A systematic comparison of results on the survival of chronic myelocytic leukemia patients reported from clinical trials and obtained from population-based cancer registries is presented. The extent of and potential reasons for survival differences are explored.
Keywords: Chronic myelocytic leukemia, Survival, SEER program, Clinical trials
Abstract
Introduction.
The survival of patients with chronic myelocytic leukemia (CML) has improved during the past decades. However, there have been discrepancies between results reported from clinical trials and population-based studies. We aimed to elucidate the extent of these discrepancies.
Methods.
We examined the 5-year survival rate of patients in clinical trials of CML treatment and compared these results with the survival of patients in the general population using the Surveillance, Epidemiology, and End Results (SEER) database, correcting for differences in the age structure of the patient populations.
Results.
Twenty-nine trials were identified for data extraction. The survival rate calculated from SEER data was lower than the survival rate in clinical trials in the corresponding period, with differences of 2.1%–50.7%. Age-adapted survival was similar for four trials, but differences up to 35.8% were seen in most. Limitations of the study include the lack of information on chemotherapy in the SEER database and possible heterogeneity of cases.
Discussion.
The survival rate in clinical trials of CML treatment is higher than the survival rate of all patients with CML. We speculate that the difference may be a result of access to better medications, selection of healthier patients for trials, and the time necessary for adoption of new treatments. This finding underscores the need for population-based studies to give a more realistic idea of survival for patients with a given malignancy in the general population.
Introduction
The gold standard for determination of the superiority (or equivalence) of one type of treatment over another is the randomized, controlled clinical trial. However, results from clinical trials cannot always be directly translated to the general population. Insufficient numbers of elderly, women, and minority participants in clinical trials have decreased the confidence that the findings can be applied to the general population [1, 2], although the importance of this factor may have decreased over time [3]. Additionally, patients in clinical trials may be systematically different from patients in the general population. For example, few clinical trials include patients with a poor performance status [4]. Patients with comorbid conditions such as prior cancers, HIV, or poor organ function are generally excluded from clinical trials as well. Treatments that work well under the “ideal conditions” of a clinical trial may be unsuitable for some patient populations or some clinical situations. Additionally, treatment decisions may be affected by comorbid conditions that greatly limit life expectancy or the ability to tolerate treatment. In such cases, aggressive treatment may be withheld as being unlikely to benefit the patient, even when highly effective treatment for the specific illness is available. Therefore, it is not possible to generalize directly from results obtained in clinical trials to results to be expected in the general population.
The survival duration of patients with chronic myelocytic leukemia (CML) has improved greatly over the past decades [5]. The advent of interferon therapy [6], hematopoietic stem cell transplant [7], and finally tyrosine kinase inhibitors (TKIs) [8] has greatly improved survival in patients with CML in clinical trials. However, population-based estimates of CML survival lag behind results from clinical trials, although survival on the population level has increased greatly in the past two decades as well [5]. In this article, we aim to provide a systematic comparison of results on CML survival reported from clinical trials and obtained from population-based cancer registries and to explore the extent and potential reasons for survival differences.
Methods
Data on survival of CML patients in the general U.S. population were extracted from the Surveillance, Epidemiology, and End Results 9 (SEER9) limited-use database for the calendar years 1973–2006 [9]. This database includes data from the nine registries that have been included in the SEER database since 1973: Connecticut, Hawaii, Iowa, New Mexico, Utah, Detroit, San Francisco/Oakland, Seattle/Puget Sound, and Atlanta, which together cover a population of 30 million people. All patients diagnosed with CML during each relevant period were identified in the database and the observed survival rate was calculated. The diagnosis of CML was determined by inclusion in the SEER “recode” classification of 35022, which includes the International Classification of Diseases for Oncology, Third Revision, histology codes of 9863, 9875, 9876, 9945, and 9946. The recode classification was used because it is available for all dates and therefore avoids possible bias resulting from changes in codes over time.
Peer-reviewed, randomized controlled trials of treatments for patients with CML were identified by searching the PubMed database up to June, 2010 using the key terms “chronic myelocytic leukemia” and “chronic myeloid leukemia” with the limitation that only clinical trials be shown. Trials for which patients were recruited between 1973 and 2006 with full text in either English or German were selected for analysis. Only prospective clinical trials for which 5-year survival data were available were included in the analysis. When results from a single trial were reported in the literature more than once, the publication of the final results of the trial was used. Trials that were not limited to CML patients were not included. Only trials comparing one form of treatment with another in which survival for ≥5 years was the primary outcome were included. Trials for which survival data or years of recruitment could not be determined were excluded. Trials for which the age of participants could not be determined are listed in the results but complete comparisons could not be made for these trials.
For each trial, the first author, year of publication, country of origin, type of trial, treatments being compared, years of recruitment, number of patients included in the trial, median age (if stated) and age range of the patient population, whether the trial included patients with advanced disease, and the 5-year survival (if calculated or possible to estimate from survival curves) in each arm were extracted.
For comparison, the observed survival rate for patients in the SEER database during the time period in which patients were recruited for the trial in question was analyzed. For the SEER population, the survival time was calculated the using date of diagnosis and the date of death, last date on which patient was known to be alive, or cutoff date for the study comparison. Patients diagnosed by death certificate or autopsy only were excluded. Additionally, patients with a prior malignancy were excluded because they are excluded from most clinical trials. Data on the specific stage, that is, chronic phase (CP), accelerated phase (AP), or blast crisis (BC), are not available in the SEER database and therefore no distinction among these groups could be made. Absolute survival was calculated because most clinical trials report absolute rather than relative survival.
Initially, all CML patients diagnosed in the same period of time were included. Because the prognosis of CML patients varies strongly with age, the age structures of the patient populations being compared should be as similar as possible. Because of a lack of detailed data on age distribution reported in most trials, direct age adjustment of survival was not possible. However, based on the age range of patients commonly reported in clinical trials, an “age adapted” survival estimate was calculated for patients in the SEER database diagnosed in the same period of time in the age range included in each study, that is, if the age range of the patients in the study was 18–65 then only patients aged 18–65 were included in the calculation of survival using the SEER database. When the age range was not given in the trial, the age range in the SEER analysis was selected in such a way that the median age was the same as in the trial and the width of the range was 50 years centered on the median age (i.e., the age range used was the median age ± 25 years). The latter criterion was defined based on the observation that the width of the age range varied in the range of 38–70 years when reported, with the median range being about 50 years.
In order to assess the effect of publication of studies on survival in patients with CML, we calculated the age-adapted 5-year survival rate for the 5 years prior to and including the year of publication and the 5 years following publication for the identified studies as well. Because changes in the treatment of CML and therefore in survival in patients treated in the community would likely start after the publication of the first data from a trial, the date of first publication of results was used for this calculation.
All analyses were performed with the SAS software package, version 9.2 (SAS Institute, Inc., Cary, NC).
Results
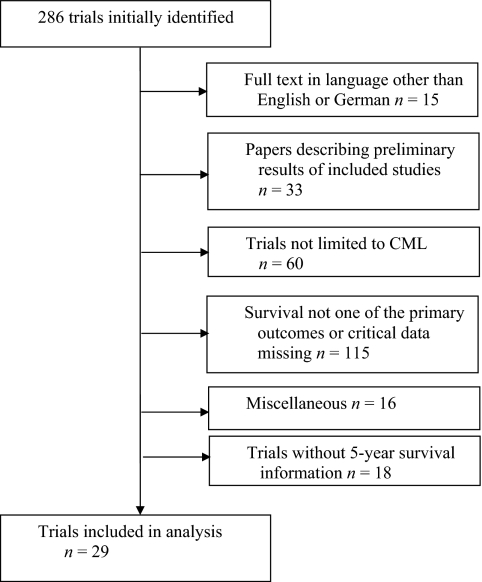
Two hundred eighty-six articles were initially identified in the search (Fig. 1). Fifteen articles were excluded because they were not in a language spoken by at least one of the authors. Thirty-three articles were excluded because they were preliminary reports of data for which final data were published in an article included in the final analysis. Sixty trials were excluded because the trials were not limited to CML patients. One hundred fifteen articles were excluded because the primary outcome addressed in the trial or analysis was not survival in CML patients or critical data were missing. Eighteen were excluded because of a lack of data on 5-year survival. Sixteen articles were excluded for other reasons (e.g., trials limited to children).
Figure 1.
Diagram of clinical trials identified, excluded, and included in this study.
Abbreviation: CML, chronic myelocytic leukemia.
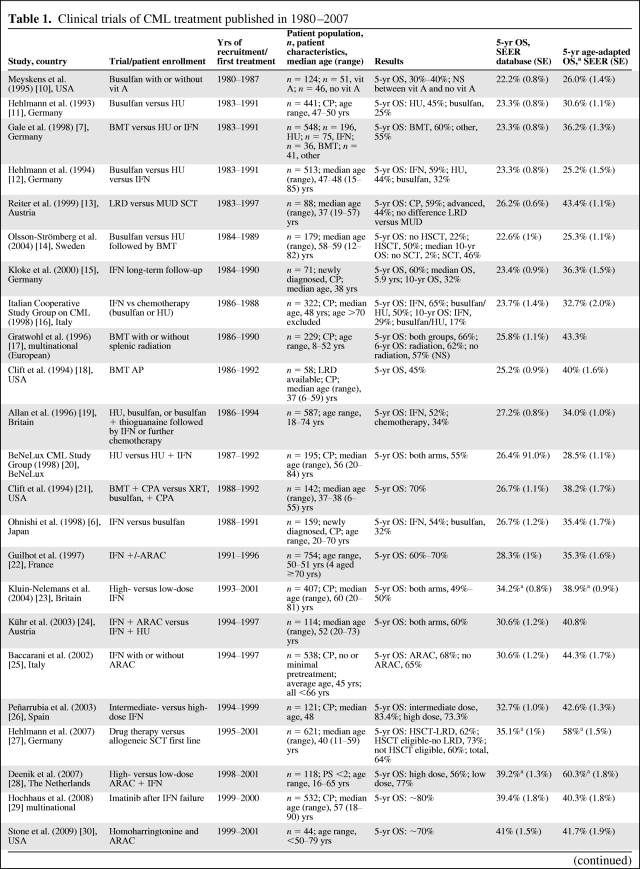
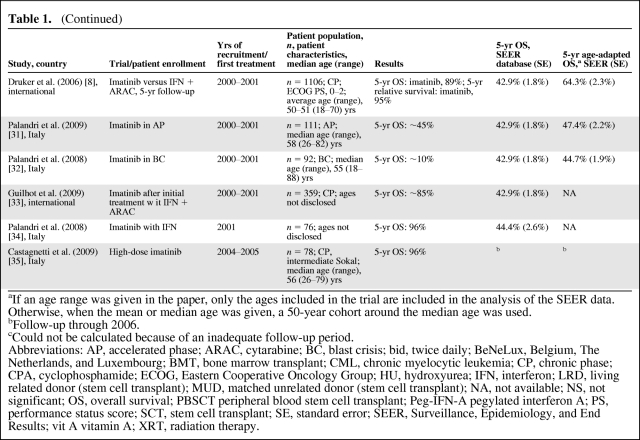
Twenty-nine trials of treatment for CML were identified (Table 1) [6–8, 10–35]. The years of recruitment of these clinical trials were 1980–1987 to 2004–2005. Trial size varied widely, in the range of 40–1,106 patients. The median age of patients in clinical trials was in the range of 37–60 years, compared with a median age of 62 years for patients in the SEER database. Most trials involved the treatment of patients in CP and, when performance status was mentioned, patients had an Eastern Cooperative Oncology Group performance status score of 0–2. Only two trials of patients in AP or BC that included 5-year survival data were identified. No major differences in the patient population or outcomes were observed when comparing trials based in the U.S. with those based in other countries.
Table 1.
Clinical trials of CML treatment published in 1980–2007
Table 1a.
(Continued)
aIf an age range was given in the paper, only the ages included in the trial are included in the analysis of the SEER data. Otherwise, when the mean or median age was given, a 50-year cohort around the median age was used.
bFollow-up through 2006.
cCould not be calculated because of an inadequate follow-up period.
Abbreviations: AP, accelerated phase; ARAC, cytarabine; BC, blast crisis; bid, twice daily; BeNeLux, Belgium, The Netherlands, and Luxembourg; BMT, bone marrow transplant; CML, chronic myelocytic leukemia; CP, chronic phase; CPA, cyclophosphamide; ECOG, Eastern Cooperative Oncology Group; HU, hydroxyurea; IFN, interferon; LRD, living related donor (stem cell transplant); MUD, matched unrelated donor (stem cell transplant); NA, not available; NS, not significant; OS, overall survival; PBSCT peripheral blood stem cell transplant; Peg-IFN-A pegylated interferon A; PS, performance status score; SCT, stem cell transplant; SE, standard error; SEER, Surveillance, Epidemiology, and End Results; vit A vitamin A; XRT, radiation therapy.
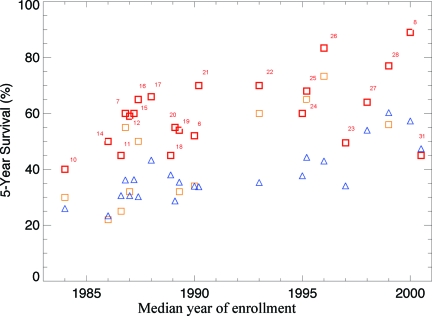
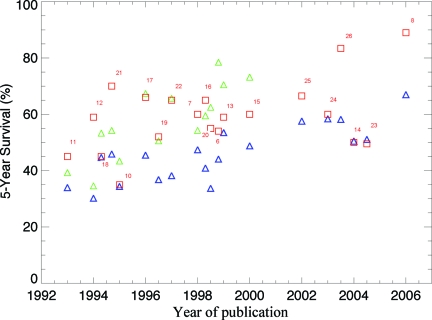
The 5-year survival rate increased with year of recruitment, from a range of 30%–40% in the earliest trials to 96% in some trials of TKIs (Table 1, Fig. 2). The 5-year survival rate in the general population calculated from the SEER database for the corresponding calendar periods ranged from 22.2% in 1980–1987 to 44.4% in 2001. Overall, the 5-year survival rate was higher in patients enrolled in clinical trials than in patients treated outside a clinical trial in the same years as in the trial enrolment. Age-adapted survival showed less variation but, with the exception of trials of AP or BC patients, the survival rate was still higher in patients in clinical trials than in the general population (Table 1).
Figure 2.
The 5-year survival rate of patients participating in clinical trials (squares) and age-adapted survival for patients in the Surveillance, Epidemiology, and End Results database diagnosed in the same time period as patients in the related clinical trial (triangles) are shown. In trials in which there was a difference in survival between arms of the trial, the higher survival rate is given as a thicker, red square; the lower survival rate is shown as a lighter, orange square. Reference numbers for the relevant trial are given next to the red squares.
Changes in the standard of care based on results of clinical trials can occur only after the trial results are published, and therefore comparison of survival immediately before and after the dates of publication of clinical trials is of interest. Figure 3 compares the age-adapted population-based 5-year survival rate of the patients diagnosed in the 5 years before (including the year of) and 5 years after the publication of trial results with the 5-year survival rate of patients in the better arm of the relevant trial (recruitment of patients completed 2–15 years prior to publication of each trial). The 5-year survival rate for the years before and including the year of publication was calculated for 20 studies (not calculated for nine because the publication date was too recent to allow calculation of a 5-year survival rate using the SEER database.) In 17 of the 20 studies, the survival rate was higher in the best arm of the study than in the general population, as calculated from the SEER database (Fig. 3). Two of the exceptions showed a marginally higher survival rate in the SEER database, whereas one, which was published in 2004 but enrolled patients in the 1980s, had a clearly higher survival rate for patients in the SEER database.
Figure 3.
The 5-year survival rate of patients participating in clinical trials (squares) and the age-adapted survival rate for patients in the Surveillance, Epidemiology, and End Results database for the 5 years prior to and including the year of publication of the relevant trial (blue triangles) and the 5 years following publication of the relevant trial (green triangles) are shown. If survival differed in different arms of the clinical trial, the arm with the best survival was used. Reference numbers for the relevant trials are included next to squares representing trials.
The 5-year survival rate for the patients in the SEER database in the 5 years following publication of the relevant comparison study was calculated for 14 studies (not calculated for 15 because the publication date was too recent to allow calculation of the 5-year survival rate using the SEER database). The 5-year survival rate for the 5 years following the publication of the relevant clinical trial was higher than in the preceding 5 years in all cases. However, the survival rate calculated from the SEER database was still lower than or similar to the survival rate in the relevant trial for eight of 14 studies (Fig. 3).
Discussion
Controversy exists as to what extent survival in clinical trials can be generalized to survival in patients in the general community and whether participation in a clinical trial is, in itself, a positive prognostic factor [36, 37]. Our results suggest that the difference in survival can range from minimal to very large, depending on the particular characteristics of a given trial, both in terms of the treatment being tested and patients involved (i.e., their age, disease stage, and general health).
Overall, patients with CML treated in clinical trials tend to be younger and are more likely to be in CP and have a good performance status than patients in the general population. In general, the 5-year survival rate for patients in the general population is lower than that for patients enrolled in clinical trials, even after adjusting for differences in the median age of patients in clinical trials.
Survival in CML patients in the SEER database has improved over time, but rarely reaches levels similar to those seen in clinical trials. When the age-adapted 5-year survival rate was compared between the best arm of a clinical trial and the general population for 5 years after the trial's publication, the survival rate from the trial was still comparable with or better than the survival rate in the general population in eight of 14 studies. It should be noted that the publications cited in this manuscript represent the final publication of a given study, so the study may have a minor effect on treatment choices of physicians treating off study even before the year cited as being the year of publication; that is, preliminary papers or abstracts presented at conferences may influence care even before the final paper is published. However, the standard of care, and therefore the treatment given to the majority of patients not participating in clinical trials, is unlikely to change prior to the publication of final results of clinical trials. Overall, this confirms that, although treatment improves over time as a result of clinical trials, survival in clinical trials is not a good predictor of survival expectations for patients who are treated outside the context of a clinical trial.
Two notable exceptions to the general trend, showing better survival in the SEER database, were two trials of survival in patients receiving imatinib in AP or BC. One of the limitations of the SEER database for hematologic malignancies is its lack of information on stage. Because the SEER population is an unselected population, it is likely that the majority of patients in the SEER database at any given time point are in the CP of disease simply because patients with CML do not spend a prolonged period of time in AP or BC. Of course, the disease may evolve into a more aggressive stage during the follow-up period.
Because of the lack of information on chemotherapy in the SEER database, most statements concerning the reasons for the differences observed are speculative. However, the difference between the survival rate in clinical trials and in the general population most likely can be accounted for by multiple, overlapping reasons. First, patients who participate in phase I–III clinical trials in oncology tend to be less “sick” overall than the general population: most trials require patients to have a good performance status and serious comorbidities disqualify patients from the vast majority of clinical trials. Although no information on comorbidities or performance status is given in the SEER database, given its nonselective nature, it is safe to assume that more people with a poor performance status or severe comorbidities are found in the SEER database than in clinical trials, which frequently require a specified performance status and organ function for participation. Thus, patients in clinical trials may be less likely to die as a result of comorbid illnesses and more likely to be able to tolerate intensive therapy. Notably, survival estimates from the SEER database tend to be close to survival rates from clinical trials of relatively low intensity therapy (i.e., hydroxyurea and TKIs), whereas they are uniformly far lower than survival rates from trials of intensive therapy (i.e., stem cell transplantation or interferon).
Practitioners may not adopt therapies shown in clinical trials to have survival advantages for a significant amount of time: initial trials may be small or limited to patient populations different from the typical patient, the advantage of the therapy may be controversial, the treatment may be unsuitable for the practitioner's patients, or practitioners may simply not be aware of the trials' findings or have immediate access to the relevant medication. Even in cases when the advantage is clear and well publicized, adoption of the new therapy is not instantaneous. For example, the use of imatinib in leukemia increased linearly between 2001 and 2005 in a study of drug uptake in several European countries [38]. Hence, uptake of new medications or techniques may not be complete even 5 years after clinical trials demonstrating their efficacy. However, because of the lack of chemotherapy data in the SEER database, the extent to which this contributes to the observed differences could not be directly evaluated. Finally, patients who become involved in clinical trials may be self-selected for better compliance and therefore better outcomes. A recent study of compliance with imatinib in patients with CML showed that approximately one third of patients taking imatinib, a relatively simple and well-tolerated medication, as part of standard therapy for CML were nonadherent to their planned medication and that nonadherence was associated with a lower rate of complete response [39].
In interpreting our data, some limitations must be considered. First, as mentioned earlier, the SEER database does not include data on stage or chemotherapy and biological therapy, and therefore no direct comparison of patients receiving a given therapy on or off protocol can be made and survival cannot be stratified based on stage. Second, because the SEER database is based on U.S. data only, whereas many of the clinical trials were performed in Europe, Canada, or Asia, with many being multinational, some differences in survival observed in clinical trials may be a result of differences in survival rates in the country or countries the trial took place in versus the U.S. Because of the collaborative nature of the SEER database, there is no central pathology review of cases in the database, which can introduce bias through pathological misclassification. Additionally, with the exception of prior cancers, the SEER database does not contain information on comorbidities, making it difficult to judge the overall health status of patients in the database.
The literature review has a number of limitations. The initial screening identified papers based on whether they were classified in PubMed as “clinical trials” or not. It is possible that some papers were miscategorized and therefore improperly excluded from the analysis. Additionally, the review was limited to studies of CML in adults only, potentially limiting information about patients with CML treated on more general protocols. This may be most relevant to stem cell transplant protocols, which may report results for patients with more than one type of leukemia. Finally, as with the SEER data, the literature review is limited by the heterogeneity of the studies.
In conclusion, the survival rate from clinical trials of treatment for CML is higher than the survival rate of patients with CML in the general population. This difference can be attributed to access to better medications, a bias toward selecting younger, healthier patients for clinical trials, and the time necessary for new treatments to be adopted by practitioners. Additionally, socioeconomic factors, such as compliance, medical literacy, and access to medical care, may vary between patients in clinical trials and in the general population. This finding underscores the need for population-based studies to give a more realistic idea of survival for patients with a given malignancy in the general population. The inclusion of a more diverse patient population in clinical trials, including older and less fit patients, may reduce the disparity.
Acknowledgment
The authors would like to thank Mordecai-Mark Mac Low for his assistance in generating the figures.
Author Contributions
Conception/Design: Hermann Brenner
Administrative support: Hermann Brenner
Collection and/or assembly of data: Dianne Pulte
Data analysis and interpretation: Dianne Pulte, Adam Gondos, Maria Theresa Redaniel, Hermann Brenner
Manuscript writing: Dianne Pulte, Adam Gondos, Maria Theresa Redaniel, Hermann Brenner
Final approval of manuscript: Dianne Pulte, Adam Gondos, Maria Theresa Redaniel, Hermann Brenner
References
- 1.Svensson CK. Representation of American blacks in clinical trials of new drugs. JAMA. 1989;261:263–265. [PubMed] [Google Scholar]
- 2.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 3.Yessaian A, Mendivil AA, Brewster WR. Population characteristics in cervical cancer trials: Search for external validity. Am J Obstet Gynecol. 2005;192:407–413. doi: 10.1016/j.ajog.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Begg CB, Engstrom PF. Eligibility and extrapolation in cancer clinical trials. J Clin Oncol. 1987;5:962–968. doi: 10.1200/JCO.1987.5.6.962. [DOI] [PubMed] [Google Scholar]
- 5.Brenner H, Gondos A, Pulte D. Recent trends in long-term survival of patients with chronic myelocytic leukemia: Disclosing the impact of advances in therapy on the population level. Haematologica. 2008;93:1544–1549. doi: 10.3324/haematol.13045. [DOI] [PubMed] [Google Scholar]
- 6.Ohnishi K, Tomonaga M, Kamada N, et al. A long term follow-up of a randomized trial comparing interferon-alpha with busulfan for chronic myelogenous leukemia. The Kouseisho Leukemia Study Group. Leuk Res. 1998;22:779–786. doi: 10.1016/s0145-2126(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 7.Gale RP, Hehlmann R, Zhang MJ, et al. Survival with bone marrow transplantation versus hydroxyurea or interferon for chronic myelogenous leukemia. The German CML Study Group. Blood. 1998;91:1810–1819. [PubMed] [Google Scholar]
- 8.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 9.Surveillance, Epidemiology, and End Results (SEER) Program Limited Use Data (1973–2006) Bethesda, MD: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2009. [accessed March 28, 2011]. Released April, 2009 based on November 2008 submission. Available online at http://seer.cancer.gov/data/index. [Google Scholar]
- 10.Meyskens FL, Jr, Kopecky KJ, Appelbaum FR, et al. Effects of vitamin A on survival in patients with chronic myelogenous leukemia: A SWOG randomized trial. Leuk Res. 1995;19:605–612. doi: 10.1016/0145-2126(95)00032-j. [DOI] [PubMed] [Google Scholar]
- 11.Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of busulfan and hydroxyurea in chronic myelogenous leukemia: Prolongation of survival by hydroxyurea. The German CML Study Group. Blood. 1993;82:398–407. [PubMed] [Google Scholar]
- 12.Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064–4077. [PubMed] [Google Scholar]
- 13.Rieter E, Greinix HT, Keil F, et al. Long-term follow-up of patients after related- and unrelated-donor bone marrow transplantation for chronic myelogenous leukemia. Ann Hematol. 1999;78:507–513. doi: 10.1007/s002770050547. [DOI] [PubMed] [Google Scholar]
- 14.Olsson-Strömberg U, Simonsson B, Ahlgren T, et al. Comparison of busulphan, hydroxyurea and allogeneic bone marrow transplantation (BMT) in chronic myeloid leukemia: BMT prolongs survival. Hematol J. 2004;5:462–466. doi: 10.1038/sj.thj.6200552. [DOI] [PubMed] [Google Scholar]
- 15.Kloke O, Opalka B, Niederle N. Interferon alfa as primary treatment of chronic myeloid leukemia: Long-term follow-up of 71 patients observed in a single center. Leukemia. 2000;14:389–392. doi: 10.1038/sj.leu.2401661. [DOI] [PubMed] [Google Scholar]
- 16.The Italian Cooperative Study Group on Chronic Myeloid Leukemia. Long-term follow-up of the Italian trial of interferon-alpha versus conventional chemotherapy in chronic myeloid leukemia. Blood. 1998;92:1541–1548. [PubMed] [Google Scholar]
- 17.Gratwohl A, Hermans J, van Biezen A, et al. Splenic irradiation before bone marrow transplantation for chronic myeloid leukaemia. Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 1996;95:494–500. doi: 10.1046/j.1365-2141.1996.d01-1929.x. [DOI] [PubMed] [Google Scholar]
- 18.Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for patients in accelerated phase of chronic myeloid leukemia. Blood. 1994;84:4368–4373. [PubMed] [Google Scholar]
- 19.Allan NC, Richards SM, Shepherd PC. UK Medical Research Council randomised, multicentre trial of interferon-alpha n1 for chronic myeloid leukaemia: Improved survival irrespective of cytogenetic response. The UK Medical Research Council's Working Parties for Therapeutic Trials in Adult Leukaemia. Lancet. 1995;345:1392–1397. doi: 10.1016/s0140-6736(95)92596-1. [DOI] [PubMed] [Google Scholar]
- 20.Randomized study on hydroxyurea alone versus hydroxyurea combined with low-dose interferon-alpha 2b for chronic myeloid leukemia. The BeNeLux CML Study Group. Blood. 1998;91:2713–2721. [PubMed] [Google Scholar]
- 21.Clift RA, Bucknew CD, Thomas ED, et al. Marrow transplantation for chronic myeloid leukemia: A randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84:2036–2043. [PubMed] [Google Scholar]
- 22.Guilhot F, Chastang C, Michallet M, et al. Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. French Chronic Myeloid Leukemia Study Group. N Engl J Med. 1997;337:223–229. doi: 10.1056/NEJM199707243370402. [DOI] [PubMed] [Google Scholar]
- 23.Kluin-Nelemans HC, Buck G, le Cessie S, et al. Randomized comparison of low-dose versus high-dose interferon-alfa in chronic myeloid leukemia: Prospective collaboration of 3 joint trials by the MRC and HOVON groups. Blood. 2004;103:4408–4415. doi: 10.1182/blood-2003-10-3605. [DOI] [PubMed] [Google Scholar]
- 24.Kühr T, Burgstaller S, Apfelbeck U, et al. A randomized study comparing interferon (IFN alpha) plus low-dose cytarabine and interferon plus hydroxyurea (HU) in early chronic-phase chronic myeloid leukemia (CML) Leuk Res. 2003;27:405–411. doi: 10.1016/s0145-2126(02)00223-0. [DOI] [PubMed] [Google Scholar]
- 25.Baccarani M, Rosti G, de Vivo A, et al. A randomized study of interferon-alpha versus interferon-alpha and low-dose arabinosyl cytosine in chronic myeloid leukemia. Blood. 2002;99:1527–1535. doi: 10.1182/blood.v99.5.1527. [DOI] [PubMed] [Google Scholar]
- 26.Peñarrubria MJ, Odriozola J, Gonzalez C, et al. A randomized study of intermediate as compared with high doses of interferon-alpha for chronic myeloid leukemia: No differences in cytogenetic responses. Ann Hematol. 2003;82:750–758. doi: 10.1007/s00277-003-0724-z. [DOI] [PubMed] [Google Scholar]
- 27.Hehlmann R, Berger U, Pfirrmann M, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007;109:4686–4692. doi: 10.1182/blood-2006-11-055186. [DOI] [PubMed] [Google Scholar]
- 28.Deenik W, van der Holt B, Verhoef GE, et al. High-vs low-dose cytarabine combined with interferon alfa in patients with first chronic phase chronic myeloid leukemia. A prospective randomized phase III study. Ann Hematol. 2007;86:117–125. doi: 10.1007/s00277-006-0186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hochhaus A, Druker B, Sawyers C, et al. Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-alpha treatment. Blood. 2008;111:1039–1043. doi: 10.1182/blood-2007-07-103523. [DOI] [PubMed] [Google Scholar]
- 30.Stone RM, Donohue KA, Stock W, et al. A phase II study of continuous infusion of homoharringtonine and cytarabine in newly diagnosed patients with chronic myeloid leukemia: CALGB study 19804. Cancer Chemother Pharmacol. 2009;63:859–864. doi: 10.1007/s00280-008-0805-8. [DOI] [PubMed] [Google Scholar]
- 31.Palandri F, Castagnetti F, Alimena G, et al. The long-term durability of cytogenetic responses in patients with accelerated phase chronic myeloid leukemia treated with imatinib 600 mg: The GIMEMA CML Working Party experience after a 7-year follow-up. Haematologica. 2009;94:205–212. doi: 10.3324/haematol.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palandri F, Castagnetti F, Testoni N, et al. Chronic myeloid leukemia in blast crisis treated with imatinib 600 mg: Outcome of the patients alive after a 6-year follow-up. Haematologica. 2008;93:1792–1796. doi: 10.3324/haematol.13068. [DOI] [PubMed] [Google Scholar]
- 33.Guilhot F, Druker B, Larson RA, et al. High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: Results from the International Randomized Study of Interferon and STI571 (IRIS) trial. Haematologica. 2009;94:1669–1675. doi: 10.3324/haematol.2009.010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palandri F, Iacobucci I, Castagnetti F, et al. Front-line treatment of Philadelphia positive chronic myeloid leukemia with imatinib and interferon-alpha: 5-year outcome. Haematologica. 2008;93:770–774. doi: 10.3324/haematol.12265. [DOI] [PubMed] [Google Scholar]
- 35.Castagnetti F, Palandri F, Amabile M, et al. Results of high-dose imatinib mesylate in intermediate Sokal risk chronic myeloid leukemia patients in early chronic phase: A phase 2 trial of the GIMEMA CML Working Party. Blood. 2009;113:3428–3434. doi: 10.1182/blood-2007-08-103499. [DOI] [PubMed] [Google Scholar]
- 36.Braunholtz DA, Edwards SJL, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect.”. J Clin Epidemiol. 2001;54:217–224. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 37.Davis S, Wright PW, Schulman SF, et al. Participants in prospective, randomized clinical trials for resected non-small cell lung cancer have improved survival compared with nonparticipants in such trials. Cancer. 1985;56:1710–1718. doi: 10.1002/1097-0142(19851001)56:7<1710::aid-cncr2820560741>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 38.Kos M, Obradovic M, Mrhar A. Accessibility to targeted drugs in Slovenia and selected European countries. Eur J Cancer. 2008;44:408–418. doi: 10.1016/j.ejca.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 39.Noens L, van Liedre MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: The ADAGIO study. Blood. 2009;113:5401–5411. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]