Abstract
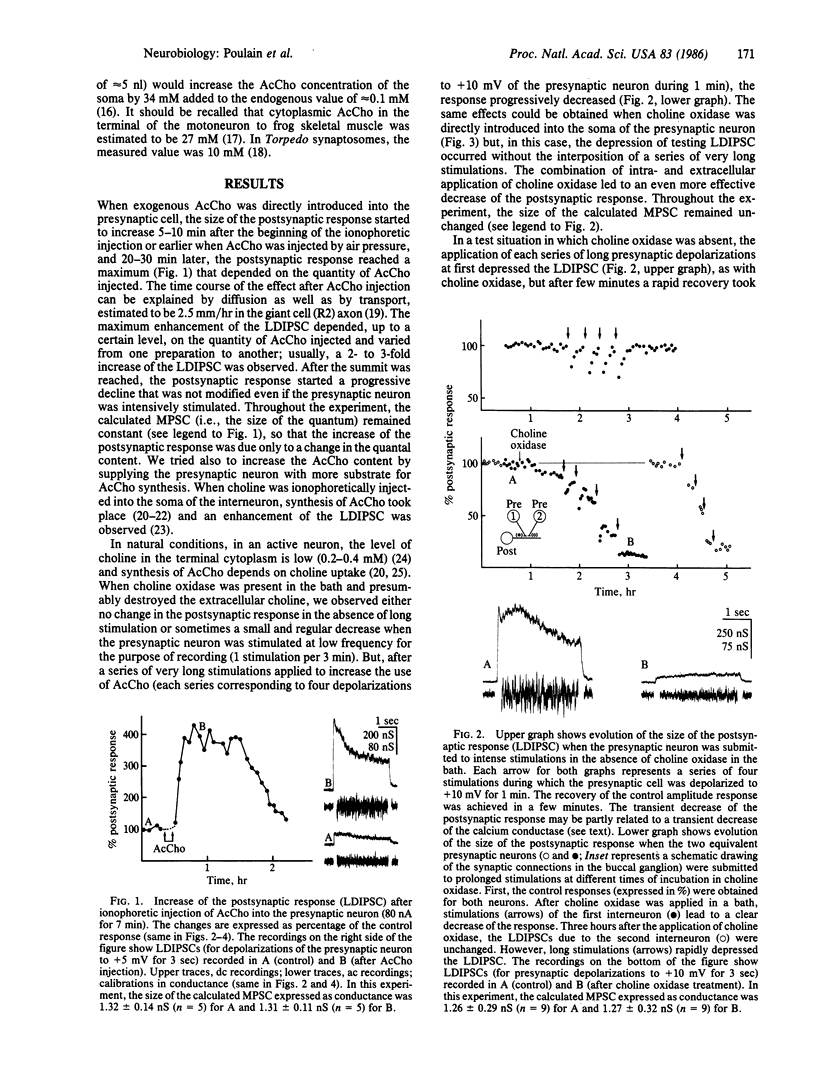
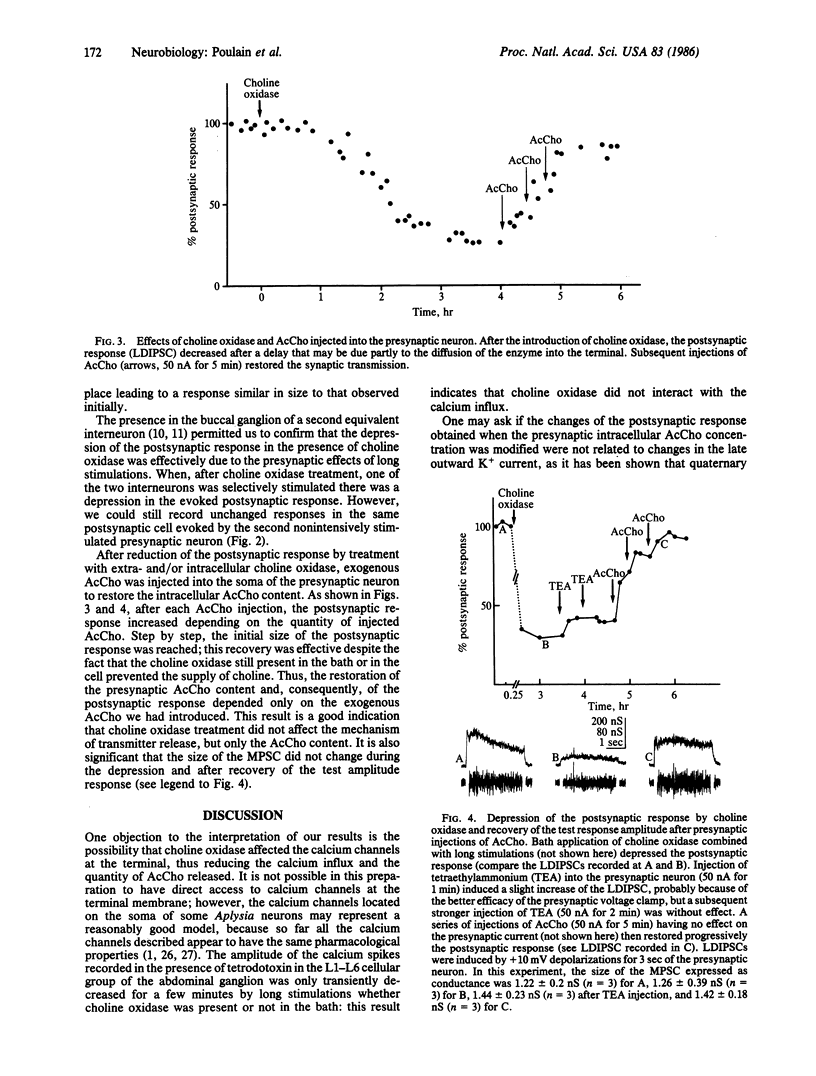
In the buccal ganglion of Aplysia the overloading of the cholinergic presynaptic neuron by exogenous acetylcholine (AcCho) led to an enhancement of the postsynaptic response. The deprivation of choline in the presynaptic neuron by extra- and/or intracellularly applied choline oxidase to prevent AcCho synthesis resulted in a decrease of the postsynaptic response. In both cases, the size of the calculated miniature postsynaptic current (i.e., the size of the quantum) remained unchanged. It was concluded that, for a given stimulation, the number of quanta released (i.e., the quantal content) is directly related to the quantity of AcCho available for release in the presynaptic neuron.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baux G., Tauc L. Carbachol can be released at a cholinergic ganglionic synapse as a false transmitter. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5126–5128. doi: 10.1073/pnas.80.16.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the storage of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):657–668. doi: 10.1113/jphysiol.1972.sp009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I. The relationship of transmitter release and storage to fine structure in a sympathetic ganglion. J Neurocytol. 1974 Jun;3(2):133–160. doi: 10.1007/BF01098386. [DOI] [PubMed] [Google Scholar]
- Collier B., Katz H. S. The synthesis, turnover and release of surplus acetylcholine in a sympathetic ganglion. J Physiol. 1971 May;214(3):537–552. doi: 10.1113/jphysiol.1971.sp009447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt M., Goldman J. E., Kandel E. R., Koike H., Koester J., Schwartz J. H. Intrasomatic injection of radioactive precursors for studying transmitter synthesis in identified neurons of Aplysia californica. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3371–3375. doi: 10.1073/pnas.70.12.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science. 1971 Aug 6;173(3996):550–553. doi: 10.1126/science.173.3996.550. [DOI] [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. Some effects of nerve stimulation andhemicholinium on quantal transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Mar;207(1):51–61. doi: 10.1113/jphysiol.1970.sp009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Koike H., Dandel E. R., Schwartz J. H. Synaptic release of radioactivity after intrasomatic injection of choline-3H into an identified cholinergic interneuron in abdominal ganglion of Aplysia californica. J Neurophysiol. 1974 Jul;37(4):815–827. doi: 10.1152/jn.1974.37.4.815. [DOI] [PubMed] [Google Scholar]
- Koike H., Nagata Y. Intra-axonal diffusion of [3H]acetylcholine and [3H]gamma-aminobutyric acid in a neurone of Aplysia. J Physiol. 1979 Oct;295:397–417. doi: 10.1113/jphysiol.1979.sp012976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaman R. E., Weinreich D., Borys H. Endogenous levels of acetylcholine and choline in individual neurons of Aplysia. J Neurochem. 1973 Aug;21(2):473–476. doi: 10.1111/j.1471-4159.1973.tb04267.x. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. Electrophysiological evidence for the second store of ACh in preganglionic nerve terminals. Brain Res. 1975 Nov 14;98(2):373–376. doi: 10.1016/0006-8993(75)90017-7. [DOI] [PubMed] [Google Scholar]
- Morel N., Israel M., Manaranche R. Determination of ACh concentration in torpedo synaptosomes. J Neurochem. 1978 Jun;30(6):1553–1557. doi: 10.1111/j.1471-4159.1978.tb10492.x. [DOI] [PubMed] [Google Scholar]
- Ono J. K., McCaman R. E. Measurement of endogenous transmitter levels after intracellular recording. Brain Res. 1979 Apr 6;165(1):156–160. doi: 10.1016/0006-8993(79)90055-6. [DOI] [PubMed] [Google Scholar]
- Potter L. T. Synthesis, storage and release of [14C]acetylcholine in isolated rat diaphragm muscles. J Physiol. 1970 Jan;206(1):145–166. doi: 10.1113/jphysiol.1970.sp009003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal mechanism of transmitter release during progressive depletion of the presynaptic stores at a ganglionic synapse. The action of hemicholinium-3 and thiamine deprivation. J Gen Physiol. 1973 Mar;61(3):342–360. doi: 10.1085/jgp.61.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Eisenstadt M. L., Cedar H. Metabolism of acetylcholine in the nervous system of Aplysia californica. I. Source of choline and its uptake by intact nervous tissue. J Gen Physiol. 1975 Mar;65(3):255–273. doi: 10.1085/jgp.65.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau M., Tauc L., Baux G. Quantal release of acetylcholine examined by current fluctuation analysis at an identified neuro-neuronal synapse of Aplysia. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1661–1665. doi: 10.1073/pnas.77.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnakre J. Action de l'hémicholinium sur une synapse centrale d'aplysie. J Physiol (Paris) 1970;62 (Suppl 3):452–453. [PubMed] [Google Scholar]
- Stinnakre J., Tauc L. Calcium influx in active Aplysia neurones detected by injected aequorin. Nat New Biol. 1973 Mar 28;242(117):113–115. doi: 10.1038/newbio242113b0. [DOI] [PubMed] [Google Scholar]
- Tauc L., Baux G. Are there intracellular acetylcholine receptors in the cholinergic synaptic nerve terminals? J Physiol (Paris) 1982;78(4):366–372. [PubMed] [Google Scholar]
- Tauc L., Hoffmann A., Tsuji S., Hinzen D. H., Faille L. Transmission abolished on a cholinergic synapse after injection of acetylcholinesterase into the presynaptic neurone. Nature. 1974 Aug 9;250(5466):496–498. doi: 10.1038/250496a0. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Schwartz J. H. Metabolism of acetylcholine in the nervous system of Aplysia californica. IV. Studies of an identified cholinergic axon. J Gen Physiol. 1977 Jun;69(6):725–741. doi: 10.1085/jgp.69.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucek S. Problems in the organization and control of acetylcholine synthesis in brain neurons. Prog Biophys Mol Biol. 1984;44(1):1–46. doi: 10.1016/0079-6107(84)90011-7. [DOI] [PubMed] [Google Scholar]


