A binary variable is introduced based on the duration of anemia, that is, a hemoglobin level <10 g/dL, during chemotherapy in advanced epithelial ovarian cancer patients for use as a potential prognostic factor for progression-free and overall survival.
Keywords: Ovarian cancer, Anemia, Prognostic factor, Survival, Chemotherapy
Learning Objectives
After completing this course, the reader will be able to:
Compare the prognostic factors for hemoglobin level in ovarian cancer patients.
Explain the prognostic relationship between the duration of anemia during chemotherapy and survival in patients with advanced epithelial ovarian cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Objective.
To propose a measure of anemia to be used as a prognostic factor for progression-free survival and overall survival in advanced epithelial ovarian cancer patients.
Patients and Methods.
Seventy-six patients with International Federation of Gynecology and Obstetrics stage III and stage IV epithelial ovarian cancer who had received at least six courses of platinum- and taxane-based systemic chemotherapy and achieved clinical or pathologic complete response were included. A novel prognostic factor based on the duration of anemia was proposed and the impact of anemia on progression-free and overall survival times was analyzed by a log-rank test and a Cox proportional hazards model.
Results.
We introduce a binary variable, Hb1020, that takes a value of 1 if the duration of a hemoglobin (Hb) level <10 g/dL is ≥20% of the total duration of chemotherapy. We propose Hb1020 as a potential prognostic factor for epithelial ovarian cancer. The 5-year progression-free survival rates were 48.4% in the Hb1020 = 0 group (duration of Hb <10 g/dL <20% of total duration) and 17.7% in the Hb1020 = 1 group (p = .026). The 5-year overall survival rates were 64.6% and 45.0%, respectively (p = .015).
Conclusions.
Hb1020, based on the duration of anemia, is a potential prognostic factor for epithelial ovarian cancer. Using Hb1020, we will be able to administer highly optimized treatment for anemia to improve patient survival. Further independent studies are needed to confirm its prognostic role.
Introduction
Since the mid-1990s, cytoreduction to <1 cm of residual tumor nodules and systemic combination chemotherapy with a platinum compound and a taxane have become the standard of treatment for epithelial ovarian cancer [1]. Although this combined therapy initially seems effective, ovarian cancer is the most common cause of death and is responsible for 6% of all cancer deaths in females [2]. Until now, stage at diagnosis, maximum residual disease following cytoreductive surgery, and performance status were the three major prognostic factors for epithelial ovarian cancer [3–5].
Anemia is a common finding in cancer patients and >30% of patients with cancer suffer from anemia [6]. Multiple factors may contribute to the development of anemia in cancer patients. Most commonly, anemia in patients with cancer is a consequence of cancer therapy, whether chemotherapy or radiotherapy. The mechanisms that cause anemia include myelosuppression, inflammatory cytokines, extracorpuscular hemolysis, catabolism with tumor burden, and deficiency of erythropoietin [7, 8].
Recently, cumulative data have indicated that anemia is associated with a poor clinical prognosis [9–11]. Also, in ovarian cancer there are several reports available regarding the prognostic impact of hemoglobin (Hb) levels before and throughout chemotherapy [11–18].
However, most of the reported data have focused on the pretreatment Hb level or a single precycle Hb level and do not reflect the cumulative effect of anemia during chemotherapy. Therefore, some reports showed partially contradictory results or statistically insignificant findings [13, 14, 16–18].
In this study, we assessed the prognostic relationship between the duration of anemia during chemotherapy and survival in patients with advanced epithelial ovarian cancer by analyzing all Hb levels checked during treatment.
Materials and Methods
Patients
This study retrospectively evaluated the medical records of all patients with histologically confirmed invasive epithelial ovarian cancer who were treated between March 2000 and December 2009 at Seoul St. Mary's Hospital in Seoul, Korea. Patients with primary tubal cancer or primary peritoneal cancer were excluded. We obtained all information by chart review. Institutional review board approval was obtained for medical record reviews.
Only International Federation of Gynecology and Obstetrics (FIGO) stage III and stage IV patients who were scheduled to receive at least six courses of systemic platinum and taxane chemotherapy—cisplatin (75 mg/m2) or carboplatin (area under the concentration versus time curve, 5) and paclitaxel (175 mg/m2) or docetaxel (75 mg/m2) on day 1 every 22 days—and achieved clinical or pathologic complete remission were included. Patients who received other regimens were excluded, as were patients with other nonmalignancy-related anemia (e.g., sickle cell anemia, thalassemia, chronic iron deficiency, and hematologic malignancy). Clinical prognostic factors assessed included patient age, tumor histology, grade, para-aortic node metastasis, optimal cytoreduction, and hematologic parameters during treatment.
Iron supplements, transfusion, and recombinant human erythropoietin administration to correct the anemia were allowed following individual treatment decisions and had to be within the protocol.
Measurement of Hb Level and Duration of Anemia
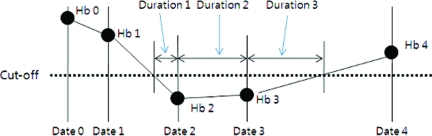
In general, we routinely measure the Hb level prior to the initiation of the corresponding chemotherapy and at least one time between sequential cycles. The duration of anemia was defined as the duration of a Hb level sustained under a specific level (cutoff) in each patient, which was calculated as follows (Fig. 1):
Figure 1.
Calculation of the duration under a specific Hb level (cutoff). Duration1 = (Date2 − Date1)*(cutoff − Hb2)/(Hb1 − Hb2). Duration2 = (Date3 − Date2). Duration3 = (Date4 − Date3) × (cutoff − Hb3)/(Hb4 − Hb3). Total duration under a specific value is obtained by adding up all the durations from the first chemotherapy cycle date to the sixth chemotherapy cycle date.
(a) If two consecutive Hb levels are higher than the cutoff level, then skip.
(b) If the first Hb level is higher (or lower) than and the second Hb value is lower (or higher) than the cutoff level, use the interpolation method. For example, duration 1 = (date2 − date1) × (cutoff − Hb2)/(Hb1 − Hb2), duration3 = (date4 − date3) × (cutoff − Hb3)/(Hb4 − Hb3).
(c) If two consecutive Hb levels are lower than the cutoff level, then calculate the duration of consecutive dates. For example, duration2 = (date3 − date2).
The total duration under a specific Hb level is obtained by adding all the durations from the first chemotherapy cycle date to the sixth chemotherapy cycle date.
Because the total duration of treatment for each patient was different, we used the ratio of the duration under a specific Hb level, which was defined as the ratio of the duration of anemia and the total duration of treatment.
Statistical Analysis
The pretreatment Hb level and the Hb levels prior to the six chemotherapy cycles are presented as mean and standard deviation (SD) values for the recurrent/nonrecurrent and dead/alive patient groups and were compared using a two-sample t-test. Descriptive statistics for the duration and ratio of anemia under a specific Hb level are also presented.
To determine the cutoff value of anemia that could optimally discriminate the recurrent/nonrecurrent and dead/alive patients, we repeatedly analyzed the progression-free and overall survival times for the ratios (from 0% to the maximum percent by 5%) of the durations at four levels (12 g/dL, 11 g/dL, 10 g/dL, and 9 g/dL Hb).
The Kaplan–Meier method, log-rank test, and Cox proportional hazards model were used to estimate and compare the effects of the clinical parameters. All reported p-values are based on two-sided tests and p < .05 was regarded as significant. SAS for Windows, release 9.1 (SAS Institute Inc., Cary, NC) was used for all statistical analyses.
Results
Clinical Characteristics
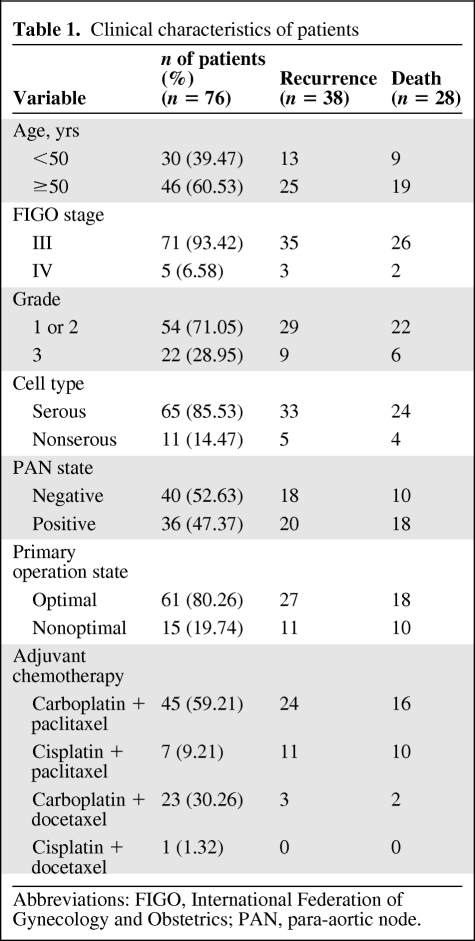
Seventy-six patients qualified for inclusion in this study. The mean age of the patients at diagnosis was 52.8 years (median, 52.0 years; SD, 10.3 years). Of the 76 patients, 71 (93.42%) were classified as FIGO stage III and five (6.58%) were classified as FIGO stage IV. Tumors were classified as serous in 65 patients (85.53%) and as nonserous in 11 patients (14.47%). Grade 1 and grade 2 tumors were reported in 54 patients (71.05%) and grade 3 tumors were reported in 22 patients (28.95%). Para-aortic node metastasis occurred in 36 patients (47.37%) and optimal cytoreduction was achieved in 61 patients (80.26%) (Table 1).
Table 1.
Clinical characteristics of patients
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; PAN, para-aortic node.
Hb Level at Baseline and Prior to Each Cycle and Survival
The mean number of Hb measurements for each patient was 23.4 ± 7.0 during chemotherapy.
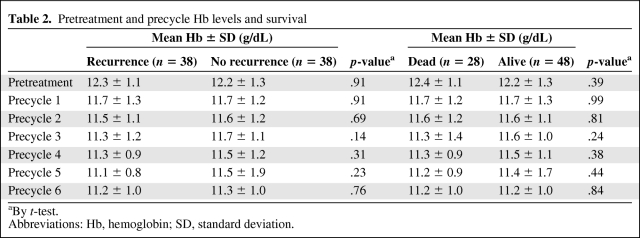
Table 2 shows the pretreatment Hb levels and the Hb levels prior to each course of chemotherapy. The mean Hb levels decreased as the number of chemotherapy cycles increased; however, there was no significant difference in Hb level between the recurrence and nonrecurrence groups, and between the deceased and survivor groups.
Table 2.
Pretreatment and precycle Hb levels and survival
aBy t-test.
Abbreviations: Hb, hemoglobin; SD, standard deviation.
Duration and Ratio of Anemia and Optimal Cutoff
The mean duration of total treatment was 131.2 ± 17.2 days (median, 127 days; minimum, 115 days; maximum, 227 days) and the mean durations of anemia were 99.6 days, 53.6 days, 15.0 days, and 3.1 days at Hb levels of 12 g/dL, 11 g/dL, 10 g/dL, and 9 g/dL, respectively. The mean ratios of the duration of anemia were 76%, 41%, 11%, and 2% at Hb levels of 12 g/dL, 11 g/dL, 10 g/dL, and 9 g/dL, respectively.
After repeatedly applying the ratio of 0% to each maximum percentage by 5% at four levels (12 g/dL, 11 g/dL, 10 g/dL, and 9 g/dL of Hb), at the 12 g/dL and 9 g/dL levels there was no significant ratio of duration that showed differences for both the progression-free survival and overall survival rates. However, 80% of the ratio of the duration at 11 g/dL and 20% of the ratio at 10 g/dL showed a significant difference in both the progression-free survival and overall survival rates, respectively.
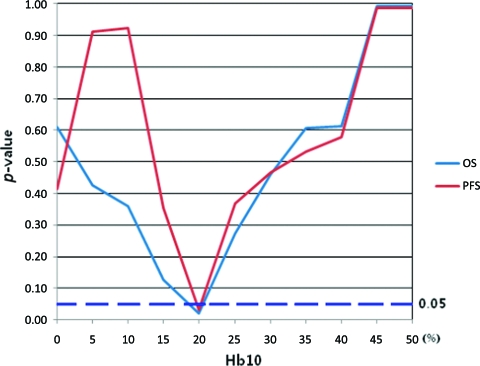
Although we could have accepted both 80% of the ratio at 11 g/dL and 20% of the ratio at 10 g/dL as competing cutoffs for anemia, 80% of the total duration (average, 127 days) equaled nearly 100 days and was thus regarded as not useful from a practical viewpoint. On the other hand, 20% of the total duration was approximately 25 days, which could be useful for the early detection of anemia. Figure 2 shows the change in p-value for the various ratios of duration of an Hb level of 10 g/dL.
Figure 2.
Change in p-value according to various ratios of duration of anemia when the cutoff hemoglobin (Hb) level is 10 g/dL (Hb10). When the ratio is 20% (Hb1020), both the progression-free survival (PFS) and overall survival (OS) rates are significantly different between the Hb1020 = 0 group and Hb1020 = 1 group (p < .05).
We used a term called “Hb1020” that takes a value of 1 if the duration of the Hb level <10 g/dL is ≥20% of the total duration of chemotherapy as the cutoff. So Hb1020 = 1 group includes patients whose duration of Hb level <10 g/dL is at least 20% of the total duration of chemotherapy and the Hb1020 = 0 group includes patients whose duration of Hb level <10 g/dL is <20% of the total duration of chemotherapy.
Prognostic Results for the Duration of Anemia
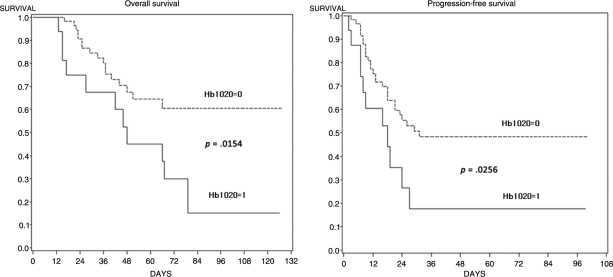
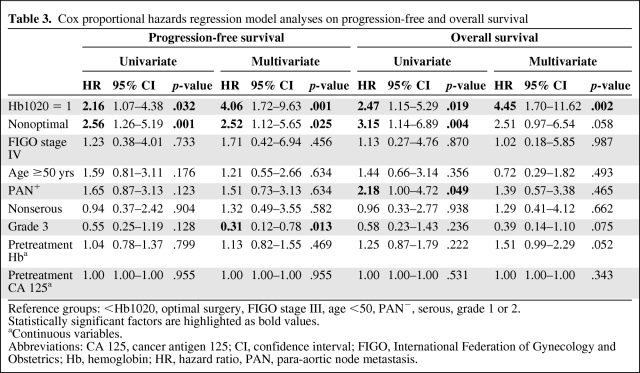
Figure 3 shows the progression-free and overall survival rates according to Hb1020 status. The 5-year progression-free survival rates were 48.4% in the Hb1020 = 0 group and 17.7% in the Hb1020 = 1 group (p = .026). The 5-year overall survival rates were 64.6% and 45.0% in the two groups, respectively (p =.015). Using a Cox proportional hazards model, Hb1020 was an independently significant prognostic factor for both progression-free survival (HR, 4.06; 95% CI, 1.72–9.63; p = .001) and overall survival (hazard ratio, 4.45; 95% confidence interval, 1.70–11.62; p = .002) (Table 3).
Figure 3.
Progression-free and overall survival according to Hb1020.
Table 3.
Cox proportional hazards regression model analyses on progression-free and overall survival
Reference groups: <Hb1020, optimal surgery, FIGO stage III, age <50, PAN−, serous, grade 1 or 2.
Statistically significant factors are highlighted as bold values.
aContinuous variables.
Abbreviations: CA 125, cancer antigen 125; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; Hb, hemoglobin; HR, hazard ratio, PAN, para-aortic node metastasis.
Discussion
According to the World Health Organization/National Cancer Institute, normal values for Hb are 12–16 g/dL in women and anemia is diagnosed when the Hb level is <12 g/dL [19]. Anemia occurs in >30% of patients with epithelial ovarian cancer before surgery [12]. In our study, anemia was present in 34.21% of patients (26 of 76) with advanced epithelial ovarian cancer at the time of initial diagnosis.
Anemia may be caused by bleeding, nutritional deficiencies, bone marrow damage, tumor infiltration of the bone marrow, and the malignant process itself. Furthermore, cancers are often associated with inflammatory cytokines, such as tumor necrosis factor-α and interleukin-1, which are both associated with direct inhibition of the proliferation of erythrocytic progenitors [12, 20]. Activation of the immune system appears to be the basis for the impairment of effective erythropoiesis. The shorter survival time of normal RBCs infused in anemic cancer patients was the first evidence of an unfavorable internal environment in this condition [21]. In addition, it must be emphasized that cancer cells themselves are also involved directly and indirectly in the suppression of erythropoiesis [22]. Anemia may be a common consequence of cancer therapy, whether it is chemotherapy or radiotherapy [20].
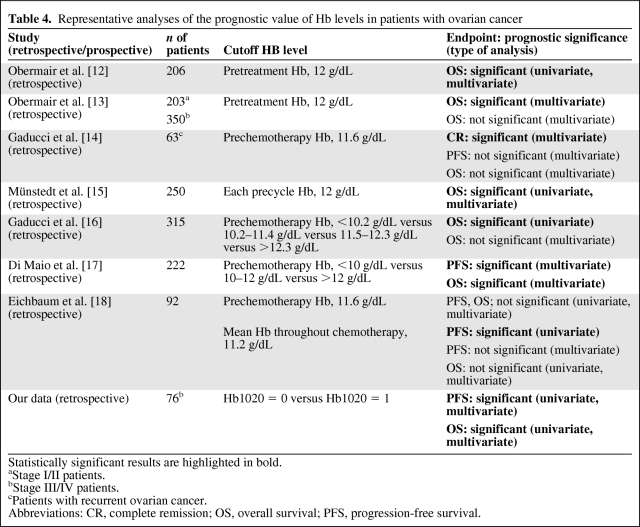
Previously reported studies on the prognostic impact of anemia for survival are shown in Table 4. Because all reported data focused on the pretreatment Hb level or a single precycle Hb level, which does not reflect the cumulative effect of anemia during chemotherapy, there are limited, partially contradictory, results in the literature [13, 14, 16–18] (Table 4). If a patient undergoes transfusion before sampling, a single Hb level does not represent the real status of the patient around sampling time. Other factors for consideration when evaluating the risk for chemotherapy-induced anemia include the nadir Hb level, the time to the nadir Hb level (estimated to be 2 weeks, but the length of time can vary), and whether an Hb measurement is pre- or postnadir [23]. Taken together, we determined the specific cutoff value Hb1020 by statistical analysis.
Table 4.
Representative analyses of the prognostic value of Hb levels in patients with ovarian cancer
Statistically significant results are highlighted in bold.
aStage I/II patients.
bStage III/IV patients.
cPatients with recurrent ovarian cancer.
Abbreviations: CR, complete remission; OS, overall survival; PFS, progression-free survival.
The novel cutoff value Hb1020 has some merits over existing cutoffs (i.e., pretreatment Hb, 12 g/dL; prechemotherapy Hb, 11.6 g/dL). First, although our study group was considerably homogeneous, the mean Hb level did not influence survival. Second, in the clinical setting, it is difficult to maintain an Hb level >12 g/dL during chemotherapy because many patients refuse blood transfusion [15]. It is, however, relatively easy to maintain a target Hb level >10 g/dL without the need for transfusion. Third, physicians can determine the critical time to treat anemia during chemotherapy using a cutoff level of Hb1020. For example, if a patient's duration of anemia under an Hb level of 10 g/dL is >26 days during chemotherapy, the anemia must be managed actively (mean duration of treatment, 131 days).
The reasons for the correlation of anemia and survival remain unclear. Patients with low serum Hb levels may have a larger number of biologically aggressive tumor cell clones than patients with higher Hb levels [12]. Recently, it has become increasingly clear that hypoxia plays a crucial role in the progression of cancer. Tumor hypoxia is not necessarily synonymous with tissue hypoxia, but anemia has been associated with impaired tumor oxygenation and numerous recent retrospective studies have documented an adverse relationship between Hb level and response to therapy in cervical, head and neck, and breast cancer patients [9, 24, 25]. Furthermore, fatigue is a common problem in cancer patients. Anemia is the most common reversible cause of cancer-related fatigue, particularly among patients receiving chemotherapy [26, 27]. The optimal management of symptomatic anemia requires an accurate diagnosis to identify potentially remediable causes. If a potentially treatable cause cannot be identified, treatment options include RBC transfusion and an erythropoietin-stimulating agent [26].
We propose the novel variable Hb1020 as a potential prognostic factor for epithelial ovarian cancer. Using Hb1020, we will be able to administer highly optimized treatment for anemia to improve patient survival. However, Hb1020 given by this retrospective analysis only allows for the development of a hypothesis. It still needs to be explored further in an independent dataset to confirm its prognostic role in patients with epithelial ovarian cancer during platinum- and taxane-based chemotherapy.
Author Contributions
Conception/Design: Joon-Mo Lee, Soo Young Hur, Sung Ha Lee, Jin Hwi Kim, Keun Ho Lee
Provision of study material or patients: Yong Seok Lee, Ki Sung Ryu, Jin Hwi Kim
Collection and/or assembly of data: Jin Hwi Kim
Data analysis and interpretation: Yong Gyu Park, Jin Hwi Kim
Manuscript writing: Yong Gyu Park, Jin Hwi Kim
Final approval of manuscript: Joon-Mo Lee
References
- 1.Kyrgiou M, Salanti G, Pavlidis N, et al. Survival benefits with diverse chemotherapy regimens for ovarian cancer: Meta-analysis of multiple treatments. J Natl Cancer Inst. 2006;98:1655–1663. doi: 10.1093/jnci/djj443. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Lund B, Williamson P. Prognostic factors for overall survival in patients with advanced ovarian carcinoma. Ann Oncol. 1991;2:281–287. doi: 10.1093/oxfordjournals.annonc.a057937. [DOI] [PubMed] [Google Scholar]
- 4.Gershenson DM, Copeland LJ, Wharton JT, et al. Prognosis of surgically determined complete responders in advanced ovarian cancer. Cancer. 1985;55:1129–1135. doi: 10.1002/1097-0142(19850301)55:5<1129::aid-cncr2820550531>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Holschneider CH, Berek JS. Ovarian cancer: Epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::aid-ssu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Tesarovà P, Kvasnicka J. [Treatment of anemia in patients with tumors.] Cas Lek Cesk. 1995;134:647–650. In Czech. [PubMed] [Google Scholar]
- 7.Macciò A, Madeddu C, Massa D, et al. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: Role of inflammation in cancer-related anemia. Blood. 2005;106:362–367. doi: 10.1182/blood-2005-01-0160. [DOI] [PubMed] [Google Scholar]
- 8.Mercadante S, Gebbia V, Marrazzo A, et al. Anaemia in cancer: Pathophysiology and treatment. Cancer Treat Rev. 2000;26:303–311. doi: 10.1053/ctrv.2000.0181. [DOI] [PubMed] [Google Scholar]
- 9.Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 10.Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol. 2002;13:965–973. doi: 10.1093/annonc/mdf122. [DOI] [PubMed] [Google Scholar]
- 11.Van Belle SJ, Cocquyt V. Impact of haemoglobin levels on the outcome of cancers treated with chemotherapy. Crit Rev Oncol Hematol. 2003;47:1–11. doi: 10.1016/s1040-8428(03)00093-3. [DOI] [PubMed] [Google Scholar]
- 12.Obermair A, Handisurya A, Kaider A, et al. The relationship of pretreatment serum hemoglobin level to the survival of epithelial ovarian carcinoma patients: A prospective review. Cancer. 1998;83:726–731. [PubMed] [Google Scholar]
- 13.Obermair A, Petru E, Windbichler G, et al. Significance of pretreatment serum hemoglobin and survival in epithelial ovarian cancer. Oncol Rep. 2000;7:639–644. doi: 10.3892/or.7.3.639. [DOI] [PubMed] [Google Scholar]
- 14.Gadducci A, Cosio S, Fanucchi A, et al. Is pretreatment hemoglobin level a predictor of complete response to salvage chemotherapy for recurrent platinum-pretreated ovarian carcinoma? Eur J Gynaecol Oncol. 2003;24:405–410. [PubMed] [Google Scholar]
- 15.Münstedt K, Kovacic M, Zygmunt M, et al. Impact of hemoglobin levels before and during chemotherapy on survival of patients with ovarian cancer. Int J Oncol. 2003;23:837–843. [PubMed] [Google Scholar]
- 16.Gadducci A, Sartori E, Landoni F, et al. Pre-chemotherapy hemoglobin levels and survival in patients with advanced epithelial ovarian cancer who received a first-line taxane/platinum-based regimen: Results of a multicenter retrospective Italian study. Gynecol Oncol. 2005;98:118–123. doi: 10.1016/j.ygyno.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Di Maio M, Pisano C, Tambaro R, et al. The prognostic role of pre-chemotherapy hemoglobin level in patients with ovarian cancer. Front Biosci. 2006;11:1585–1590. doi: 10.2741/1906. [DOI] [PubMed] [Google Scholar]
- 18.Eichbaum MH, Weiss LM, Bruckner T, et al. Prognostic impact of hemoglobin levels before and during carboplatin/taxane-based chemotherapy in patients with primary invasive epithelial ovarian cancer. Med Sci Monit. 2009;15:CR156–CR163. [PubMed] [Google Scholar]
- 19.Rodgers GM, 3rd, Becker PS, Bennett CL, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw. 2008;6:536–564. doi: 10.6004/jnccn.2008.0042. [DOI] [PubMed] [Google Scholar]
- 20.Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys. 2005;63:25–36. doi: 10.1016/j.ijrobp.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Hyman GA. Anemia in malignant neoplastic disease. J Chronic Dis. 1963;16:645–666. doi: 10.1016/0021-9681(63)90003-1. [DOI] [PubMed] [Google Scholar]
- 22.Spivak JL. The anaemia of cancer: Death by a thousand cuts. Nat Rev Cancer. 2005;5:543–555. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- 23.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 24.Becker A, Stadler P, Lavey RS, et al. Severe anemia is associated with poor tumor oxygenation in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2000;46:459–466. doi: 10.1016/s0360-3016(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 25.Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: Pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001;28(suppl 8):29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cancer- and Chemotherapy-Induced Anemia, Version 2.2011. [accessed February 17, 2011]. Available at http://www.nccn.org.
- 27.Dy SM, Lorenz KA, Naeim A, et al. Evidence-based recommendations for cancer fatigue, anorexia, depression, and dyspnea. J Clin Oncol. 2008;26:3886–3895. doi: 10.1200/JCO.2007.15.9525. [DOI] [PubMed] [Google Scholar]