Randomized trials and a meta-analysis of postoperative radiation therapy in non-small cell lung cancer patients are discussed along with studies evaluating adjuvant chemoradiation in this patient population.
Keywords: Non-small cell lung cancer, Post-operative radiotherapy, Adjuvant treatment, Mediastinal involvement
Learning Objectives
After completing this course, the reader will be able to:
Identify patients with non-small cell lung cancer who may benefit from postoperative radiotherapy based upon current evidence.
Summarize the results of analyses of both older and more recent data regarding postoperative radiotherapy in NSCLC patients.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
In completely resected non-small cell lung cancer (NSCLC) patients with pathologically involved mediastinal lymph nodes (N2), administration of adjuvant platinum-based chemotherapy is now considered the standard of care, based on level 1 evidence. The role of postoperative radiation therapy (PORT) in this group of patients remains controversial. The PORT meta-analysis published in 1998 concluded that adjuvant radiotherapy was detrimental to patients with early-stage completely resected NSCLC, but that the role of PORT in the treatment of tumors with N2 involvement was unclear, and that further research was warranted. Recent retrospective and nonrandomized studies, as well as subgroup analyses of recent randomized trials evaluating adjuvant chemotherapy, provide evidence of the possible benefit of PORT in patients with mediastinal nodal involvement. The role of PORT is also a valid question in patients with proven N2 disease who have undergone only induction chemotherapy followed by surgery, because the local recurrence rate for such patients varies in the range of 20%–60%. Based on the currently available data, PORT should be discussed for fit patients with completely resected NSCLC with N2 nodal involvement, preferably after completion of adjuvant chemotherapy. There is a need for new randomized evidence to evaluate PORT using the modern three-dimensional conformal radiation technique, with attention paid to reducing the risk for, particularly, pulmonary and cardiac toxicity. A new large multi-institutional randomized trial evaluating PORT in this patient population is needed and now under way.
Introduction
Over one million people are diagnosed with lung cancer every year throughout the world [1]. About 80% of them have non-small cell lung cancer (NSCLC). For patients with NSCLC in the absence of distant metastases, surgical resection remains one of the treatments of choice with the best potential for curative therapy. Most long-term survivors are indeed patients having had a complete surgical resection. However, it is only achievable in about 30% of patients. Even in this highly selected group of patients, there is still a high risk for both local and distant failure. Adjuvant treatments such as chemotherapy and radiotherapy (RT) have therefore been evaluated in order to improve prognosis. For years, their use has remained a controversial issue. However, the situation has completely changed for adjuvant chemotherapy. Whereas individual trials comparing surgery alone with surgery plus adjuvant chemotherapy could not find any significant difference, the meta-analysis published in 1995 showed a modest survival benefit of 5% in completely resected patients having received postoperative cisplatin-based adjuvant chemotherapy, compared with patients not treated with chemotherapy [2]. The benefit has now been confirmed in several trials and meta-analyses published within the last few years [3–8]. The absolute benefit observed with chemotherapy in these trials varies in the range of 4%–15% in terms of the 5-year survival rate. The individual data–based meta-analysis recently published, based on 34 trials and 8,447 patients, took into consideration updated data from the old trials for the 1995 meta-analysis, all recent trials listed above, as well as recent nonpublished or negative trials [3–10]. It showed an absolute difference in the 5-year survival rate of 4% at 5 years (64% versus 60%; p < .0001). All these results have had a significant impact on the therapeutic approach of such a common cancer. Thus, most clinicians now consider adjuvant chemotherapy as standard treatment in patients with stage II and stage III completely resected lung cancer. However, longer-term follow-up of these patients is needed. Updated survival analyses of two trials were published, with contrasting results: the largest adjuvant trial questioned the durability of the benefit of adjuvant chemotherapy beyond 5 years [11], whereas another study confirmed the long-term benefit of adjuvant chemotherapy in patients with stage IB and stage II disease [12]. Furthermore, the relevance of molecular predictors of response to systemic therapies, which were recently identified in patients presenting with metastatic disease, remain to be studied both in locally advanced NSCLC and in the adjuvant setting.
However, even after complete surgical resection and adjuvant chemotherapy, 20%–40% of patients still have a local tumor failure. In view of the high proportion of patients still suffering from local tumor recurrence after a complete resection and adjuvant chemotherapy, a new interest in postoperative radiotherapy (PORT) has occurred, even though PORT remains a controversial issue.
Studies Evaluating PORT
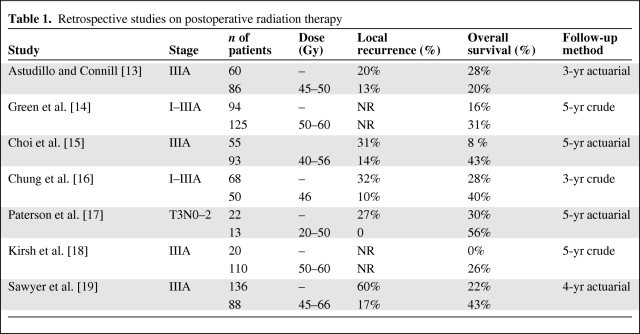
The risk for local recurrence is lower with PORT (25%–35%), as shown in several retrospective studies listed in Table 1 [13–19]. In most of these studies, there was a historical comparison with patients who had no PORT, so these figures should be interpreted with caution. Although, they suggested a potential benefit from PORT, this finding was not corroborated by most randomized trials. However, many of these prospective studies have serious design flaws. The rates of local failure at 5 years vary according to stage and, in several studies, patients at low risk for locoregional recurrence were included, possibly obscuring a radiation-induced benefit in higher-risk patients. The risk for local failure may also vary according to histology: it is higher in squamous cell lung cancer patients, whereas distant failure is observed more often in adenocarcinoma patients. Hence, some trials of the Lung Cancer Study Group addressing the question of adjuvant RT only concerned patients with squamous cell histology [20]. It is also important to state that most of the prospective studies evaluating PORT versus observation are old trials, from the 1960s and 1970s, thus conducted in the pre–computed tomography (CT) scan and pre–positron emission tomography (PET) era, with patients treated with cobalt-60 (60Co) or even orthovoltage therapy. The use of these lower energy sources resulted in greater morbidity and greater mortality [21]. The quality of RT was inferior to what is now available, with patients being currently treated with mega-voltage-energy linear accelerators. Furthermore, irradiated volumes were often large, fractionations were unusual and often >2 Gy daily, and there was no CT-based planning in most trials, with all these factors contributing to a higher morbidity. Other technical factors, such as the use of spinal cord blocks, which potentially block mediastinal disease, may explain several locoregional recurrences [22, 23]. In this review, we focus on randomized trials and the meta-analysis of PORT [24]. We then speak about studies that have evaluated adjuvant chemoradiation.
Table 1.
Retrospective studies on postoperative radiation therapy
Randomized Trials of Adjuvant RT in Stage I Resected NSCLC Patients
Van Houtte et al. [25] conducted a randomized trial in 175 patients who had a complete resection and no lymph node involvement. The 5-year survival rates were 24% in the RT arm versus 43% in the control arm. PORT was significantly deleterious, especially after pneumonectomy (16% in the PORT arm versus 43% in the control arm). They concluded that thoracic radiation therapy (TRT) should not be recommended in N0 patients. Those same authors pointed out, in a subsequent study, that more modern PORT techniques using linear accelerators instead of 60Co could improve the outcome of patients [21]. The 5-year survival rate was only 8% among patients treated with 60Co, whereas it was 30% in patients treated in modern facilities. A more recent Italian randomized trial compared PORT at a dose of 50.4 Gy with no PORT in 104 patients with pathological stage I disease [26]. All patients had a CT-planned treatment, linear accelerators were used, and treatment volumes including the bronchial stump and homolateral hilum were small. RT resulted in a significantly lower risk for local recurrence but there was no significant difference in terms of the 5-year overall survival rate (67% in the PORT group and 58% in the control group). However, several clinicians may argue that patients with pathological stage I NSCLC have a low risk for local recurrence, and routine PORT is, at present, not recommended for such patients after complete resection.
Randomized Trials of Adjuvant RT in Stage II and III Resected NSCLC Patients
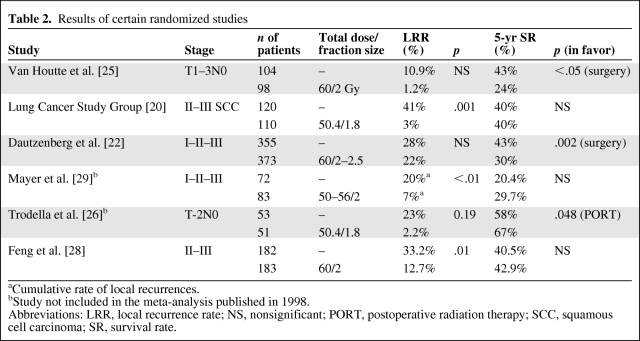
The Lung Cancer Study Group conducted a randomized study including 230 patients with stage II and stage III squamous cell carcinoma to evaluate PORT at a dose of 50 Gy [20]. PORT resulted in a significantly lower risk for local recurrence, 1%, versus 41% in the control arm (p = .001), but this effect did not translate into a demonstrable overall survival benefit (5-year survival rate, ∼40% in both arms), because most recurrences were outside the RT field or were distant failures. However, a subgroup analysis suggested that PORT could prolong the disease-free interval in patients with N2 disease. The design of the Medical Research Council (MRC) study was quite similar but also included patients with adenocarcinoma [27]. Patients with pathologically staged T1–T2, N1–N2 NSCLC were randomly assigned to receive either surgery alone or surgery and PORT at a dose of 40 Gy in 15 fractions. The results confirmed previous studies: there was no survival advantage for patients in the PORT group over those in the control arm, but in the N2 subgroup analysis there was a nonsignificant trend toward longer survival and better local control. Thus, the authors concluded that there was no indication for PORT in N1 disease, but the question remained open for N2 patients. The largest trial evaluating PORT included 728 patients (221 with stage I, 180 with stage II, and 327 with stage III disease) [22]. It demonstrated that PORT had a detrimental effect on survival: the 5-year survival rate was 43% for the control group and 30% for the RT group (p = .002). In terms of the 5-year rate without local recurrence, there was a trend in favor of PORT among N2 patients. The higher mortality rate in the RT group was a result of more intercurrent deaths, with a high incidence of cardiac and respiratory complications. In a Chinese randomized study of 366 completely resected patients with N1 or N2 nodal disease, PORT resulted in a significantly lower rate of local relapse—the local failure rate was 12.7%, versus 33.2% in the control group (p = .01)—but had no impact on survival [28]. Several of these trials are shown in Table 2 [20, 22, 25, 26, 28, 29].
Table 2.
Results of certain randomized studies
aCumulative rate of local recurrences.
bStudy not included in the meta-analysis published in 1998.
Abbreviations: LRR, local recurrence rate; NS, nonsignificant; PORT, postoperative radiation therapy; SCC, squamous cell carcinoma; SR, survival rate.
In conclusion, before the meta-analysis was published, the role of PORT remained unclear, particularly in N2 disease, because the individual trials showed conflicting and inconclusive results. They did not have the statistical power to detect moderate survival differences. Thus, the PORT meta-analysis group gathered individual data on 2,128 patients from nine randomized trials [24]. The results of the meta-analysis indicated that PORT had a significantly detrimental effect on survival, with an absolute 7% lower survival rate at 2 years, 48% versus 55% (p = .001). This overview was updated in 2005, including an additional trial by Trodella et al. [26] included in the analysis, and it still showed PORT to be detrimental, with an 18% higher relative risk for death [30]. In the original publication, subset analyses suggested that PORT could be deleterious in terms of overall survival, predominantly among patients who had a complete resection and no mediastinal involvement (either pN0 or pN1) [24]. However, they observed a 24% lower relative local recurrence rate (all stages together), so that the question of PORT in pN2 patients who have a high local recurrence rate remained valid and could warrant further research. No randomized study evaluating more modern PORT has been published since 1998. However, there have been studies on adjuvant chemoradiotherapy.
Adjuvant Chemoradiotherapy in Stage II and III Patients
Before the publication of the PORT overview, PORT was considered the standard treatment by many clinicians for stage II and stage III patients; thus, the Eastern Cooperative Oncology Group (ECOG) completed a prospective trial comparing PORT, at a dose of 50.4 Gy in 1.80-Gy fractions, with PORT plus concomitant chemotherapy combining etoposide and cisplatin [31]. The 3-year survival rates were 52% in the PORT arm and 50% in the combined treatment arm (p = .56). The locoregional recurrence rate within the radiotherapy field was around 13% in both arms and was therefore smaller than those reported in the literature. Better standardized surgical treatment may explain these results in terms of local control, with more modern radiotherapy using CT scan–based planning. The protocol asked for a thorough mediastinal lymph node dissection or sampling according to the American Thoracic Society lymph node definitions [32, 33]. There was a significant difference (p < .05) in the recurrence rate between patients with mediastinal dissection (50%) and those with mediastinal sampling (60%) [34]. Thus, the authors concluded that cisplatin and etoposide administered concomitantly with PORT did not prolong survival or modify local failures, compared with PORT alone. Since this publication, there have been several phase II trials evaluating adjuvant concomitant chemoradiation [35, 36]. In the Radiation Therapy Oncology Group 9705 phase II trial, 86 patients with completely resected NSCLC (stage II and stage IIIA) had concurrent paclitaxel plus carboplatin and PORT at a dose of 50.4 Gy [35]. The 3-year progression-free and overall survival rates were, respectively, 50% and 61%, and local failure was a component of first failure in 15% of patients. In another phase II study that included 42 patients (of whom 60% were N2) treated with a similar regimen, the 2- and 5-year Kaplan–Meier estimates of locoregional control and overall survival were 92% and 88% and 72% and 44%, respectively [36].
It should be outlined that the recently published meta-analysis based on individual data comprised a second analysis based on 13 trials and 2,660 patients, mostly stage III patients, and compared surgery plus RT with surgery plus RT and chemotherapy [8]. It showed a 4% significantly higher survival rate at 5 years (33% versus 29%). This 4% absolute benefit was also observed in trials comparing surgery with surgery and chemotherapy. Thus, the effect of chemotherapy was similar irrespective of which locoregional treatment was used: surgery alone or surgery plus PORT. The authors concluded that, because this meta-analysis was not designed to study the effect of PORT, randomized trials are needed to assess modern RT as an adjuvant treatment.
PORT: Local Control and Toxicity Issues
The meta-analysis has been criticized because the RT techniques used were considered suboptimal, resulting in higher morbidity and mortality rates in the PORT arm than in more recent studies. The majority of these trials were performed in 1965–1995 so that there was no CT scan–based planning. It should be stressed that seven of the nine trials included patients treated with 60Co equipment, which is now known to result in greater morbidity [21]. Lally et al. [37] reported on PORT based on a population-based cohort of 7,465 patients with stage II and stage III NSCLC who had surgery. They selected, from the Surveillance, Epidemiology, and End Results (SEER) database, patients treated in 1988–2002, of whom 47% received PORT. Patients who had PORT were presumably treated with more modern RT techniques than patients included in the meta-analysis. The 5-year survival rate was 20% in the no-PORT subgroup, versus 27% in the PORT group (p = .0036). The authors concluded that PORT offered a significant survival benefit for patients with N2 nodal disease, but that there was a detrimental effect for patients with N0 or N1 nodal disease. However, as with any retrospective study using a large database, one should be cautious with the results. What should be emphasized is that they came to the same conclusions as the PORT meta-analysis for patients with N0 and N1 nodal disease, even if most patients from the SEER database had more modern linear accelerator–based RT. Another recently published trial by Douillard et al. [6, 38] also pointed to the possible impact of PORT on survival in patients included in the adjuvant Navelbine International Trialist Association randomized trial. In a subgroup analysis according to nodal status, survival was longer in patients with pN2 disease who received PORT, both in the chemotherapy (median survival time, 23.8 months versus 47.4 months) and observation (median survival time, 12.7 months versus 22.7 months) arms [38]. The authors concluded that their retrospective evaluation suggested a positive effect of PORT in patients with pN2 disease and a negative effect in patients with pN1 disease when patients received adjuvant chemotherapy, and that these results supported further evaluation of PORT in prospectively randomized studies in completely resected pN2 NSCLC patients.
Considering all the studies presented, PORT should be considered only after complete resection in patients with mediastinal nodal involvement. In the study of Dautzenberg et al. [22], the risk for local recurrence was 29% lower considering only the 190 pN2 patients. In the MRC study, the local recurrence rate among pN2 patients in the control arm was 41%; this was lower, at 26%, among patients who had PORT [27]. In the retrospective study of Ichinose et al. [39], on 406 patients with pN2 nodal involvement, the local recurrence rate among the 332 evaluated patients was 39.2%, with most of these recurrences located in the mediastinum. Some interesting issues concerning local control have been outlined by retrospective studies. Kelsey et al. [40] studied 61 patients who, after a complete resection and no adjuvant treatment, presented with a local failure. They observed that left-sided tumors tended to recur in the contralateral mediastinum more frequently than did right-sided tumors, and this may be explained by the surgical technique because left-sided lymph node exploration is considered more difficult than right-sided lymph node exploration [34]. A retrospective study recently evaluated PORT according to the number of lymph node stations involved [41]. PORT efficacy was dependent on the number of lymph nodes involved, and thus of more benefit to patients with multiple stations involved. Sawyer et al. [19] tried to divide pN2 patients into three different subgroups according to their respective risk for failure: high risk, for cases with multiple distant mediastinal nodes involved; intermediate risk, for cases of involvement of inferior nodes or superior nodes with eventually invasion of hilar nodes; and low risk, if there is no hilar node involvement. However, as with any retrospective study, one should be cautious with the results, which were very much in favor of PORT, and this delineates the importance of a new randomized study comparing PORT with no PORT in such a common cancer as NSCLC, as stressed in most recent articles on PORT in patients with N2 disease.
Even if the techniques used were different from one trial to another, most of the trials included in the meta-analysis used elective nodal irradiation including the two supraclavicular regions, the whole mediastinum, and the homolateral hilar region up to a dose of 36–42 Gy. A boost to a more limited region was delivered with oblique opposed fields or with anterior–posterior reduced fields with a spinal block. Many of these trials used suboptimal techniques, leading to poor local control and eventually greater toxicity [23]. There was a significant underdosage in the nodal area at risk, especially in trials recommending the spinal block technique [27].
The excess toxicity (mostly cardiac and pulmonary) and noncancer-related deaths observed after PORT in the meta-analysis trials can probably be explained by excessive volumes of radiation, old radiation techniques, too large doses and fraction sizes, and no CT scan–based planning. Unfortunately, the authors could not collect data on toxicity or causes of intercurrent deaths in the different studies. Late cardiac complications described after mediastinal RT are linked to the total dose, the fraction size, the irradiated volume, the technique of irradiation, as well as comorbidities (tobacco use, overweight) [42, 43]. Several authors have underlined the importance of the RT technique to lower this risk [21, 44]. Pulmonary complications, such as pneumonitis and lung fibrosis, can also be observed, but they occur earlier and there are strong volume and fractionation effects [45, 46]. The administration of certain radiosensitizing drugs may increase this risk.
Presently, patients should have conformal RT based on CT planning, contributing to less toxicity and possibly better local control [21, 44, 47–50]. Miles et al. [50] elaborated on a mathematical model to describe the tumor stage- and field size-dependent risks and benefits of PORT and showed that RT-induced mortality was strongly dependent on field size. In the largest published randomized trial, Dautzenberg et al. [22] were able to determine that the use of fraction sizes >2 Gy resulted in a high risk for late toxicity. There were more intercurrent deaths with higher daily doses (5-year rate, 16%–18% if daily fraction ≤2Gy and 26% with higher daily fractions). There was a correlation between fractionation and morbidity. The risks for noncancer-related death were 7% in the control group, 16%–18% among patients who had TRT with daily fractions ≤2 Gy, and 26% among those who had higher doses per fraction. A retrospective study published on a selected patient population focused on toxicity issues, showing that PORT could be administered safely if patients were treated with more modern treatment techniques, a more limited volume of irradiation, a daily fraction size ≤2 Gy, and a total dose ≤54 Gy [44]. The ECOG also reported on the 4-year actuarial rate of death from intercurrent disease (DID) for patients treated with PORT within the E3590 trial, which was 12,9%, not significantly different from the 10.1% expected rate of DID observed in a control population matched for age and gender and corrected for smoking status [51]. A SEER data–based study analyzed deaths from heart disease in a group of 6,148 pN1 or pN2 patients operated on in 1983–1993 who were followed up until 2005 [52]. Among these patients, 58% had PORT. PORT delivery was indeed associated with a greater hazard for heart disease. However, this was only significant in patients treated in 1988–1993. For the authors, this reflected the impact of the more modern RT techniques used in the second half of the 1990s on morbidity.
Importance of Surgery and Preoperative Staging in the Perspective of Modern Adjuvant RT
New data in the reassessment of PORT should take into consideration the quality of surgery and the progress made in terms of preoperative staging. In past years, there has been an important collaborative effort of thoracic surgeons to define lymph node exploration and complete resection. The European Society of Thoracic Surgeons proposed guidelines for appropriate intraoperative and preoperative lymph node staging [53, 54]. The International Association for the Study of Lung Cancer staging committee proposed a definition of complete resection [55]. All resection margins, including bronchial, venous, and arterial stumps and peribronchial soft tissue, should be microscopically free from disease. Systematic nodal examination should comprise at least three intrapulmonary and hilar nodes and at least 3 nodes from the following mediastinal nodal stations according to the location of the primary tumor:
Right upper and middle lobe: subcarinal nodes (station 7) and two of the following three stations—superior paratracheal (station 2), inferior paratracheal, and pretracheal (station 4);
Right lower lobe: subcarinal nodes (station 7) and right inferior paratracheal (station 4) and either the paraesophageal or pulmonary ligament nodes (station 8 and station 9);
Left upper lobe: subcarinal nodes (station 7) and subaortic and anterior mediastinal nodes (station 5 and station 6);
Left lower lobe: subcarinal nodes (station 7), paraesophageal and pulmonary ligament nodes (station 8 and station 9).
There is no consensus on whether or not the highest mediastinal node that has been explored and removed should be negative. It is also unclear whether the extent of mediastinal exploration can affect long-term survival. Even if randomized trials have been performed comparing these two mediastinal approaches, there still is debate between advocates of radical mediastinal node dissection, who claim not only a potential prognostic benefit but better tumor staging, and those who oppose the radical approach because of higher morbidity and mortality as a result of the extent of surgery and possibly because of a negative effect on survival resulting from impaired local immunity [56–58].
Repositioning PORT in 2010 in Patients at High Risk for Local Recurrence
The 5-year survival rate of patients with mediastinal nodal involvement who have had a complete resection of NSCLC (pN2) varies in the range of 20%–35% in large surgical series according to the number of lymph node stations involved [33, 59]. The importance of mediastinal node involvement seems the best and most consensual prognostic factor. Their risk for metastatic dissemination is high. Local control is also an important issue, with reported local failure rates around 30%–40% [20, 27, 28, 39].
In 2010, most patients considered for surgery are much better selected based on PET scanning and brain imaging. PET–CT is highly sensitive and specific in detecting mediastinal nodal spread and extracranial metastases [60–62]. After induction chemotherapy for patients with N2 involvement, repeated fluorodeoxyglucose PET may help in the selection of surgical candidates among patients with mediastinal downstaging or persistent minor disease [63]. Many clinicians treat patients with clinical N2 involvement with preoperative chemotherapy. Several studies as well as a meta-analysis based on the literature have indeed suggested a benefit in terms of survival in favor of preoperative chemotherapy [64–66]. Mediastinal downstaging is a very important prognostic factor [67, 68]; however, the recurrence rate can be quite high, as seen in the updated results of a Swiss phase II trial showing that, among N2 patients treated with neoadjuvant chemotherapy, at a 5-year follow-up, 60% of patients had a local relapse [69]. The question of PORT is then also valid among patients who have histologically proven N2 disease before preoperative chemotherapy, whatever their response: persistent mediastinal involvement or mediastinal downstaging (from histologically proven N2 to pN0 or pN1). There is no randomized study on this issue.
Implications for a New Trial Evaluating PORT
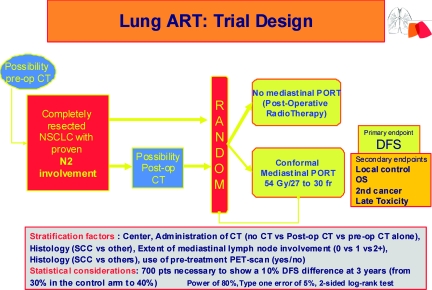
At present, based on level 1 evidence, patients who have had a complete resection of their primary tumor with mediastinal lymph node dissection showing no mediastinal involvement (pN0 and pN1) should not have PORT. The issue of PORT is not as clear among pN2 patients and warrants further studies with more modern techniques [24]. The indication for PORT is currently debated for each individual patient with mediastinal involvement. Some clinicians never consider PORT for pN2 patients, others consider it standard in pN2 patients, and others restrict their indication for PORT to patients with multiple N2 nodal involvement or cases of extracapsular extension. However, in order to avoid the errors of previous studies, it is very important to describe well the modalities of surgery (most particularly, the lymph node exploration) as well as the RT modalities in terms of volume, fields, and dose prescription. Conformational RT should be mandatory. The irradiation volume should take into account data from a thoracic CT scan and the eventual PET scan data before surgery, as well as the description of the mediastinal exploration and histopathological results. Thus, if one considers a series of surgical patients with pN2 nodal disease, the risk for lymph node involvement is 48% around the trachea and 41% in the subcarenal region [70]. Consequently, paratracheal nodes, subcarenal nodes, as well as the homolateral hilar region should be systematically included in the irradiation volume [71, 72]. A recent study has shown that there are wide variations in target volume definition for PORT even among TRT oncologists [71]. Based on previous studies, it seems reasonable to treat only involved lymph node stations and uninvolved stations at high risk to better protect surrounding normal structures and consequently minimize treatment-related mortality [40, 50, 72, 73, 74]. In the ongoing, phase III, randomized Lung Adjuvant Radiotherapy Trial (ART), the irradiation volume includes the lymph node stations involved according to the pathological report as well as the lymph node stations considered at high risk for involvement according to tumor location, with station 4 and station 7 and the homolateral hilar region always involved in the conformal treatment volume (Fig. 1) [75]. All patients who have had a complete resection for NSCLC with proven mediastinal nodal metastases, irrespective of whether they have had preoperative or postoperative chemotherapy, can be included.
Figure 1.
Lung Adjuvant Radiotherapy Trial (ART) design.
Abbreviations: CT, computed tomography; DFS, disease-free survival; NSCLC, non-small cell lung cancer; PET, positron emission tomography; PORT, postoperative radiation therapy; post-op, postoperative; pre-op, preoperative; SCC, squamous cell carcinoma.
Stratification factors comprise the modalities of adjuvant treatment (preoperative alone versus postoperative versus none), number of mediastinal lymph node stations involved (0 versus 1 versus ≥2), histology (squamous cell carcinoma versus others), use of pretreatment PET scanning (yes versus no), and center. There is no stratification according to modality of lymph node exploration (mediastinal dissection or sampling) or clinical versus incidental N2 involvement because no uniform definition of these entities exists in the participating centers. Such data will be collected and subsequently analyzed by a panel of surgeons and thoracic oncologists.
The primary endpoint of the Lung ART trial is disease-free survival and secondary endpoints are overall survival, patterns of relapse, local failure, second cancers, and treatment-related toxicity. Because of toxicity reported in old trials, quality assurance for conformal RT as well as translational research (predictive factors of toxicity and toxicity) programs are planned. For lymph node station delineation, the atlas of CT-based definition of thoracic lymph node stations may be helpful [76]. Statistical considerations are based on data issued from large randomized trials having evaluated adjuvant chemotherapy in completely resected patients with N2 involvement. Because the disease-free survival (DFS) rate among these patients was about 30% at 3 years, 700 patients would allow for the observation of a 10% difference in terms of the 3-year DFS rate with a bilateral test (power, 80%; risk α, 5%).
Because the standard treatment for patients with mediastinal involvement has changed in the last 10 years from surgery plus adjuvant RT to surgery plus chemotherapy, and because selection of surgical candidates has evolved with PET–CT, it is of utmost importance to evaluate whether modern adjuvant RT can improve survival in patients after complete resection and adjuvant chemotherapy. Such a study could also prove that PORT can produce significantly better local control with low morbidity (using modern RT techniques). Such a study would contribute to an optimization of standard care in operable patients with mediastinal involvement.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Non-Small Cell Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 3.Arriagada R, Bergman B, Dunant A, et al. International Adjuvant Lung cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 4.Strauss GM, Herndon JE, 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group study groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winton T, Livingston R, Johnson D, et al. National Cancer Institute of Canada Clinical Trials Group; National Cancer Institute of the United States Intergroup JBR.10 Trial Investigators. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 7.Pignon JP, Tribodet H, Scagliotti GV, et al. LACE Collaborative Group. Lung Adjuvant Cisplatin Evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 8.NSCLC Meta-analyses Collaborative Group. Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scagliotti GV, Fossati R, Torri V, et al. Adjuvant Lung Project Italy/European Organisation for Research Treatment of Cancer-Lung Cancer Cooperative Group Investigators. Randomized study of adjuvant chemotherapy for completely resected stage I, II or IIIA non-small-cell lung cancer. J Natl Cancer Inst. 2003;95:1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 10.Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: The surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26:173–182. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 12.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: Updated survival analysis of JBR-10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astudillo J, Conill C. Role of postoperative radiation therapy in stage IIIa non-small cell lung cancer. Ann Thorac Surg. 1990;50:618–623. doi: 10.1016/0003-4975(90)90200-p. [DOI] [PubMed] [Google Scholar]
- 14.Green N, Kurohara SS, George FW, 3rd, et al. Postresection irradiation for primary lung cancer. Radiology. 1975;116:405–407. doi: 10.1148/116.2.405. [DOI] [PubMed] [Google Scholar]
- 15.Choi NC, Grillo HC, Gardiello M, et al. Basis for new strategies in postoperative radiotherapy of bronchogenic carcinoma. Int J Radiat Oncol Biol Phys. 1980;6:31–35. doi: 10.1016/0360-3016(80)90199-6. [DOI] [PubMed] [Google Scholar]
- 16.Chung CK, Stryker JA, O'Neill M, Jr, et al. Evaluation of adjuvant postoperative radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys. 1982;8:1877–1880. doi: 10.1016/0360-3016(82)90444-8. [DOI] [PubMed] [Google Scholar]
- 17.Paterson R, Russell MH. Clinical trials in malignant disease. IV–Lung cancer. Value of post-operative radiotherapy. Clin Radiol. 1962;13:141–144. doi: 10.1016/s0009-9260(62)80036-1. [DOI] [PubMed] [Google Scholar]
- 18.Kirsh MM, Sloan H. Mediastinal metastases in bronchogenic carcinoma: Influence of postoperative irradiation, cell type, and location. Ann Thorac Surg. 1982;33:459–463. doi: 10.1016/s0003-4975(10)60786-2. [DOI] [PubMed] [Google Scholar]
- 19.Sawyer TE, Bonner JA, Gould PM, et al. Effectiveness of postoperative irradiation in stage IIIA non-small cell lung cancer according to regression tree analyses of recurrence risks. Ann Thorac Surg. 1997;64:1402–1408. doi: 10.1016/S0003-4975(97)00908-9. [DOI] [PubMed] [Google Scholar]
- 20.The Lung Cancer Study Group. Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. N Engl J Med. 1986;315:1377–1381. doi: 10.1056/NEJM198611273152202. [DOI] [PubMed] [Google Scholar]
- 21.Phlips P, Rocmans P, Vanderhoeft P, et al. Post-operative radiotherapy after pneumonectomy: Impact of modern treatment facilities. Int J Radiat Oncol Biol Phys. 1993;27:525–529. doi: 10.1016/0360-3016(93)90375-6. [DOI] [PubMed] [Google Scholar]
- 22.Dautzenberg B, Arriagada R, Chammard AB, et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Groupe d'Etude et de Traitement des Cancers Bronchiques. Cancer. 1999;86:265–273. doi: 10.1002/(sici)1097-0142(19990715)86:2<265::aid-cncr10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.DiBiase SJ, Werner-Wasik M, Croce R, et al. Standard off-cord lung oblique fields do not include the entire mediastinum: A computed tomography simulator study. Am J Clin Oncol. 2000;23:249–252. doi: 10.1097/00000421-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 24.PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352:257–263. [PubMed] [Google Scholar]
- 25.Van Houtte P, Rocmans P, Smets P, et al. Postoperative radiation therapy in lung cancer: A controlled trial after resection of curative design. Int J Radiat Oncol Biol Phys. 1980;6:983–986. doi: 10.1016/0360-3016(80)90105-4. [DOI] [PubMed] [Google Scholar]
- 26.Trodella L, Granone P, Valente S, et al. Adjuvant radiotherapy in non-small cell lung cancer with pathological stage I: Definitive results of a phase III randomized trial. Radiother Oncol. 2002;62:11–19. doi: 10.1016/s0167-8140(01)00478-9. [DOI] [PubMed] [Google Scholar]
- 27.Stephens RJ, Girling DJ, Bleehen NM, et al. Medical Research Council Lung Cancer Working Party. The role of post-operative radiotherapy in non-small-cell lung cancer: A multicentre randomised trial in patients with pathologically staged T1–2, N1–2, M0 disease. Br J Cancer. 1996;74:632–639. doi: 10.1038/bjc.1996.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng QF, Wang M, Wang LJ, et al. A study of postoperative radiotherapy in patients with non-small-cell lung cancer: A randomized trial. Int J Radiat Oncol Biol Phys. 2000;47:925–929. doi: 10.1016/s0360-3016(00)00509-5. [DOI] [PubMed] [Google Scholar]
- 29.Mayer R, Smolle-Juettner FM, Szolar D, et al. Postoperative radiotherapy in radically resected non-small cell lung cancer. Chest. 1997;112:954–959. doi: 10.1378/chest.112.4.954. [DOI] [PubMed] [Google Scholar]
- 30.Burdett S, Stewart L PORT Meta-analysis Group. Postoperative radiotherapy in non-small-cell lung cancer: Update of an individual patient data meta-analysis. Lung Cancer. 2005;47:81–83. doi: 10.1016/j.lungcan.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Keller SM, Adak S, Wagner H, et al. Eastern Cooperative Oncology Group. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. N Engl J Med. 2000;343:1217–1222. doi: 10.1056/NEJM200010263431703. [DOI] [PubMed] [Google Scholar]
- 32.Mountain CF. A new international staging system for lung cancer. Chest. 1986;89(4 suppl):225S–233S. doi: 10.1378/chest.89.4_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 33.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 34.Keller SM, Adak S, Wagner H, et al. Eastern Cooperative Oncology Group. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Ann Thorac Surg. 2000;70:358–365. doi: 10.1016/s0003-4975(00)01673-8. discussion 365–366. [DOI] [PubMed] [Google Scholar]
- 35.Bradley JD, Paulus R, Graham MV Radiation Therapy Oncology Group. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: Promising long-term results of the Radiation Therapy Oncology Group—RTOG 9705. J Clin Oncol. 2005;23:3480–3487. doi: 10.1200/JCO.2005.12.120. [DOI] [PubMed] [Google Scholar]
- 36.Feigenberg SJ, Hanlon AL, Langer C, et al. A phase II study of concurrent carboplatin and paclitaxel and thoracic radiotherapy for completely resected stage II and IIIA non-small cell lung cancer. J Thorac Oncol. 2007;2:287–292. doi: 10.1097/01.JTO.0000263710.54073.b3. [DOI] [PubMed] [Google Scholar]
- 37.Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2006;24:2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 38.Douillard JY, Rosell R, De Lena M, et al. Adjuvant Navelbine International Trialist Association. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 39.Ichinose Y, Kato H, Koike T, et al. Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: Questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung Cancer. 2001;34:29–36. doi: 10.1016/s0169-5002(01)00207-0. [DOI] [PubMed] [Google Scholar]
- 40.Kelsey CR, Light KL, Marks LB. Patterns of failure after resection of non-small-cell lung cancer: Implications for postoperative radiation therapy volumes. Int J Radiat Oncol Biol Phys. 2006;65:1097–1105. doi: 10.1016/j.ijrobp.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Matsuguma H, Nakahara R, Ishikawa Y, et al. Postoperative radiotherapy for patients with completely resected pathological stage IIIA-N2 non-small cell lung cancer: Focusing on an effect of the number of mediastinal lymph node stations involved. Interact Cardiovasc Thorac Surg. 2008;7:573–577. doi: 10.1510/icvts.2007.174342. [DOI] [PubMed] [Google Scholar]
- 42.Adams MJ, Hardenbergh PH, Constine LS, et al. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 43.Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76(3 suppl):S77–S85. doi: 10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 44.Machtay M, Lee JH, Shrager JB, et al. Risk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non-small-cell lung carcinoma. J Clin Oncol. 2001;19:3912–3917. doi: 10.1200/JCO.2001.19.19.3912. [DOI] [PubMed] [Google Scholar]
- 45.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 46.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 suppl):S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong J, McGibney C. The impact of three-dimensional radiation on the treatment of non-small cell lung cancer. Radiother Oncol. 2000;56:157–167. doi: 10.1016/s0167-8140(00)00207-3. [DOI] [PubMed] [Google Scholar]
- 48.Senan S, De Ruysscher D, Giraud P, et al. Radiotherapy Group of European Organization for Research and Treatment of Cancer. Literature-based recommendations for treatment planning and execution in high-dose radiotherapy for lung cancer. Radiother Oncol. 2004;71:139–146. doi: 10.1016/j.radonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 49.von Lieven H, Burkhardt E. Postoperative radiotherapy of NSCLC—outcome after 3-D treatment planning. Strahlenther Onkol. 2001;177:302–306. doi: 10.1007/pl00002412. [DOI] [PubMed] [Google Scholar]
- 50.Miles EF, Kelsey CR, Kirkpatrick JP, et al. Estimating the magnitude and field-size dependence of radiotherapy-induced mortality and tumor control after postoperative radiotherapy for non-small-cell lung cancer: Calculations from clinical trials. Int J Radiat Oncol Biol Phys. 2007;68:1047–1052. doi: 10.1016/j.ijrobp.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 51.Wakelee HA, Stephenson P, Keller SM, et al. Post-operative radiotherapy (PORT) or chemoradiotherapy (CPORT) following resection of stages II and IIIA non-small cell lung cancer (NSCLC) does not increase the expected risk of death from intercurrent disease (DID) in Eastern Cooperative Oncology Group (ECOG) trial E3590. Lung Cancer. 2005;48:389–397. doi: 10.1016/j.lungcan.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Lally BE, Detterbeck FC, Geiger AM, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy. Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2007;110:911–917. doi: 10.1002/cncr.22845. [DOI] [PubMed] [Google Scholar]
- 53.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006;30:787–792. doi: 10.1016/j.ejcts.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 54.De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 55.Rami-Porta R, Wittekind C, Goldstraw P International Association for the Study of Lung Cancer (IASLC) staging committee. Complete resection of lung cancer surgery: Proposed definition. Lung Cancer. 2005;49:25–33. doi: 10.1016/j.lungcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: Results of a prospective randomized trial. Ann Surg. 1998;227:138–144. doi: 10.1097/00000658-199801000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer. 2002;36:1–6. doi: 10.1016/s0169-5002(01)00445-7. [DOI] [PubMed] [Google Scholar]
- 58.Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung cancer: Systematic review and meta-analysis of randomised controlled trials. Thorax. 2006;61:597–603. doi: 10.1136/thx.2005.051995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:4–9. [PubMed] [Google Scholar]
- 60.Cerfolio RJ, Ojha B, Bryant AS, et al. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg. 2004;78:1017–1023. doi: 10.1016/j.athoracsur.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 61.Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: A meta-analysis. Ann Intern Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 62.Ung YC, Maziak DE, Vanderveen JA, et al. 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: A systematic review. J Natl Cancer Inst. 2007;99:1753–1767. doi: 10.1093/jnci/djm232. [DOI] [PubMed] [Google Scholar]
- 63.Dooms C, Verbeken E, Stroobants S, et al. Prognostic stratification of stage IIIA-N2 non-small-cell lung cancer after induction chemotherapy: A model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol. 2008;26:1128–1134. doi: 10.1200/JCO.2007.13.9550. [DOI] [PubMed] [Google Scholar]
- 64.Martini N, Kris MG, Flehinger BJ, et al. Preoperative chemotherapy for stage IIIa (N2) lung cancer: The Sloan-Kettering experience with 136 patients. Ann Thorac Surg. 1993;55:1365–1373. doi: 10.1016/0003-4975(93)91072-u. discussion 1373–1374. [DOI] [PubMed] [Google Scholar]
- 65.André F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: Evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–2989. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- 66.Burdett S, Stewart LA, Rydzewska L. A systematic review and meta-analysis of the literature: Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. J Thorac Oncol. 2006;1:611–621. [PubMed] [Google Scholar]
- 67.Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: A multicenter phase II trial. J Clin Oncol. 2003;21:1752–1759. doi: 10.1200/JCO.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 68.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. European Organisation for Research and Treatment of Cancer-Lung Cancer Group. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 69.Betticher DC, Hsu Schmitz SF, Totsch M, et al. Swiss Group for Clinical Cancer Research (SAKK) Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer. 2006;94:1099–1106. doi: 10.1038/sj.bjc.6603075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watanabe Y, Shimizu J, Tsubota M, et al. Mediastinal spread of metastatic lymph nodes in bronchogenic carcinoma. Mediastinal nodal metastases in lung cancer. Chest. 1990;97:1059–1065. doi: 10.1378/chest.97.5.1059. [DOI] [PubMed] [Google Scholar]
- 71.Spoelstra FO, Senan S, Le Péchoux C, et al. Lung Adjuvant Radiotherapy Trial Investigators Group. Variations in target volume definition for postoperative radiotherapy in stage III non-small-cell lung cancer: Analysis of an international contouring study. Int J Radiat Oncol Biol Phys. 2010;76:1106–1113. doi: 10.1016/j.ijrobp.2009.02.072. [DOI] [PubMed] [Google Scholar]
- 72.Kiricuta IC, Mueller G, Stiess J, et al. The lymphatic pathways of non-small cell lung cancer and their implication in curative irradiation treatment. Lung Cancer. 1994;11:71–82. doi: 10.1016/0169-5002(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 73.Emami B, Kaiser L, Simpson J, et al. Postoperative radiation therapy in non-small cell lung cancer. Am J Clin Oncol. 1997;20:441–448. doi: 10.1097/00000421-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Belderbos JS, Kepka L, Spring Kong FM, et al. Report from the International Atomic Energy Agency (IAEA) consultants' meeting on elective nodal irradiation in lung cancer: Non-small-cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 2008;72:335–342. doi: 10.1016/j.ijrobp.2008.04.081. [DOI] [PubMed] [Google Scholar]
- 75.Le Péchoux C, Dunant A, Pignon JP, et al. Need for a new trial to evaluate adjuvant postoperative radiotherapy in non-small-cell lung cancer patients with N2 mediastinal involvement. J Clin Oncol. 2007;25:e10–e11. doi: 10.1200/JCO.2006.09.6263. [DOI] [PubMed] [Google Scholar]
- 76.Chapet O, Kong FM, Quint LE, et al. CT-based definition of thoracic lymph node stations: An atlas from the University of Michigan. Int J Radiat Oncol Biol Phys. 2005;63:170–178. doi: 10.1016/j.ijrobp.2004.12.060. [DOI] [PubMed] [Google Scholar]