Results of a double-blind, randomized, phase III clinical trial evaluating time to progression and overall survival in women with metastatic breast cancer who received sialyl-TN keyhole limpet hemocyanin vaccine are reported.
Keywords: Metastatic breast cancer, MUC-1 antibody, Antiestrogen, Vaccine, Chemotherapy
Abstract
Purpose.
This double-blind, randomized, phase III clinical trial evaluated time to progression (TTP) and overall survival in women with metastatic breast cancer (MBC) who received sialyl-TN (STn) keyhole limpet hemocyanin (KLH) vaccine. Secondary endpoints included vaccine safety and immune response.
Experimental design.
The study population consisted of 1,028 women with MBC across 126 centers who had previously received chemotherapy and had had either a complete or a partial response or no disease progression. All women received one-time i.v. cyclophosphamide (300 mg/m2) 3 days before s.c. injection of 100 μg STn-KLH plus adjuvant (treatment group) or 100 μg KLH plus adjuvant (control group) at weeks 0, 2, 5, and 9. Subsequently, STn-KLH without adjuvant or KLH without adjuvant was then administered monthly for 4 months, and then quarterly until disease progression, without cyclophosphamide.
Results.
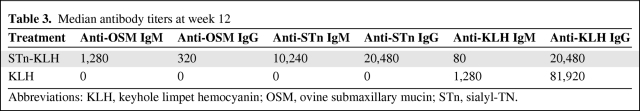
STn-KLH vaccine was well tolerated; patients had mild to moderate injection-site reactions and reversible flu-like symptoms. Week-12 antibody testing revealed high specific IgG titers and a high rate of IgM-to-IgG seroconversion; the median IgG titers in STn-KLH recipients were 320 (anti-ovine submaxillary mucin) and 20,480 (anti-STn), with no detectable antimucin antibodies in the control group. The TTP was 3.4 months in the treatment group and 3.0 months in the control group. The median survival times were 23.1 months and 22.3 months, respectively.
Conclusions.
Although STn-KLH was well tolerated in this largest to date metastatic breast cancer vaccine trial, no overall benefit in TTP or survival was observed. Lessons were learned for future vaccine study designs.
Introduction
Breast cancer is the most common type of cancer in women, with 1.05 million new cases diagnosed annually [1]. It was estimated that, in 2009, in the U.S., 192,370 women would be diagnosed with breast cancer and 40,170 women would die as a result of this disease; in Europe, the corresponding estimates for 2006 were 429,900 women diagnosed and 131,900 deaths [2, 3]. The 5-year survival rate for women with distant metastasis from breast cancer is only 26% in the U.S. and <20% in Europe [2, 4].
Therapeutic cancer vaccines are increasingly being studied in the treatment of breast cancer; they may induce relevant humoral and/or cell-mediated immunity to tumor cells. These cancer vaccines can consist of whole tumor cells/lysates or defined tumor antigens [5, 6]. One potentially important tumor-associated antigen is sialyl-Tn (STn), a carbohydrate epitope found on a variety of glycoproteins, including cancer-associated mucins [7, 8]. STn expression is associated with a poor prognosis in metastatic colorectal, gastric, ovarian, and breast cancer patients [9–13]. A synthetic STn antigen was generated for use as a therapeutic cancer vaccine antigen, and tests in animal models and human studies showed the antigen to be safe and to produce a strong immune response [8, 14–17]. STn-keyhole limpet hemocyanin (KLH) (Theratope®; Biomira, Inc., Edmonton, Canada), a synthetic STn conjugated to the KLH carrier protein, has been used in conjunction with Enhanzyn™ (previously called Detox B stable emulsion; Corixa Corp., Hamilton, MA) adjuvant therapy (hereafter referred to as “adjuvant”) [15]. The immune response to administered antigens may be augmented using “immunomodulatory” doses of a variety of cytotoxic agents, such as cyclophosphamide, which have been posited to exert their effect by inhibiting putative suppressor T regulatory cells (Treg) or by increasing the humoral response to STn-KLH vaccine [17–19].
The efficacy of and clinical outcomes associated with cyclophosphamide and STn-KLH treatment in patients with metastatic breast cancer (MBC) have been measured in several studies by examining patients' antimucin antibodies and T-cell responses and correlating these immune responses with overall survival [8, 16, 20, 21]. In addition, a phase II multicenter trial of STn-KLH plus adjuvant demonstrated STn-KLH's safety and immunogenicity [22]. The primary goals of this phase III trial were to evaluate the effects of STn-KLH vaccine compared with a control vaccine on TTP and overall survival in a large international cohort of women with MBC. Secondary endpoints were vaccine safety, serum antibody response, and self-reported quality of life (QoL).
Materials and Methods
Patient Eligibility, Randomization, and Stratification
This double-blind, randomized study was conducted across 126 centers in 10 countries. The study was approved by the independent ethics committee or institutional review board at each site. Patients provided institutionally approved informed consent before being enrolled in the study.
Eligible patients were aged ≥18 years, had histologically/cytologically proven breast cancer, and had either no evidence of disease (NED) or no progressive disease (NPD) following first-line chemotherapy for MBC. The chemotherapy had to have begun no later than 40 weeks and ended no later than 3 weeks before study entry. Stable disease was required for at least 24 weeks for study entry. Patients were also required to have a neutrophil count ≥1.0 × 109/L, a platelet count ≥75 × 109/L, a hemoglobin level ≥9 g/dL, and an Eastern Cooperative Oncology Group performance status score ≤2. Women with bone metastases as the only metastatic site of disease were eligible for inclusion in the trial.
In total, 1,030 women were enrolled in the trial but two patients experienced progression of disease prior to initiation of treatment and were excluded; therefore, 1,028 patients were randomized to either the STn-KLH (test) or KLH (control) groups following evaluation, receipt of informed consent, and determination of eligibility. The investigators and patients were blinded to test versus control therapy assignments.
Additionally, patients were stratified as NED or NPD according to their disease status following first-line chemotherapy. As a result of slow study accrual, a protocol amendment was implemented that allowed the inclusion of women receiving concomitant hormone therapy to treat their MBC. All but the first 150 patients were enrolled in the study under this amended protocol. To ensure the statistical integrity of the study, two additional strata were added prior to patient randomization: no hormone therapy or concomitant anticancer hormone therapy.
Vaccine Formulation and Clinical Treatment Protocol
Preclinical studies demonstrated that a higher conjugation ratio of STn to KLH led to greater specific antibody titers (internal Biomira results). An optimized formulation of STn-KLH was therefore developed in an attempt to increase the immune response in patients. Prior to the phase III trial, the week-12 antibody response to the optimized vaccine formulation was studied in a phase II trial of 36 MBC patients, confirming the immunogenicity of the new formulation (BR-103 bridging study; data not published). The new formulation is chemically identical to that used in the early phase I/II studies, but has a greater conjugation ratio of STn to KLH of approximately 9%, compared with the conjugation ratio of approximately 5% found in the STn-KLH vaccine used in previous phase I/II studies [15–17, 23].
Patients were evaluated for study eligibility within 14 days prior to receiving a single i.v. dose of 300 mg/m2 cyclophosphamide. Three days following this pretreatment, patients in the KLH group (n = 505) received 100 μg KLH and patients in the STn-KLH group (n = 523) received 100 μg STn-KLH administered s.c. Treatments were given on weeks 0, 2, 5, 9, 13, 17, 21, 25, and 37, and every 12 weeks thereafter. Patients were evaluated for disease status, both clinically and radiologically, by repeat studies on weeks 12, 24, 36, 48, and 60, or as clinically indicated.
For the initial treatment period (weeks 0, 2, 5, and 9), both STn-KLH and KLH were admixed with adjuvant for s.c. injection (with a half dose delivered to each of two body sites: a deltoid muscle of the upper arm and/or the anterolateral region of the upper thigh). The adjuvant was withdrawn in the event of a significantly higher rate of ulceration at the injection sites or ulceration not ameliorated by withholding the adjuvant [15]. The adjuvant was omitted after week 12 to ameliorate potential ulceration at injection sites; thus, vaccine without adjuvant was administered to patients continuing in the subsequent treatment period (weeks 13, 17, 21, and 25, and every 3 months thereafter). Primary safety, tumor, and immune response evaluations were done at week 12; however, data on the sustainability of the antibody response beyond week 12 were not available. Patients were withdrawn from the trial at the first signs of disease progression, as determined by the investigator. Patients were also withdrawn from the study at the discretion of the investigator or at the request of the patient.
Measurement of Primary Endpoints
TTP was defined as the time between the first vaccination and disease progression, patient death, or last patient contact. Overall survival was defined as the time between the first vaccination and patient death or last patient contact. To determine disease progression, patient radiologic images were reviewed by radiologists on the Response Evaluation Committee, who remained blind to treatment assignments. World Health Organization criteria were used to define disease progression: an increase ≥25% in the product of the two largest perpendicular diameters of a bidimensionally measurable lesion; a 25% increase in a single diameter of a unidimensionally measurable lesion; or the appearance of a new lesion upon clinical examination or imaging scan, including computed tomography radiograph, ultrasonography, or plain film radiograph of the bone. A conservative approach was taken for the statistical analysis of disease progression by using the earliest date of progression as determined by the investigator or the Response Evaluation Committee. Disease response was not examined.
Measurement of Secondary Endpoints
The investigators were blinded to the treatment assignments and the safety, QoL, and immunologic testing results. Safety evaluations were conducted by the clinical research team at each site during each vaccination visit. These evaluations consisted of physical examinations (including injection site inspections), standard clinical laboratory tests, and reports of adverse events (AEs). In addition, a data safety monitoring board analyzed the safety evaluations and ensured the statistical robustness of the sample size estimates following enrollment of 300, 600, 800, and 1,000 patients and during the TTP, interim survival, and final survival analyses.
QoL data were collected at baseline (day of first cyclophosphamide injection), week 12, and then every 3 months using the 30-item European Organization for Research and Treatment of Cancer QLQ C30 [24] cancer survey and a 23-item breast cancer-specific module [24]. QoL data gathering was discontinued upon disease progression or patient death.
To determine the patients' immune response, serum titers of antiovine submaxillary mucin (anti-OSM), which contains both clustered and unclustered forms of the STn epitope and is believed to be representative of native STn present on tumor-associated mucin [25], in addition to anti-STn and anti-KLH IgM and IgG antibodies were measured at week 12 using an enzyme-linked immunosorbent assay as previously described [13].
Statistical Analyses
Event-based TTP and interim survival analyses were performed in September 2002, with a final survival analysis in June 2003. The primary TTP analysis occurred following 800 progression events (achieving 87% power to observe a 30% longer TTP), and the interim survival analysis occurred following 488 patient deaths (achieving 67% power). The final survival analysis occurred after at least 650 deaths (to allow an 89% power to observe a 30% longer overall survival time). The α level was adjusted based on the actual number of events at the time of analysis. The Lan–DeMets [26] implementation of the O'Brien–Fleming [27] method of calculating an adjusted α level was used. The p-values adopted were <.0145 and <.0357 for the interim and final analyses, respectively.
For both the TTP and survival time, the differences between the STn-KLH and KLH groups were evaluated following an initial analysis using the Cox proportional hazards regression model; a secondary analysis was performed using an overall log-rank test. The Cox model was conducted using the stratification variables as factors and adjusting for a specific prognostic variable (time from initial diagnosis to first metastasis). The protocol assumptions were that 20% of patients would be categorized in the NED group and 80% would be categorized in the NPD group.
An overall p < .05 was considered statistically significant. This was achieved using a significance level of .01 for the TTP differences and a significance level of .04 for the survival differences between treatment groups.
Descriptive statistics were used for injection site reactions, AEs, QoL, and antimucin or anti-KLH antibody titers. Patients were considered evaluable for the immunologic endpoints if they provided a blood sample following the first four vaccinations (week 12). Treatment effect analyses were also performed by treatment group using the slope of the QoL curve score over time (time-adjusted area under the change-from-baseline curve).
Results
Clinical Study and Patient Characteristics
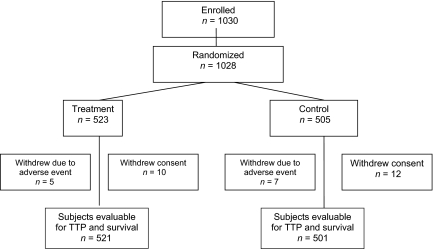
This phase III trial was the largest ever randomized, double-blind vaccine study in women with MBC. The trial began enrollment in November 1998 and the final analysis of all endpoints concluded in June 2003, with follow-up of surviving patients ongoing at the time of this writing. One thousand twenty-eight patients were randomized to the study. Data were missing for six patients. Therefore, 1,022 patients were evaluable for TTP and survival time analyses (Fig. 1). However, during the course of the study, 34 patients were taken off the protocol because of AEs or withdrawal of consent. The data from these patients were considered censored at the time of withdrawal.
Figure 1.
CONSORT diagram.
Abbreviation: TTP, time to progression.
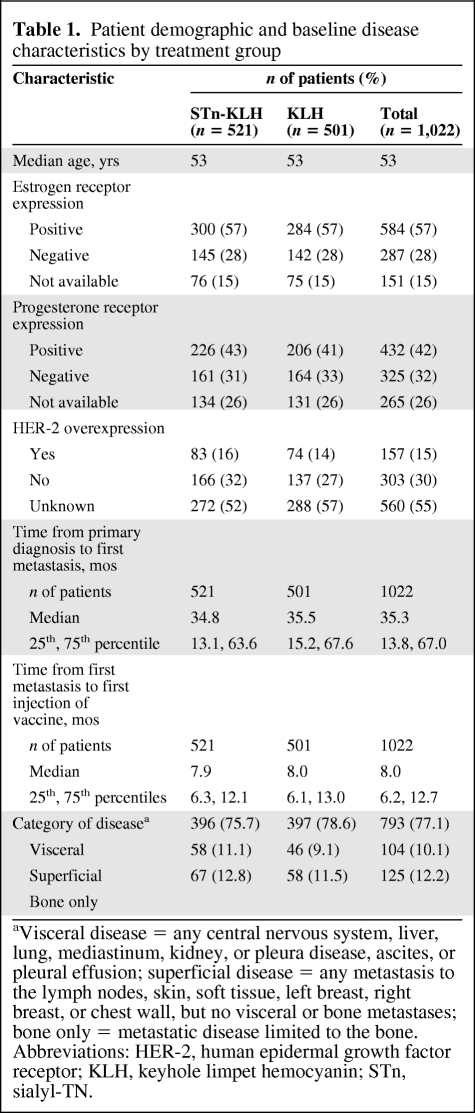
Table 1 summarizes the patient demographic and baseline disease characteristics. There were no significant differences between the STn-KLH and KLH groups. The patients' median age at diagnosis of their primary cancer was 53 years.
Table 1.
Patient demographic and baseline disease characteristics by treatment group
aVisceral disease = any central nervous system, liver, lung, mediastinum, kidney, or pleura disease, ascites, or pleural effusion; superficial disease = any metastasis to the lymph nodes, skin, soft tissue, left breast, right breast, or chest wall, but no visceral or bone metastases; bone only = metastatic disease limited to the bone.
Abbreviations: HER-2, human epidermal growth factor receptor; KLH, keyhole limpet hemocyanin; STn, sialyl-TN.
There were no significant differences in disease characteristics between the treatment groups. The most common sites of metastasis included the bone (61%), liver (42%), and lungs (34%). Most of the patients in both the STn-KLH (75.7%) and KLH (78.6%) groups had visceral disease (supplemental online Table A).
Prior to study entry, most participants (90%) had NPD (56% had a partial response, 23% had stable disease, and 11% had a minor response), whereas 10% had NED following first-line chemotherapy. Following the protocol amendment, 31% of the patients received hormone therapy in addition to the study medication; these patients were stratified equally between the treatment groups.
Primary Endpoints: TTP and Overall Survival
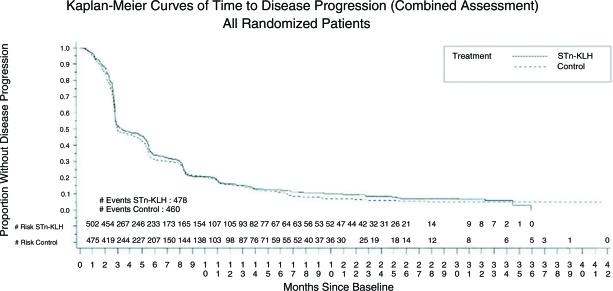
There were no significant differences in the median TTP or overall survival time between the treatment groups. The median TTP was 3.4 months for the STn-KLH group and 3.0 months for the KLH group (Cox proportional hazards model p = .353; log-rank test p = .305) (Fig. 2).
Figure 2.
Kaplan–Meier curves of time to disease progression (combined assessment).
Abbreviations: KLH, keyhole limpet hemocyanin; STn, sialyl-TN.
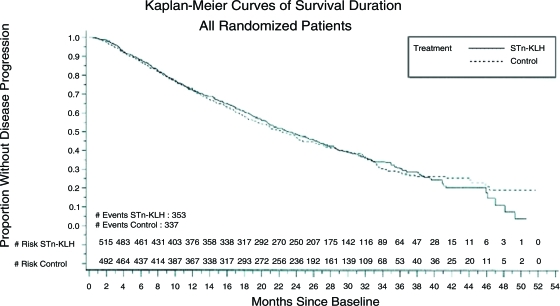
At the final analysis, the median overall survival times of the patients in the STn-KLH and KLH groups were 23.1 months and 22.3 months, respectively (Cox p = .916). A secondary analysis of survival duration using log-rank statistics revealed a similar lack of a significant difference (p = .972) (Fig. 3). Likewise, there were no significant differences in TTP or overall survival time according to disease status (NED or NPD) or hormone therapy use.
Figure 3.
Kaplan–Meier curves of survival duration.
Abbreviations: KLH, keyhole limpet hemocyanin; STn, sialyl-TN.
Secondary Endpoints: Safety and AEs, QoL, and Antibody Response
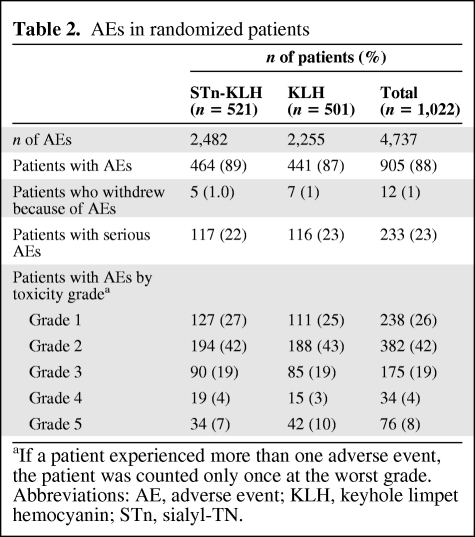
In the STn-KLH group, 2,482 AEs were reported in 464 (89%) patients, and in the KLH group, 2,255 AEs were reported in 441 (87%) patients. Five patients (1%) in the STn-KLH group and seven patients (1.4%) in the KLH group withdrew as a result of AEs (supplemental online Table B). There were 298 and 278 AEs (mostly grade 1 or 2 nausea and/or vomiting) ascribed to cyclophosphamide prior to the first injection of STn-KLH and KLH, respectively.
Following the first four vaccinations, injection site reactions, including burning/stinging, erythema, pain, itching, and indurations, were common. Ulcerations at the injection site were seen in 91 (9%) and 61 (12%) patients in the STn-KLH and KLH groups, respectively (supplemental online Table C). Following discontinuation of the adjuvant in affected patients, the incidence of ulcerations dropped to <1.5%.
The vaccine was well tolerated, with only 233 patients experiencing one or more serious events—117 patients in the STn-KLH group and 116 patients in the KLH group (Table 2).
Table 2.
AEs in randomized patients
aIf a patient experienced more than one adverse event, the patient was counted only once at the worst grade.
Abbreviations: AE, adverse event; KLH, keyhole limpet hemocyanin; STn, sialyl-TN.
The most common AEs are shown in supplemental online Table D. Patient-reported QoL was not significantly different between the STn-KLH and KLH groups (data not shown).
Week-12 median titers of antimucin and anti-KLH antibodies better illustrate the specificity of the humoral immune responses in the two treatment groups (Table 3 and supplemental online Table E). Patients in the STn-KLH group developed high titers of IgM and IgG antibodies to OSM. The median survival duration for the 367 STn-KLH patients with an antibody determination followed a dose response–like outcome: an OSM IgG response less than the median, 24 months; equal to the median, 28.4 months; and greater than the median, 31.9 months (Cox p = .1794).
Table 3.
Median antibody titers at week 12
Abbreviations: KLH, keyhole limpet hemocyanin; OSM, ovine submaxillary mucin; STn, sialyl-TN.
Discussion
Herein, we report the results of the largest and, to our knowledge, the only double-blind, randomized, multicenter phase III trial of a candidate therapeutic cancer vaccine in patients with MBC. Although the primary endpoints were not met, many clinical and immunological lessons were learned.
The therapeutic cancer vaccine—STn-KLH—did not appear to be detrimental to the intent-to-treat population, but neither did it provide a survival benefit or longer TTP, despite a vigorous and specific humoral response to the STn antigen. To our knowledge, no results have been published regarding survival following a therapeutic cancer vaccine in patients with MBC previously treated with chemotherapy.
The development of a specific anticancer–antigen humoral immune response is thought to be important in fighting metastatic cancer [28–30]. In this trial, STn-KLH vaccine led to high levels of anti-OSM and anti-STn antibodies and seroconversion from IgM to IgG; however, patients who received KLH did not have significant antimucin responses. Anti-KLH antibody responses were present in both groups, but at lower median levels in the STn-KLH group. This is to be expected, because one would predict a higher response to the hapten (STn) than to the carrier (KLH) in an appropriately designed vaccine. Despite a successful antibody response to STn-KLH, there was no evidence that STn-KLH had a significant effect on the primary endpoints. Perhaps the tumor-specific antibody response to STn did not occur in time to prevent disease progression, particularly because the patients in this study had advanced metastatic disease, or perhaps its clinical benefit was blurred by a nontumor-specific immune response against KLH in the control arm. In other words, if a placebo arm was used instead of the KLH control arm, a significantly longer TTP could not be ruled out.
The design of this study was such that all patients stopped treatment upon disease progression or toxicity; we cannot therefore exclude the possibility that continued vaccination beyond primary progression might have been advantageous. An STn-KLH vaccine trial in women with early-stage breast cancer, in whom the tumor burden is smaller, may better address this issue.
Future designs with antimucin antibodies should verify the potential clinical interaction between mucin (MUC)-1 and antiestrogen regulatory pathways, as the clinical outcome of this study hinted to. Supported by preclinical studies that detailed the modulation by and interaction of MUC-1 with estrogen receptors and various other relevant gene pathways [31, 32], we may, therefore, justify prospective clinical studies to address this issue. Furthermore, patients in this study received only one dose of cyclophosphamide infusion as a Treg suppressor [33, 34]. It was previously demonstrated that patients who received low-dose cyclophosphamide infusion versus no cyclophosphamide achieved significantly higher IgG and IgM titers to STN-KLH after four treatments (weeks 0, 2, 5, and 9) and a lower rate of disease progression at week 9 [16, 17]. It is not clear from this study whether the clinical benefit was a direct effect of cyclophosphamide infusion, although all published data imply that the dose of cyclophosphamide used (300 mg/m2) is of no substantial clinical effect. Whether the Treg suppressive effect of cyclophosphamide resulted in enhancing the immunological antitumor response—and therefore, maintaining the use of this immune-suppressive dose of cyclophosphamide would have brought a better clinical outcome—remains to be seen.
Consistent with all other previous trials of STn-KLH, we also found that STn-KLH had an acceptable safety profile [19, 21]. Patient tolerance of both STn-KLH and KLH was very good, with similar QoL data in both treatment arms.
The optimal method for detecting carbohydrate epitopes is unclear, and as a consequence, estimates of the expression of STn in breast tumors have varied considerably (16%–91%). Although patient selection based on the level of STn expression in the tumor seems prudent, the lack of an established method for determining STn expression at the time of the study design led to the decision to enroll patients without attempting to phenotype tumors. Future trials with targeted therapies may benefit from this acknowledged limitation of our study, and restrict patient enrollment to those who express the target in question.
The main concern in evaluating the relationship between an immune response and antitumor activity was the possibility of ascribing a causal relationship to what might simply have been a casual relationship—for example, some patients may have been more capable of generating a higher immune response or may have had a smaller tumor burden, or perhaps an unknown molecular pathway associated with a more favorable outcome in terms of progression-free and overall survival may have been present. Perhaps testing for antibody responses over time and correlating these responses with the trial endpoints could have addressed this unknown. This exercise may have been particularly enlightening if tumor blocks had been available for post hoc STn expression testing, which was not the case for our study.
Although modest improvements in TTP and survival have been noted as a consequence of novel chemotherapeutic combinations or the addition of biologic therapies, MBC remains largely incurable [35]. Novel therapeutic strategies are clearly required. Despite the disappointing results of this randomized, controlled phase III trial, there continues to be a place for better-designed and targeted therapeutic cancer vaccine trials. Patients should be selected based on the targeted antigen, and the vaccine should be administered alone or in conjunction with standard treatments, engaging the immune systems of cancer patients to improve clinical outcomes. This study confirmed the safety, tolerability, and humoral immune–stimulating capabilities of STn-KLH in a large international group of women with MBC. Although promising in preclinical and phase II trials, STn-KLH vaccine did not show any overall efficacy in this larger and randomized study. Selection of patients with minimal tumor burden and indolent disease (e.g., those with estrogen receptor–positive tumors) and a longer duration of vaccination with continued Treg suppression should be explored in future designs.
Summary
Despite the knowledge of many cancer-related antigens and the potential development of therapeutic vaccines, this has not yet been translated into clinical use. This is the first randomized trial using an anti–MUC-1 vaccine in the management of MBC. Although it did not meet its primary objectives, we did show that it can be safely administered.
Subsequent preclinical research suggests modulation of the estrogen receptor by MUC-1 and a resultant downstream signaling effect on cell proliferation. Although antiestrogen was given in combination with the vaccine in a subset of patients, a subset analysis was not planned or powered to address this modulation effect.
Our report may represent a true incentive for additional translational research to explore the potential benefit of combining an antiestrogen with a MUC-1 vaccine to validate the preclinical data, supported by the safety of this approach as shown in this manuscript.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the MD Anderson Department of Scientific Publications for editorial support, Cynthia Griffin for secretarial assistance, and all patients in the trial.
Funding for conducting this study, data analysis, and reporting was provided by Biomira, Inc., Edmonton, AB, Canada; Chiron Corporation, Emeryville, CA, USA, and Merck KGaA, Darmstadt, Germany.
N. K. Ibrahim had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Joanne Parker is currently at IBT Laboratories, Lenexa, Kansas.
Author Contributions
Conception/Design: Nuhad K. Ibrahim, David Miles, Joanne Parker, James L. Murray
Administrative support: Nuhad K. Ibrahim, David Miles, Joanne Parker
Provision of study material or patients: Nuhad K. Ibrahim, David Miles, Henri Roché, Miguel Martin, Timothy J. Perren, David A. Cameron, John Glaspy, David Dodwell, José Mayordomo, Alejandro Tres, James L. Murray
Collection and/or assembly of data: Nuhad K. Ibrahim, David Miles
Data analysis and interpretation: Nuhad K. Ibrahim, David Miles
Manuscript writing: Nuhad K. Ibrahim, David Miles
Final approval of manuscript: Nuhad K. Ibrahim
The authors take full responsibility for the content of the paper but thank Dr. Sheela Hota-Mitchell (Write On Science) and Dory Smith (Biomera) for assistance with the manuscript.
References
- 1.Parkins DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2005. Atlanta, GA: American Cancer Society, Inc.; 2005. pp. 1–60. [Google Scholar]
- 3.Ferlay J, Autier P, Boniol M, et al. Estimates of cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 4.Sant M, Allemani C, Berrino F, et al. Breast carcinoma survival in Europe and the United States. Cancer. 2004;100:715–722. doi: 10.1002/cncr.20038. [DOI] [PubMed] [Google Scholar]
- 5.Jäger E, Jäger D, Knuth A. Clinical cancer vaccine trials. Curr Opin Immunol. 2002;14:178–182. doi: 10.1016/s0952-7915(02)00318-7. [DOI] [PubMed] [Google Scholar]
- 6.Bitton RJ. Cancer vaccines: A critical review on clinical impact. Curr Opin Mol Ther. 2004;6:17–26. [PubMed] [Google Scholar]
- 7.Musselli C, Livingston PO, Ragupathi G. Keyhole limpet hemocyanin conjugate vaccines against cancer: The Memorial Sloan Kettering experience. J Cancer Res Clin Oncol. 2001;127(suppl 2):R20–R26. doi: 10.1007/BF01470995. [DOI] [PubMed] [Google Scholar]
- 8.Holmberg LA, Sandmaier BM. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev Vaccines. 2004;3:655–663. doi: 10.1586/14760584.3.6.655. [DOI] [PubMed] [Google Scholar]
- 9.Miles DW, Happerfield LC, Smith P, et al. Expression of sialyl-Tn predicts the effect of adjuvant chemotherapy in node-positive breast cancer. Br J Cancer. 1994;70:1272–1275. doi: 10.1038/bjc.1994.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles DW, Linehan J, Smith P, et al. Expression of sialyl-Tn in gastric cancer: Correlation with known prognostic factors. Br J Cancer. 1995;71:1074–1076. doi: 10.1038/bjc.1995.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinney AY, Sahin A, Vernon SW, et al. The prognostic significance of sialyl-Tn antigen in women treated with breast carcinoma treated with adjuvant chemotherapy. Cancer. 1997;80:2240–2249. doi: 10.1002/(sici)1097-0142(19971215)80:12<2240::aid-cncr4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Kolbayashi H, Terao T, Kawashima Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J Clin Oncol. 1992;10:95–101. doi: 10.1200/JCO.1992.10.1.95. [DOI] [PubMed] [Google Scholar]
- 13.Longenecker BM, Reddish M, Koganty R, et al. Immune responses of mice and human breast cancer patients following immunization with synthetic sialyl-Tn conjugated to KLH plus detox adjuvant. Ann N Y Acad Sci. 1993;690:276–291. doi: 10.1111/j.1749-6632.1993.tb44016.x. [DOI] [PubMed] [Google Scholar]
- 14.Itzkowitz SH, Yuan M, Montgomery CK, et al. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 15.MacLean GD, Reddish M, Koganty RR, et al. Immunization of breast cancer patients using a synthetic sialyl-Tn glycoconjugate plus Detox adjuvant. Cancer Immunol Immunother. 1993;36:215–222. doi: 10.1007/BF01740902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLean GD, Reddish MA, Koganty RR, et al. Antibodies against mucin-associated sialyl-Tn epitopes correlate with survival of metastatic adenocarcinoma patients undergoing active specific immunotherapy with synthetic STn vaccine. J Immunother Emphasis Tumor Immunol. 1996;19:59–68. doi: 10.1097/00002371-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Miles DW, Towlson KE, Graham R, et al. A randomised phase II study of sialyl-Tn and DETOX-B adjuvant with or without cyclophosphamide pretreatment for the active specific immunotherapy of breast cancer. Br J Cancer. 1996;74:1292–1296. doi: 10.1038/bjc.1996.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bass KK, Mastrangelo MJ. Immunopotentiation with low-dose cyclophosphamide in the active specific immunotherapy of cancer. Cancer Immunol Immunother. 1998;47:1–12. doi: 10.1007/s002620050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLean GD, Miles DW, Rubens RD, et al. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Holmberg LA, Sandmaier BM. Theratope® vaccine (STn-KLH) Expert Opin Biol Ther. 2001;1:881–891. doi: 10.1517/14712598.1.5.881. [DOI] [PubMed] [Google Scholar]
- 21.Reddish MA, MacLean GD, Poppema S, et al. Pre-immunotherapy serum CA27.29 (MUC-1) mucin level and CD69+ lymphocytes correlate with effects of Theratope sialyl-Tn-KLH cancer vaccine in active specific immunotherapy. Cancer Immunol Immunother. 1996;42:303–309. doi: 10.1007/s002620050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogel C, Sandmaier BM, Burris H, et al. Theratope® (STn-KLH) with Detox™-B stable emulsion for treatment of MBC following first-line chemotherapy. Presented at the 1999 Annual Meeting of the American Society of Clinical Oncology; May 15–18, 1999; Atlanta, Georgia. [Google Scholar]
- 23.Miles D, Papazisis K. Rationale for the clinical development of STn-KLH (Theratope) and anti-MUC-1 vaccines in breast cancer. Clin Breast Cancer. 2003;3(suppl 4):S134–S138. doi: 10.3816/cbc.2003.s.002. [DOI] [PubMed] [Google Scholar]
- 24.Fayers P, Aaronson N, Bjordal K, et al. Brussels, Belgium: EORTC Study Group on Quality of Life; 1995. EORTC QLQ-C30 Scoring Manual. [Google Scholar]
- 25.Zhang S, Walberg LA, Ogata S, et al. Immune sera and monoclonal antibodies define two configurations for the sialyl Tn tumor antigen. Cancer Res. 1995;55:3364–3368. [PubMed] [Google Scholar]
- 26.Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:654–663. [Google Scholar]
- 27.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 28.Foy TM, Fanger GR, Hand S, et al. Designing HER2 vaccines. Semin Oncol. 2002;29(suppl 11):53–61. doi: 10.1053/sonc.2002.34056. [DOI] [PubMed] [Google Scholar]
- 29.Cristofanilli M, Hortobagyi GN. New horizons in treating metastatic disease. Clin Breast Cancer. 2001;1:276–287. doi: 10.3816/CBC.2001.n.002. [DOI] [PubMed] [Google Scholar]
- 30.Reilly RT, Emens LA, Jaffee EM. Humoral and cellular immune responses: Independent forces or collaborators in the fight against cancer? Curr Opin Investing Drugs. 2001;2:133–135. [PubMed] [Google Scholar]
- 31.Pitroda SP, Khodarev NN, Beckett MA, et al. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc Natl Acad Sci U S A. 2009;106:5837–5841. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21:295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: Depletion of CD4+, 2H4+ suppressor inducer T-cell. Cancer Res. 1988;48:1671–1675. [PubMed] [Google Scholar]
- 34.Berd D, Mastrangelo MJ, Engstrom PF, et al. Augmentation of the human immune response by cyclophosphamide. Cancer Res. 1982;42:4862–4866. [PubMed] [Google Scholar]
- 35.Sànchez-Muñoz A, Pérez-Ruiz E, Ribelles N, et al. Maintenance treatment in metastatic breast cancer. Expert Rev Anticancer Ther. 2008;8:1907–1912. doi: 10.1586/14737140.8.12.1907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.