Abstract
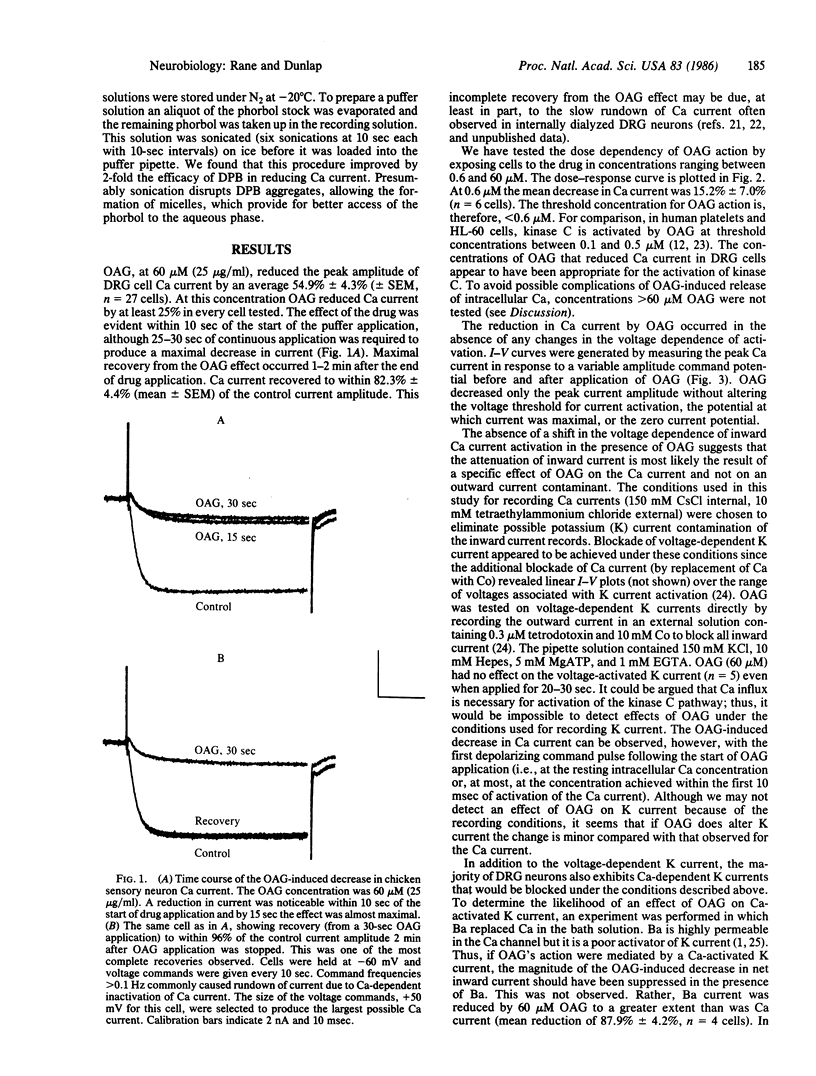
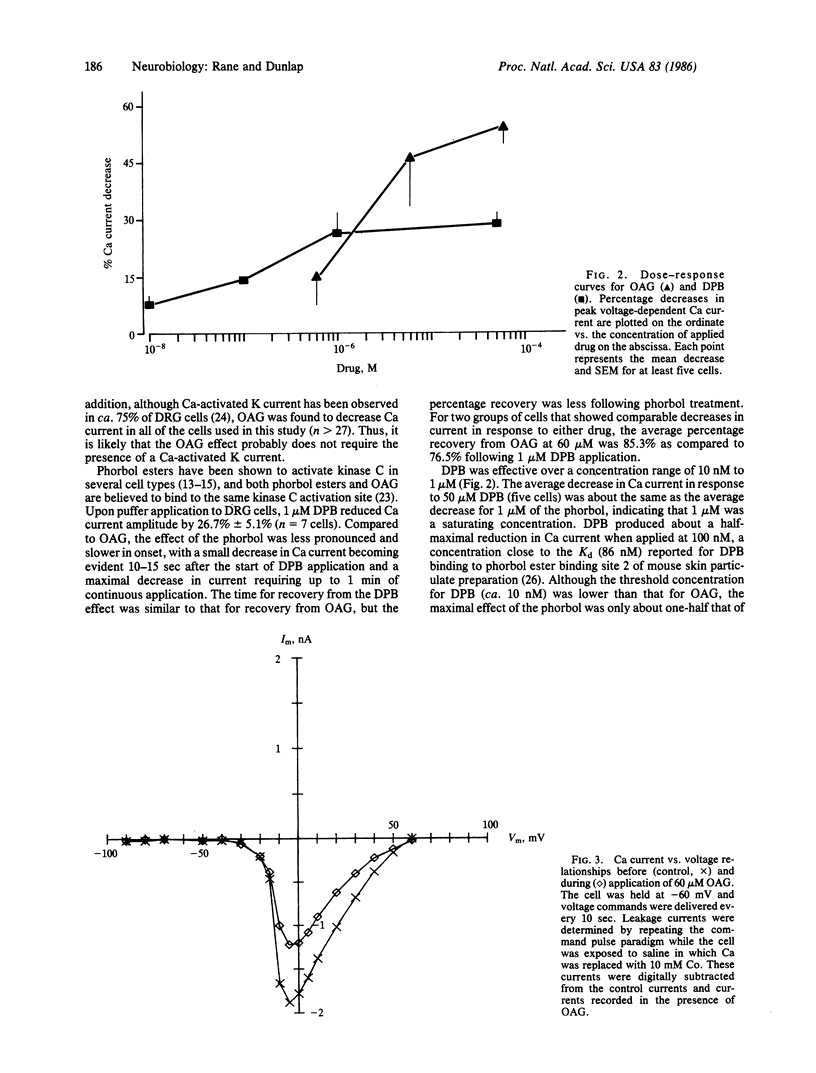
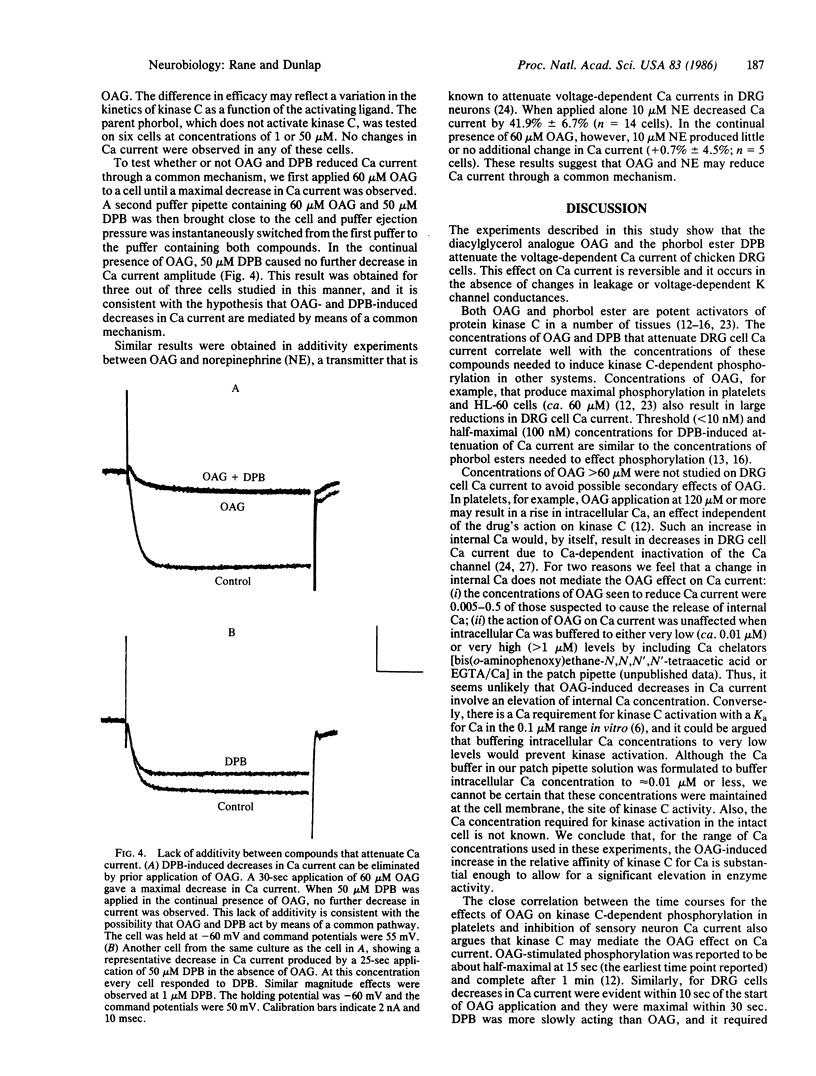
The diacylglycerol analogue 1,2-oleoylacetylglycerol (OAG) and the phorbol ester 12-deoxyphorbol 13-isobutyrate (DPB) were tested for their effects on the voltage-dependent calcium (Ca) current in embryonic chicken dorsal root ganglion neurons in vitro. OAG (0.6-60 microM) and DPB (0.01-50 microM) produced reversible decreases in Ca current. Neither drug affected resting membrane conductance, the voltage-dependent potassium current, or the Ca current-voltage relationship. The concentrations of OAG and DPB that reduced Ca current correlate well with those concentrations that have been shown, in other systems, to activate protein kinase C-dependent phosphorylation. The time course for OAG action on Ca current is also consistent with an involvement of kinase C. Incubation of dorsal root ganglion cells in 60 microM OAG prevented further reductions in Ca current by either 50 microM DPB or 10 microM norepinephrine, a known modulator of the voltage-dependent Ca channel in these cells. This evidence suggests that protein kinase C may play a role in modulating Ca channel function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baraban J. M., Snyder S. H., Alger B. E. Protein kinase C regulates ionic conductance in hippocampal pyramidal neurons: electrophysiological effects of phorbol esters. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2538–2542. doi: 10.1073/pnas.82.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Choi D. W., Farb D. H., Fischbach G. D. Chlordiazepoxide selectively augments GABA action in spinal cord cell cultures. Nature. 1977 Sep 22;269(5626):342–344. doi: 10.1038/269342a0. [DOI] [PubMed] [Google Scholar]
- DeRiemer S. A., Strong J. A., Albert K. A., Greengard P., Kaczmarek L. K. Enhancement of calcium current in Aplysia neurones by phorbol ester and protein kinase C. Nature. 1985 Jan 24;313(6000):313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. A., Blumberg P. M. Specific binding of [20-3H]12-deoxyphorbol 13-isobutyrate to phorbol ester receptor subclasses in mouse skin particulate preparations. Cancer Res. 1983 Oct;43(10):4632–4637. [PubMed] [Google Scholar]
- Ebeling J. G., Vandenbark G. R., Kuhn L. J., Ganong B. R., Bell R. M., Niedel J. E. Diacylglycerols mimic phorbol diester induction of leukemic cell differentiation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):815–819. doi: 10.1073/pnas.82.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Forscher P., Oxford G. S. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 1985 May;85(5):743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P. R., Mazzei G. J., Wood J. G., Kuo J. F. Polyclonal antibodies to phospholipid/Ca2+-dependent protein kinase and immunocytochemical localization of the enzyme in rat brain. Proc Natl Acad Sci U S A. 1985 May;82(9):3030–3034. doi: 10.1073/pnas.82.9.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J., Walaas S. I., Greengard P. Neuronal phosphoproteins: physiological and clinical implications. Science. 1984 Sep 21;225(4668):1357–1364. doi: 10.1126/science.6474180. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Giles W., Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nat New Biol. 1972 Dec 6;240(101):181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- Yamanishi J., Takai Y., Kaibuchi K., Sano K., Castagna M., Nishizuka Y. Synergistic functions of phorbol ester and calcium in serotonin release from human platelets. Biochem Biophys Res Commun. 1983 Apr 29;112(2):778–786. doi: 10.1016/0006-291x(83)91529-2. [DOI] [PubMed] [Google Scholar]


