expression of a panel of differentiation and other tumor-related molecular markers in human ES and USEF specimens, and (c) developing an ES experimental model for future studies to enhance our understanding of ES molecular determinants while developing more effective therapeutic strategies.
Patients and Methods
Clinical Database
This study was conducted with institutional review board (IRB) approval from The University of Texas MD Anderson Cancer Center (MDACC). A pathology archive search revealed patients with ES or USEF seen in January 1992 to May 2009 (n = 220). Patients receiving only clinical or pathological second opinions were excluded (n = 104). Patients treated and followed up at MDACC were further evaluated; only those with unequivocal ES or USEF histology confirmed by a sarcoma pathologist (A.J.L.) were included in the final dataset (n = 116). ES was diagnosed according to standard pathology criteria. USEF cases lacked defining features of carcinoma or sarcomatoid carcinoma and were diagnosed as unclassified sarcomas with the unusual feature of having more epithelioid rather than spindle cell morphology, but not considered to fall within the formal diagnostic category of ES. These cases lacked association with internal organs and arose in compartments where carcinomas are rare, and lacked a history of a prior or concurrent carcinoma. A database was constructed containing patient, tumor, treatment, and follow-up information.
Tissue Microarray Construction
Archived paraffin blocks from 27 localized ES, 17 localized USEF, and eight primary breast cancer surgical samples (control for epithelial-related markers) were assembled in a tissue microarray (TMA) as previously described [17]. Hematoxylin and eosin (H&E)-stained sections were reviewed from each tumor block by a sarcoma pathologist (A.J.L.) to define areas of homogeneous, viable tumor. Using an automated TMA apparatus (ATA-27; Beecher Instruments, Sun Prairie, WI), 0.6-mm punch samples (two per case) were obtained from donor blocks and formatted into a recipient block (104 total cores). Sections (4 μm) were cut and verified by H&E staining.
IHC
Commercially available antibodies against vimentin (Dako, Carpenteria, CA), pan-cytokeratin (BD Biosciences, San Jose, CA and Zymed, Carlsbad, CA), epithelial membrane antigen (EMA) (Dako), CD34 (BD Biosciences), integrase interactor (INI)-1 (BAF47; BD Biosciences), cancer antigen (CA)-125 (Dako), p53 (Dako), vascular endothelial growth factor (VEGF)-A (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), VEGF-C (Santa Cruz Biotechnology), β-catenin (BD Biosciences), and E-cadherin (Zymed Laboratories, Inc., South San Francisco, CA) were used for IHC, conducted as previously described [18, 19]. Positive and negative controls were run in parallel. Labeling intensity was scored by a soft tissue pathologist (A.J.L.) as none, low, or high, and the percentage of positive tumor cells was estimated.
Cell Lines and ES Experimental Model
The human ES cell line VAESBJ (ATCC, Manassas, VA) and the Epi-544 cell line, established per below, were maintained in Dulbecco's medium supplemented with 10% fetal bovine serum, nonessential amino acids, and penicillin. Epi-544 isolation was conducted with approval from the MDACC IRB and informed patient consent as previously described [20]. For STR DNA fingerprinting, DNA was prepared from cells and tumor tissue using the DNA Maxi kit as per the manufacturer's protocol (Qiagen, Valencia, CA). DNA fingerprints were obtained using the AmpFlSTR Identifiler polymerase chain reaction (PCR) Amplification kit (Applied Biosystems, Foster City, CA) as per the manufacturer's protocol [20]. For the chromosome analysis, karyotyping was conducted at the University of Texas MDACC Cytogenetics Core Facility. The soft agar colony formation assay was performed as previously described [20]. Migration/invasion assays were conducted as described previously [21]. The Western blot analysis was performed by standard methods. Briefly, 25–50 μg of proteins extracted from cultured cells were separated by SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blocked and blotted with the anti–INI-1 antibody described above; antiactin antibody was used for the loading control. Horseradish peroxidase–conjugated secondary antibodies were detected by ECL chemiluminesence (Amersham Biosciences, Plc., Cardiff, U.K.). Reverse transcription (RT)-PCR was conducted as previously described [22]. PCR primers were designed using primer 3 software. The primers used were: INI-1, 5′-AGCCACTGTGGAAGAGAGGA-3′ and 5′-CTGTTCCTCTTGGCCTTCTG-3′; glyceraldehyde-3-phosphate dehydrogenase, 5′-GAGCCACATCGCTCAGAC-3′ and 5′-CTTCTCATGGTTCACACCC-3′. For the in vivo growth experiment, Epi-544 or VAESBJ cells were injected s.c. (1 × 106 cells/mouse) into the flank of 6-week-old female severe combined immunodeficient (SCID) mice. Mice were followed for tumor growth. The study was terminated when tumors reached 1.5 cm in largest dimension. Tumors were then resected, preserved in formalin, and paraffin embedded.
Statistical Analysis
The statistical analysis was conducted as previously described [17]. Patient demographic characteristics, clinical characteristics, and molecular marker expression levels were summarized using medians or proportions as applicable. The two-sample t-test, χ2 test, or Fisher's exact test were used to compare ES- and USEF-related variables. Disease-specific survival (DSS) was estimated using the Kaplan–Meier method (with a 95% confidence interval). Event time to occurrence was computed as the date of presentation at MDACC to the date the event was recorded, or as the date of last follow-up assessment in event-free patients. Kaplan–Meier curves were used to determine the DSS time; log-rank testing was used to compare DSS between patient subgroups. Univariate Cox proportional hazards models were fit to assess patient characteristics' abilities to predict DSS. A multivariable Cox model was obtained by performing backward elimination with a .05 p-value cutoff. All computations were carried out in SAS (SAS Institute Inc., Cary, NC).
Results
Clinicopathological Variables and Outcome of ES Patients
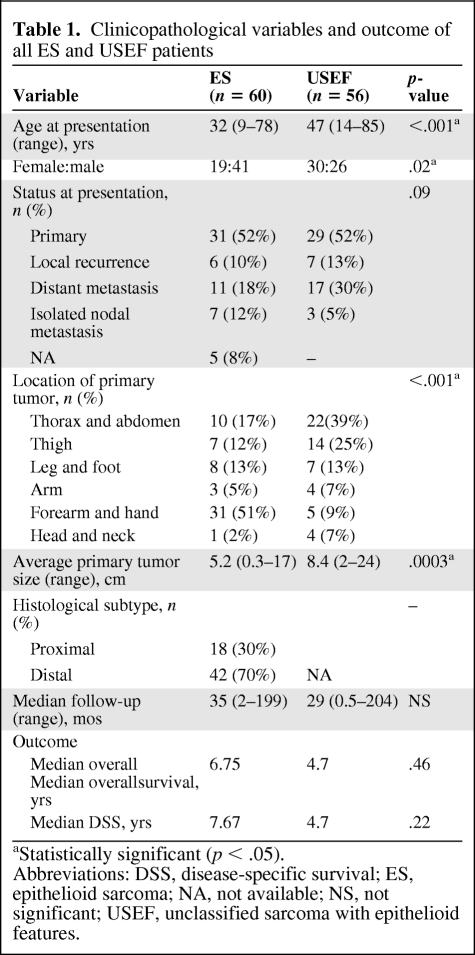
Sixty ES patients were initially analyzed (Table 1). The median age was 32 years (range, 9–78 years). There were more males than females. The majority of patients (62%) presented with localized disease—31 with primary and six with recurrent disease. Seven patients presented with lymph node (LN) only metastatic disease. Eleven patients exhibited systemic metastasis, most commonly pulmonary; five of these also presented with LN metastasis (i.e., lymphatic metastases were found in 20% of patients). The most prevalent ES primary location sites were the forearm and hand. The average tumor size was 5.2 cm. Histologically, 70% of the lesions exhibited classic distal-type ES features; 30% were proximal-type ES. Treatment variables for localized disease patients are discussed below. Metastatic patients were all initially treated with doxorubicin-based chemotherapy. During a median follow-up of 35 months (range, 2–199 months), the median overall survival (OS)and DSS times were 6.75 years and 7.67 years, respectively. Patients presenting with metastatic disease had significantly worse outcomes, with a median survival of 1.9 years, versus 8.7 years for localized disease patients (p < .001) (Fig. 1A).
Table 1.
Clinicopathological variables and outcome of all ES and USEF patients
aStatistically significant (p < .05).
Abbreviations: DSS, disease-specific survival; ES, epithelioid sarcoma; NA, not available; NS, not significant; USEF, unclassified sarcoma with epithelioid features.
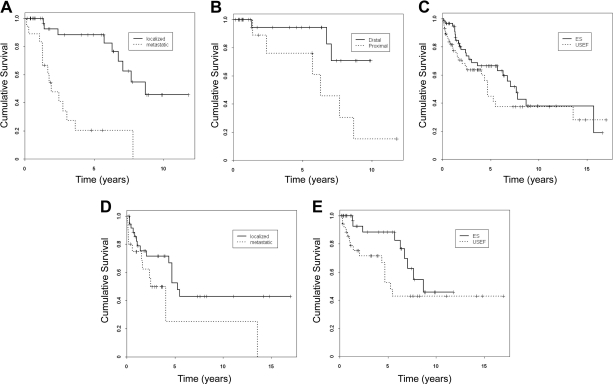
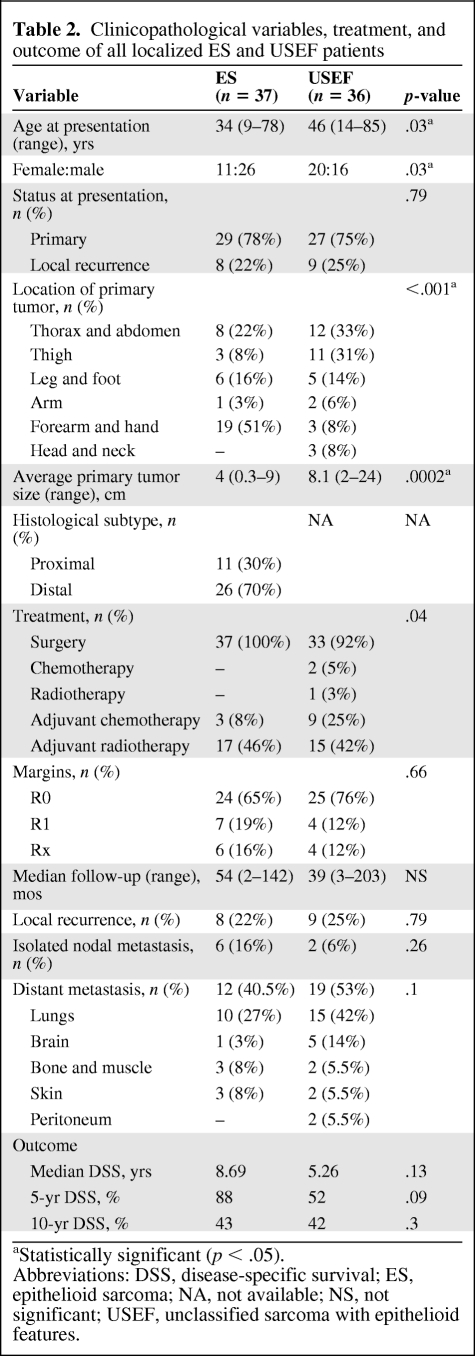
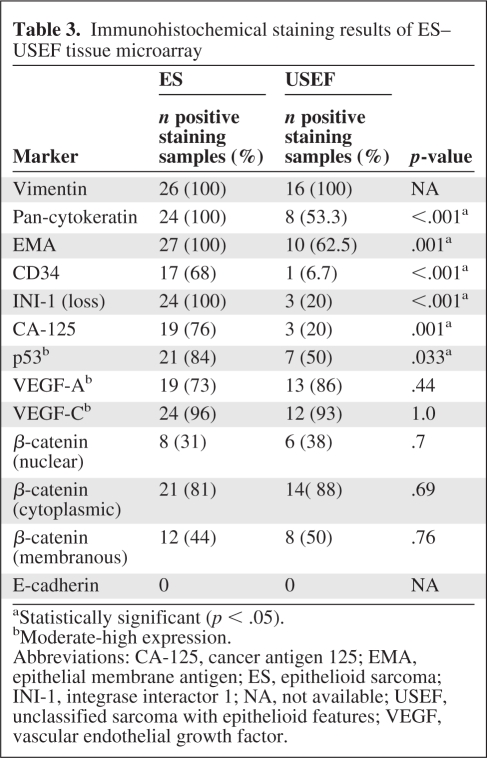
Figure 1.
Epithelioid sarcoma (ES) and unclassified sarcoma with epithelioid features (USEF) outcome analysis. Kaplan–Meier disease-specific survival curves stratified for patients with localized versus metastatic ES (p < .0001) (A), patients with localized distal ES versus localized proximal ES (p = .03) (B), patients with ES versus USEF (p = .22) (C), patients with localized versus metastatic USEF (p = .05) (D), and patients with localized ES versus localized USEF (p = .09) (E).
To better understand the outcomes of ES patients, those presenting with localized disease were analyzed (Table 2). Twenty-four patients underwent local excision; amputation (mainly ray amputation) was done in 13. Complete macroscopic resection was achieved in all patients; microscopic surgical margins were negative in 24 and positive in seven cases (margin status could not be determined in six patients). Seventeen patients underwent adjuvant radiotherapy (including all the microscopically positive surgical margin cases); three patients received adjuvant chemotherapy. The median follow-up duration was 54 months (range, 2–142 months). Eight patients (22%) developed local recurrence that was treated with re-excision with or without radiation and chemotherapy. Six patients developed nodal disease as their sole metastases; an additional seven developed synchronous metastases elsewhere. Twelve patients (32%) developed systemic (predominantly lung) metastasis; other sites included the brain, bone, soft tissue, and skin. Interestingly, >66% of patients who developed metastasis did so >5 years after localized tumor treatment. The median DSS time was 8.7 years, the 5- and 10-year DSS rates were 88% and 43%, respectively. Kaplan–Meier and univariate and multivariate analyses were conducted to identify variables impacting DSS: only proximal-type ES histology (hazard ratio [HR], 4.2; p = 0.04) and proximal disease location (HR, 16; p = .04) were predictive of outcome (Fig. 1B).
Table 2.
Clinicopathological variables, treatment, and outcome of all localized ES and USEF patients
aStatistically significant (p < .05).
Abbreviations: DSS, disease-specific survival; ES, epithelioid sarcoma; NA, not available; NS, not significant; USEF, unclassified sarcoma with epithelioid features.
Clinicopathological Variables and Outcome of USEF Patients and Comparison With ES Patients
After comprehensive pathological and IHC analyses, 56 patients were diagnosed as USEF; clinicopathological variables are depicted in Table 1. Their average age was older than that of ES patients (47 years; p < .001). There was no sex predilection, in contrast to the greater ES rate observed in males (p = .02). MDACC USEF patient presentation was similar to that of ES patients; the majority harbored localized disease. Three patients presented with nodal disease as the single metastatic site; five additional patients had LN metastasis synchronous with systemic (mainly pulmonary) disease. USEF occurred more commonly in central or truncal locations (p < .001). The average size at diagnosis was significantly larger than that of ES tumors (8.4 cm; p = .0003). The median USEF follow-up time was 29 months. The median OS and DSS times were both 4.7 years, which, although shorter than ES patient survival, were not statistically significantly different (Fig. 1C). As expected, patients presenting with metastatic USEF had significantly worse outcomes (median DSS time, 2.5 years versus 5.3 years in patients with localized disease; p = .05) (Fig. 1D).
Clinicopathological variables of localized USEF patients are summarized in Table 2. The therapeutic approaches for USEF and ES patients were similar—surgery was the mainstay of therapy. The tumor was nonresectable in three patients; these were treated with chemotherapy or radiotherapy. All other patients received complete surgical resection; six patients underwent amputation (hemipelvectomy in one case). Adjuvant doxorubicin-based chemotherapeutic regimens were administered to 19% of patients and 42% received adjuvant radiotherapy.
The median follow-up duration was 39 months. Local recurrence developed in nine patients, isolated lymphatic metastasis developed in two patients, and systemic metastasis (mainly lung) developed in 19 patients. Five systemic metastasis patients also had nodal involvement. Interestingly, five patients developed brain metastasis. In the majority of cases (82%), metastasis occurred within the first 5 years after initial treatment. The median DSS time and 5-year DSS rate were 5.26 years and 52%, respectively. The local, regional, and systemic failure rates and DSS (Fig. 1E) were not statistically different in USEF and ES patients. Univariate and multivariate analyses failed to identify markers predictive of USEF patient DSS.
ES and USEF Molecular Markers
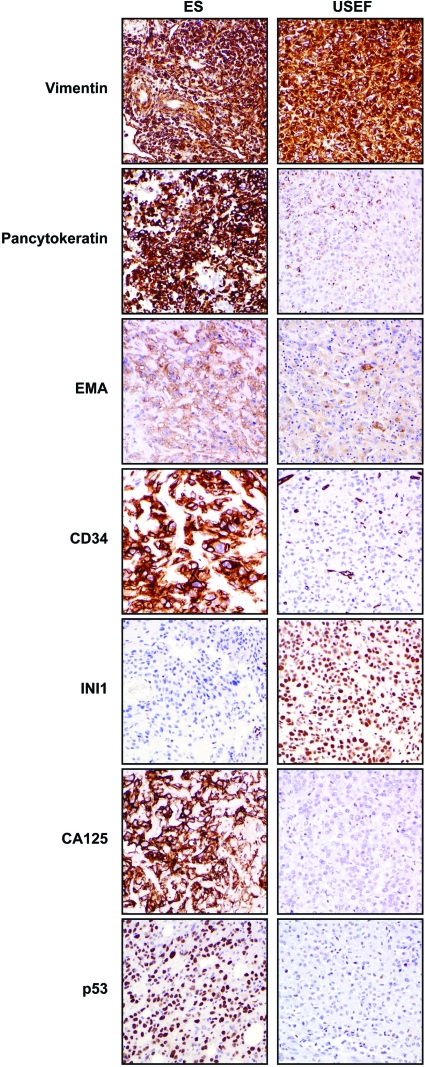
A human ES–USEF TMA was used to evaluate candidate molecular marker expression (Table 3, Fig. 2). Differentiation markers were initially evaluated: all ES and USEF samples expressed vimentin. In contrast, cytokeratin and EMA were more common in ES than in USEF samples (100% and 100% in ES samples, versus 53% and 66% in USEF samples, respectively). All USEF samples expressed at least one of the two epithelial markers. CD34 immunoreactivity was found in 17 ES samples (68%), but only in one USEF sample. Loss of INI expression was recently identified as a marker of ES [19, 23–25]. All evaluable ES samples on our TMA exhibited INI-1 loss. In contrast, only three (20%) USEF samples were INI-1−. CA-125 expression has also been suggested as an ES marker [26]. Most ES samples (76%) exhibited moderate to high CA-125 expression; no expression was seen in six cases. No expression was observed in the 12 evaluable USEF samples (80%), whereas three samples exhibited moderate to high expression; a direct correlation between INI-1 loss and CA-125 expression was found in USEF samples.
Table 3.
Immunohistochemical staining results of ES–USEF tissue microarray
aStatistically significant (p < .05).
bModerate-high expression.
Abbreviations: CA-125, cancer antigen 125; EMA, epithelial membrane antigen; ES, epithelioid sarcoma; INI-1, integrase interactor 1; NA, not available; USEF, unclassified sarcoma with epithelioid features; VEGF, vascular endothelial growth factor.
Figure 2.
Biomarker expression in ES and USEF. Immunohistochemical images depicting representative marker expression in human ES and USEF samples included in the tissue microarray. All original images were captured at 200× magnification.
Abbreviations: INI-1, integrase interactor 1; EMA, epithelial membrane antigen; ES, epithelioid sarcoma; USEF, unclassified sarcoma with epithelioid features.
Next, the expression of tumorigenic markers was assessed in ES and USEF samples. Moderate to high nuclear p53 was seen in 84% of ES and 50% of USEF samples (p = .033). VEGF-A and VEGF-C were highly expressed in both ES and USEF samples and of interest given the unusual propensity for these sarcomas to show systemic and lymphatic dissemination. Similarly, β-catenin expression was found in all ES and USEF samples, with similar intracellular distribution patterns. Nuclear β-catenin was found in 31% and 38% of ES and USEF samples, respectively. Interestingly, although >40% of ES and USEF samples expressed membranous β-catenin, no samples exhibited E-cadherin expression, in contrast to the high E-cadherin expression levels observed in control breast cancer samples.
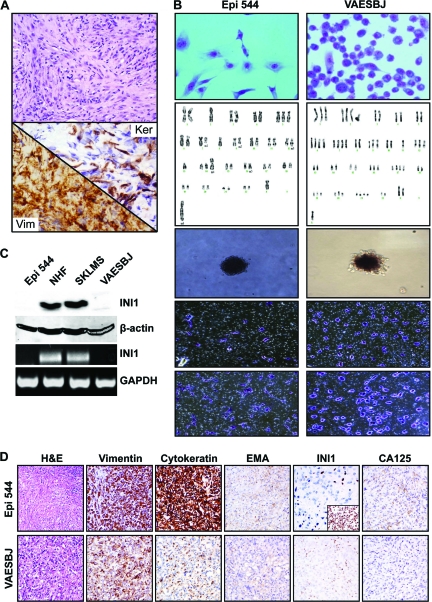
ES-Related Experimental Model
The cell line Epi-544 originated from a primary ES of the foot (Fig. 3A). Epi-544 has grown without interruption for >24 months with >60× passages. DNA fingerprinting by STR analysis confirmed the origin to be from the human tumor, demonstrating that no crosscontamination had occurred in culture. Epi-544 and the human ES cell line VAESBJ (ATCC) were further evaluated (Fig. 3B). Karyotypic analysis of Epi-544 identified a modal chromosomal number of 45 (range, 42–45), monosomy of chromosomes 2, 8, 13, and X, trisomy of chromosome 5, and the following structural abnormalities: del 7q, del 9q, del 12p, 16q+, t(9q;14q), and t(2q;?). The karyotype of VAESBJ was consistent with previously published data (http://www.atcc.org/ATCCAdvancedCatalogSearch/ProductDetails/tabid/452/Default.aspx?ATCCNum=CRL-2138&Template=cellBiology). Cell doubling time was determined by counting and was found to be ∼32 hours and ∼48 hours for VAESBJ and Epi-544, respectively. Both cell lines formed colonies in soft agar (cloning efficiency, 5% and 12.4%, respectively); both have migratory and invasive phenotypes. Next, INI-1 protein and mRNA expression were evaluated. As shown in Figure 3C, no INI-1 expression was found in our ES cells, in contrast to controls (normal human fibroblasts and a leiomyosarcoma cell line). Lastly, we evaluated ES cell growth in SCID mice. s.c. injection of Epi-544 cells (passage 25; 2 × 106 cells/mouse) (n = 6) resulted in tumor growth in four mice (tumor take, 66%) after a latency period of 30 ± 2.5 weeks. After tumor establishment, lesions demonstrated reproducible growth and reached 1.5 cm in largest dimension at 64 ± 20 days. VAESBJ injection (1 × 106 cells/mouse) exhibited better growth dynamics: 90% tumor take (nine of 10), 24 ± 10 days latency period, and an average of 26 days to reach 1.5 cm. H&E staining of Epi-544 xenograft tissue samples demonstrated histological appearances resembling the original tumor and that of VAESBJ, confirming an ES diagnosis (Fig. 3D). Both tumors exhibited positive staining for human vimentin, cytokeratin, and EMA; INI-1 loss was identified in both xenografts. CA-125 expression was found in Epi-544 samples but no expression was seen in VAESBJ samples.
Figure 3.
Characterization of the human ES cell lines Epi-544 and VAESBJ. (A): H&E, pan-cytokeratin, and vimentin immunohistochemical staining of the original human sample from which Epi-544 was derived. (B): Panel (from top) depicting cell morphology, karyotype, growth in soft agar, migration, and invasion. (C): ES cells do not express INI-1 protein (Western blot, upper panel) or mRNA (RT-PCR; lower panel) in contrast to NHFs and the leiomyosarcoma cell line SKLMS1, which were used as controls. (D): Epi-544 and VAESBJ preserve ES characteristics in vivo. Immunohistochemical staining of tumor xenografts is depicted (inset represents an INI-1+ soft tissue sarcoma xenograft).
Abbreviations: H&E, hematoxylin and eosin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; INI-1, integrase interactor 1; EMA, epithelial membrane antigen; ES, epithelioid sarcoma; NHF, normal human fibroblast; USEF, unclassified sarcoma with epithelioid features.
Discussion
Given our lack of targeted therapies directed at the >50 STS histological subtypes, most are managed similarly, with surgery being the mainstay of therapy. The dramatic progress made in the diagnosis and management of gastrointestinal stromal tumor, an STS histological subtype that, until a decade ago, was indistinguishable from leiomyosarcoma [27], attests to the importance of STS histologic and molecular subclassification.
First described almost 50 years ago, ES remains a clinical enigma. Excluding publications consisting of small/single case studies, our series expand the number of ES patients reported to ∼1,000, suggesting the rarity of ES [4]. Together, these reports highlight several unique ES features distinguishing it from other STS subtypes: (a) ES is mainly a disease of young adults in the third and fourth decades of life, (b) ES exhibits a strong male predilection, (c) ES is the most common hand and forearm STS, and (d) ES exhibits a unique propensity for lymphatic spread—whereas LN metastases develop in <3% of STS cases, nodal involvement rates of 20%–50% are reported for ES [14, 15, 28]. In our study, 35% of localized ES cases developed lymphatic metastasis during follow-up. Given the high rate of ES local and systemic failure, it is surprising that patient outcome is a favorable 60%–75% 5-year OS rate, compared with other STS types [15, 29–31]. We observed an 88% 5-year DSS rate for localized ES patients. It is pertinent to point out that this relatively favorable outcome of resectable ES patients in our study might reflect the impact of treatment of rare cancers in high-volume institutions experienced in the management of these diseases. However, the 10-year ES DSS rate was much worse (43.5%). In contrast to other types of STS, in which 80% of local and systemic recurrences occur within 2 years of initial treatment [32], we observed that ES tends to disseminate late in the course of disease; long-term follow-up and repeated imaging are thus indicated. With the limitation of a small patient cohort, only metastatic disease, proximal histology, and proximal location were identified as independent DSS prognosticators in our study. Older age, male gender, proximal or axial location, depth, and tumor size were previously identified as adverse prognostic factors [12].
USEF patients comprise an STS cohort that would generally be designated as unclassified pleomorphic sarcoma/malignant fibrous histocytoma (UPS/MFH) under World Health Organization criteria, although lacking pleomorphic, spindle morphology [33]. These exhibit some pathological and IHC similarities to ES, but to an insufficient degree for a diagnosis of ES. We found that, compared with ES, USEF is diagnosed at an older age, does not exhibit gender predilection, develops more commonly in central locations such as the trunk and proximal thigh, and is of a larger average size at diagnosis. No significant difference in local recurrence and systemic failure rates were found, yet a statistical trend (p = .09) toward worse 5-year outcomes was noted for USEF patients. This possibly reflects the early development of metastasis in patients treated for localized USEF; in contrast, no difference in the 10-year DSS rate was identified. In total, ∼19% of USEF patients developed lymphatic metastasis and 14% developed brain metastasis, a metastatic pattern that is different from that of UPS/MFH, suggesting that a tailored follow-up approach may be needed for USEF patients [34]. However, these observations are limited by our relatively small patient number, and larger scale validation is needed.
TMA has found wide acceptance as a powerful research tool [35]. Using our ES–USEF TMA, we showed that (as previously reported) ES samples all diffusely expressed both mesenchymal and epithelial differentiation markers [36]. All USEF samples expressed vimentin and at least one epithelial marker (pan-cytokeratin and/or EMA), yet the staining pattern was patchy, scattered, and weak rather than diffuse. CD34 immunoreactivity was previously found to occur in ∼50% of ES cases [5, 37, 38]; this was confirmed in our study. Our results further demonstrate that USEFs are generally CD34−. Loss of INI-1, the product of the hSNF5/INI1/SMARCB1/BAF47 gene first identified as a tumor suppressor gene in malignant rhabdoid tumors, was recently shown to occur in >90% of ES cases [19]. In contrast, INI-1 expression was found to be retained in most benign lesions and epithelioid neoplasms that otherwise resemble ES [19, 27]. The only other potential ES mimics that showed loss of INI-1 expression are epithelioid malignant peripheral nerve sheath tumor (50% of cases) and myoepithelial carcinoma of soft tissue (9% of cases). All ES samples in our study exhibited loss of INI-1. This finding may be attributable to the conservative approach we employ when making an ES diagnosis—only samples conforming to all histological and IHC criteria are designated ES. Three of the samples diagnosed as USEF in our study exhibited INI-1 loss. USEF is a diagnosis of exclusion, and it is thus possible that these three tumors should be reclassified as ES. At a time when specific molecular targeted therapies will become available for ES, such reclassification will be crucially important. Our findings support inclusion of INI-1 IHC in routine ES workup. Furthermore, using our newly established preclinical cell line and xenograft model, future studies will enable incisive study of the molecular underpinnings of INI-1 and its intriguing role in tumor biology. Investigations to that end are currently ongoing in the laboratory. CA-125, a serum antigen commonly used as a marker for ovarian cancer, was recently shown to be overexpressed in ES tumors [11, 26], and our study further supports this observation: 76% of ES samples exhibited moderate to high CA-125 expression. Furthermore, in two small patient series, an elevated serum CA-125 level was identified in ∼90% of ES patients; serum levels correlated with therapeutic response and disease progression [39, 40]. Together, these studies suggest the potential usefulness of CA-125 as a diagnostic and follow-up marker for ES. Interestingly, in our study, only three USEF samples (20%) expressed CA-125, and these corresponded to tumors with INI-1 loss, further supporting their diagnosis as unusual forms of ES lacking classic, characteristic features and further suggesting an inverse correlation between these two proteins. Studies evaluating CA-125 expression regulation by INI-1 are ongoing. The current study aimed to describe novel bioresources developed for comprehensive study of ES and the expression of molecular markers to expand the ES diagnostic armamentarium. Our ultimate goal is to use the described bioresources for the identification of deregulated “drugable” molecular targets and test the impact of their blockade on tumor growth and progression.
Summary
Clinical and molecular insights stemming from our study enhance our understanding of ES and USEF. At this juncture, it is critical to translate these ES clinical and pathological tissue-based observations into benchside investigations. Enhanced knowledge of ES molecular determinants and their mechanisms of action will hopefully aid the development of urgently needed targeted therapies. Accordingly, it is essential to develop experimental models that robustly recapitulate the characteristics of clinical ES. The human cell lines and established animal models described here embody ES morphological and biochemical characteristics, rendering them useful for further in-depth investigation of this disease. Preclinical testing of several currently available tyrosine kinase inhibitors and other small molecule inhibitors is under way.
Acknowledgments
We thank Ms. Vu for aid in figure preparation and Mr. Cuevas for his assistance with manuscript preparation and submission.
This work was supported in part by the National Cancer Institute at the National Institutes of Health (RO1CA138345 grant to D.L.) and an Amschwand Foundation Seed Grant (to P.Z.). The MD Anderson Cancer Center cell line characterization and cytogenetic Core Facilities are both supported by a National Institutes of Health Cancer Center Support Grant (CA#16672).
Author Contributions
Conception/Design: Dina Lev, Aniket Sakharpe
Provision of study material or patients: Dina Lev, Priya Rao, Svetlana Bolshakov, Raphael E. Pollock, Alexander J. Lazar
Collection and/or assembly of data: Dina Lev, Jason L. Hornick, Priya Rao, Theresa Nguyen, Svetlana Bolshakov, Pingyu Zhang, Xianbiao Xie, Alexander J. Lazar, Roman Belousov, Aniket Sakharpe, Guy Lahat, Taher Gulamhusein, Eric Young
Data analysis and interpretation: Dina Lev, Jason L. Hornick, Raphael E. Pollock, Alexander J. Lazar, Aniket Sakharpe, Guy Lahat, Ping Liu
Manuscript writing: Dina Lev, Raphael E. Pollock, Alexander J. Lazar, Ping Liu
Final approval of manuscript: Dina Lev, Jason L. Hornick, Priya Rao, Theresa Nguyen, Svetlana Bolshakov, Pingyu Zhang, Xianbiao Xie, Raphael E. Pollock, Alexander J. Lazar, Roman Belousov, Aniket Sakharpe, Guy Lahat, Taher Gulamhusein, Eric Young, Ping Liu
References
- 1.Enzinger FM. Epitheloid sarcoma. A sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029–1041. doi: 10.1002/1097-0142(197011)26:5<1029::aid-cncr2820260510>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Molenaar WM, DeJong B, Dam-Meiring A, et al. Epithelioid sarcoma or malignant rhabdoid tumor of soft tissue? Epithelioid immunophenotype and rhabdoid karyotype. Hum Pathol. 1989;20:347–351. doi: 10.1016/0046-8177(89)90044-0. [DOI] [PubMed] [Google Scholar]
- 3.Laskowski J. Sarcoma aponeuroticum. Nowotory. 1961;11:61–67. [Google Scholar]
- 4.de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: Still an only surgically curable disease. Cancer. 2006;107:606–612. doi: 10.1002/cncr.22037. [DOI] [PubMed] [Google Scholar]
- 5.Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. Clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130–146. doi: 10.1097/00000478-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: A clinicopathologic study of 20 cases. Mod Pathol. 2001;14:655–663. doi: 10.1038/modpathol.3880368. [DOI] [PubMed] [Google Scholar]
- 7.Chase DR, Enzinger FM, Weiss SW, et al. Keratin in epithelioid sarcoma: An immunohistochemical study. Am J Surg Pathol. 1984;8:435–441. doi: 10.1097/00000478-198406000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Manivel JC, Wick MR, Dehner LP, et al. Epithelioid sarcoma: An immunohistochemical study. Am J Clin Pathol. 1987;87:319–326. doi: 10.1093/ajcp/87.3.319. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt D, Harms D. Epithelioid sarcoma in children and adolescents: An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1987;410:423–431. doi: 10.1007/BF00712762. [DOI] [PubMed] [Google Scholar]
- 10.Enzinger FM, Weiss SW. Epithelioid sarcoma: Malignant tumors of uncertain histogenesis. In: Enzinger FM, Weiss SW, editors. Soft Tissue Tumors. Third Edition. St Louis: Mosby; 1995. pp. 1074–1083. [Google Scholar]
- 11.Weisskopf M, Mn̈ker R, Hermanns-Sachweh B, et al. Epithelioid sarcoma in the thoracic spine. Eur Spine J. 2006;15(suppl 5):604–609. doi: 10.1007/s00586-006-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chase DR, Enzinger FM. Epithelioid sarcoma: Diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985;9:241–263. [PubMed] [Google Scholar]
- 13.Callister MD, Ballo MT, Pisters PW, et al. Epithelioid sarcoma: Results of conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:384–391. doi: 10.1016/s0360-3016(01)01646-7. [DOI] [PubMed] [Google Scholar]
- 14.Wolf PS, Flum DR, Tanas MR, et al. Epithelioid sarcoma: The University of Washington experience. Am J Surg. 2008;196:407–412. doi: 10.1016/j.amjsurg.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Jawad MU, Extein J, Min ES, et al. Prognostic factors for survival in patients with epithelioid sarcoma: 441 cases from the SEER database. Clin Orthop Relat Res. 2009;467:2939–2948. doi: 10.1007/s11999-009-0749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800–1808. doi: 10.1002/1097-0142(19871015)60:8<1800::aid-cncr2820600822>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Zhan M, Hannay JA, et al. Wild-type p53 inhibits nuclear factor-kappaB-induced matrix metalloproteinase-9 promoter activation: Implications for soft tissue sarcoma growth and metastasis. Mol Cancer Res. 2006;4:803–810. doi: 10.1158/1541-7786.MCR-06-0201. [DOI] [PubMed] [Google Scholar]
- 19.Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542–550. doi: 10.1097/PAS.0b013e3181882c54. [DOI] [PubMed] [Google Scholar]
- 20.Lahat G, Zhu QS, Huang KL, et al. Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies. PLoS One. 2010;5:e10105. doi: 10.1371/journal.pone.0010105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Liu H, Radisky DC, Nelson CM, et al. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc Natl Acad Sci U S A. 2006;103:4134–4139. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Z, Lahat G, Korchin B, et al. Midkine enhances soft-tissue sarcoma growth: A possible novel therapeutic target. Clin Cancer Res. 2008;14:5033–5042. doi: 10.1158/1078-0432.CCR-08-0092. [DOI] [PubMed] [Google Scholar]
- 23.Chbani L, Guillou L, Terrier P, et al. Epithelioid sarcoma: A clinicopathologic and immunohistochemical analysis of 106 cases from the French sarcoma group. Am J Clin Pathol. 2009;131:222–227. doi: 10.1309/AJCPU98ABIPVJAIV. [DOI] [PubMed] [Google Scholar]
- 24.Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012–4019. doi: 10.1158/0008-5472.CAN-04-3050. [DOI] [PubMed] [Google Scholar]
- 25.Orrock JM, Abbott JJ, Gibson LE, et al. INI1 and GLUT-1 expression in epithelioid sarcoma and its cutaneous neoplastic and nonneoplastic mimics. Am J Dermatopathol. 2009;31:152–156. doi: 10.1097/DAD.0b013e31818a5c4f. [DOI] [PubMed] [Google Scholar]
- 26.Kato H, Hatori M, Kokubun S, et al. CA125 expression in epithelioid sarcoma. Jpn J Clin Oncol. 2004;34:149–154. doi: 10.1093/jjco/hyh027. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): A review. Eur J Cancer. 2002;38(suppl 5):S39–S51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 28.Herr MJ, Harmsen WS, Amadio PC, et al. Epithelioid sarcoma of the hand. Clin Orthop Relat Res. 2005;431:193–200. doi: 10.1097/01.blo.0000150317.50594.96. [DOI] [PubMed] [Google Scholar]
- 29.Baratti D, Pennacchioli E, Casali PG, et al. Epithelioid sarcoma: Prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2007;14:3542–3551. doi: 10.1245/s10434-007-9628-9. [DOI] [PubMed] [Google Scholar]
- 30.Ross HM, Lewis JJ, Woodruff JM, et al. Epithelioid sarcoma: Clinical behavior and prognostic factors of survival. Ann Surg Oncol. 1997;4:491–495. doi: 10.1007/BF02303673. [DOI] [PubMed] [Google Scholar]
- 31.Spillane AJ, Thomas JM, Fisher C. Epithelioid sarcoma: The clinicopathological complexities of this rare soft tissue sarcoma. Ann Surg Oncol. 2000;7:218–225. doi: 10.1007/BF02523657. [DOI] [PubMed] [Google Scholar]
- 32.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher C, Van Der Berg E, Molenaar W. Pleomorphic malignant fibrous histiocytoma/undifferentiated high grade pleomorphic sarcoma. In: Fletcher CD, Unni K, Mertens K, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Washington: IARC Press; 2002. pp. 120–122. [Google Scholar]
- 34.Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: An analysis of 200 cases. Cancer. 1978;41:2250–2266. doi: 10.1002/1097-0142(197806)41:6<2250::aid-cncr2820410626>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.Horvath L, Henshall S. The application of tissue microarrays to cancer research. Pathology. 2001;33:125–129. doi: 10.1080/003130201200338791. [DOI] [PubMed] [Google Scholar]
- 36.Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens—evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol. 1994;7:82–90. [PubMed] [Google Scholar]
- 37.Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: An immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934–942. doi: 10.1016/s0046-8177(99)90247-2. [DOI] [PubMed] [Google Scholar]
- 38.Tardío JC. CD34-reactive tumors of the skin. An updated review of an ever-growing list of lesions. J Cutan Pathol. 2009;36:89–102. doi: 10.1111/j.1600-0560.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 39.Kato H, Hatori M, Watanabe M, et al. Epithelioid sarcomas with elevated serum CA125: Report of two cases. Jpn J Clin Oncol. 2003;33:141–144. doi: 10.1093/jjco/hyg030. [DOI] [PubMed] [Google Scholar]
- 40.Kikuchi E, Kinoshita I, Yamazaki K, et al. Epithelioid sarcoma presenting as pulmonary cysts with cancer antigen 125 expression. Respirology. 2006;11:826–829. doi: 10.1111/j.1440-1843.2006.00925.x. [DOI] [PubMed] [Google Scholar]