The multitargeted tyrosine-kinase inhibitor sunitinib has emerged as one of the standards of care for good and intermediate metastatic renal cell carcinoma. Fatigue, diarrhea, anorexia, oral changes, hand-foot syndrome and other skin toxicity, thyroid dysfunction, myelotoxicity, and hypertension seem to be the most common and clinically relevant toxicities of sunitinib. The proactive assessment and consistent and timely management of sunitinib-related side effects is critical to ensure optimal treatment benefit by allowing appropriate drug dosing and prolonged treatment periods.
Keywords: Sunitinib, Toxicity, Side effect management, Treatment optimization
Abstract
The multitargeted tyrosine-kinase inhibitor sunitinib has emerged as one of the standards of care for good- and intermediate-risk metastatic renal cell carcinoma. Although generally associated with acceptable toxicity, sunitinib exhibits a novel and distinct toxicity profile that requires monitoring and management. Fatigue, diarrhea, anorexia, oral changes, hand-foot syndrome and other skin toxicity, thyroid dysfunction, myelotoxicity, and hypertension seem to be the most common and clinically relevant toxicities of sunitinib. Drug dosing and treatment duration are correlated with response to treatment and survival. Treatment recommendations for hypertension have been published but, currently, no standard guidelines exist for the management of noncardiovascular side effects. To discuss the optimal management of noncardiovascular side effects, an international, interdisciplinary panel of experts gathered in November 2009. Existing literature on incidence, severity, and underlying mechanisms of side effects as well as on potential treatment options were carefully reviewed and discussed. On the basis of these proceedings and the thorough review of the existing literature, recommendations were made for the monitoring, prevention, and treatment of the most common noncardiovascular side effects and are summarized in this review. The proactive assessment and consistent and timely management of sunitinib-related side effects are critical to ensure optimal treatment benefit by allowing appropriate drug dosing and prolonged treatment periods.
Introduction
The introduction of targeted therapies has substantially changed the treatment landscape for patients with metastatic renal cell carcinoma (mRCC). Sunitinib, a member of the rapidly expanding family of vascular endothelial growth factor receptor (VEGFR) multitargeted tyrosine kinase inhibitors (TKIs), is considered one of the standards for first-line treatment of metastatic clear cell type renal cell carcinoma [1–3]. Sunitinib inhibits not only VEGFRs (VEGFR-1, VEGFR-2, and VEGFR-3) but also other tyrosine kinases including platelet-derived growth factor receptors (PDGFR-α and PDGFR-β), stem cell factor receptor (KIT), and FMS-like tyrosine kinase-3 receptor at nanomolar concentrations [4, 5].
It is now widely accepted that TKIs have a unique mechanism of action and are associated with a distinct pattern of toxicities not previously encountered in clinical oncology. Although sunitinib generally has an acceptable toxicity profile, some side effects require careful monitoring and treatment to achieve optimal patient outcomes. In clinical practice, the most common side effects of sunitinib treatment are fatigue/asthenia, anorexia/loss of appetite, hypothyroidism, hand-foot syndrome (HFS; also called hand-foot skin reaction), stomatitis/taste changes, diarrhea/abdominal pain, myelosuppression, and hypertension.
Three key interlinked areas have emerged as being essential for the optimal use of sunitinib in mRCC: dosing and schedule, treatment duration, and proactive side effect management. Only when all of these three key areas are optimally managed can the maximum benefit be achieved for each patient. Clear dose-response and dose-survival relationships have been documented for sunitinib, demonstrating the importance of maintaining the maximum dose of sunitinib [6]. The continuous treatment administration with only short breaks is important because tumor progression may occur during periods of treatment interruption or discontinuation. This continuous treatment application makes side effect management critical, and such long-term therapy requires individualized management of the delicate balance between toxicity and dose intensity to maximize patient benefit, which in some cases may extend for several years.
Knowledge about and optimal proactive management of acute side effects is therefore essential and may help to reduce patient discomfort and avoid unnecessary dose reductions, treatment interruptions during treatment, or even early treatment discontinuation of sunitinib treatment. Patients receiving therapy with sunitinib should be monitored by a qualified physician and/or oncology nurse experienced in the use of anticancer agents and should be counseled on the potential for treatment-related side effects, including the importance of maintaining optimal dose and therapy duration.
This article summarizes the current knowledge about the pathophysiology of the predominant noncardiovascular side effects and provides treatment recommendations developed by a multidisciplinary expert panel that consisted of medical oncology, dermatology, endocrinology, oral medicine, palliative care medicine, and oncology nursing.
Methods
A multidisciplinary panel of experts gathered on November 13, 2009, in Boston to discuss management of the side effects of sunitinib treatment. Each topic was reviewed by one or more experts prior to the meeting, including a review of the available literature. At the meeting, each topic and the available data were introduced by presentation of data, provided by Pfizer Inc., from the sunitinib versus interferon (IFN)-α randomized trial (RCC study 1034; NCT00083889) and the sunitinib expanded-access program (RCC study 1037; NCT00130897). Study 1034 was a phase III trial designed to compare the efficacy and safety of sunitinib versus IFN-α as first-line treatment in 750 patients with mRCC (375 patients in each treatment group) [1]. Study 1037 enabled patients with mRCC access to sunitinib who were not eligible to participate in clinical trials [7]. A total of 4,564 patients with mRCC were enrolled in this international, open-label program. The data included side effect incidence rates, side effect development over time, frequency of dose reductions, and toxicity-related treatment discontinuation. Toxicity in both studies was graded according to the NCI Common Toxicity Criteria for Adverse Events [NCI CTCAE], version 3 [8].
The objectives of the meeting were to present and discuss the underlying mechanisms of key adverse events including fatigue/asthenia, hypothyroidism, HFS and other dermatologic events, oral toxicities, and myelotoxicity. Existing treatment recommendations were reviewed, and whenever possible, a consensus treatment recommendation/treatment algorithm was developed.
Dosing and Schedule
The approved and recommended starting dose for sunitinib is 50 mg daily for 4 weeks on-treatment followed by 2 weeks off-treatment (schedule 4/2). Lower starting doses should be considered only if there are significant concerns about potential toxicity. The standard dose reduction schema consists of 37.5 mg daily and 25 mg daily (schedule 4/2). However, a significant relationship between sunitinib exposure and efficacy/toxicity has been identified [6]. Patients with the highest exposure to sunitinib displayed not only a higher probability of a response and tumor shrinkage but also longer time to progression and, most importantly, longer overall survival. This underscores the great importance of maintaining patients on a 50-mg dose of sunitinib and striving to avoid any unnecessary dose reductions during treatment. Furthermore, minimizing the time off-therapy is important, because tumor progression may occur rapidly during treatment interruption. For patients who are unable to tolerate the 50 mg (schedule 4/2), the use of alternative dosing schedules could be guided by the above observations. Ideally the 50-mg dose should be maintained, but treatment duration may be reduced to 2–3 weeks and the period off-therapy may be reduced to 1 week. Such schedule changes have been studied in small subsets of patients [9–11]. On the basis of clinical experience, a 2 weeks on-treatment/1 week off-treatment schedule, which delivers the same cumulative sunitinib dose over a 6-week period, appears to be better tolerated by the majority of patients than the traditional 4/2 schedule. However, these schedules need to be confirmed in prospective studies and should currently not be used as standard schedules but be reserved for those patients who struggle with tolerability. Studies are ongoing using a 37.5-mg continuous dose.
Fatigue and Asthenia
Incidence
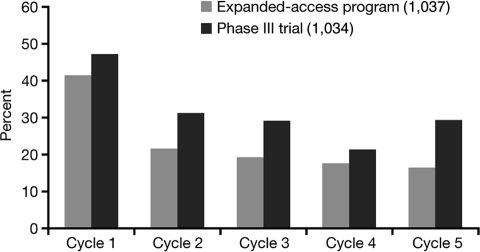
Fatigue and asthenia represent some of the most frequently encountered sunitinib-related side effects [2, 12, 13]. Fatigue is an acute or chronic condition marked by extreme tiredness and inability to function due to lack of energy. Asthenia includes weakness, lack of energy, and lack of strength. Approximately 50%–75% of mRCC patients complain about fatigue, although only 7%–11% experience severe fatigue interfering with the activities of daily living (NCI CTCAE grade 3) (Figure 1).
Figure 1.
Incidence of fatigue/asthenia decreases over cycles in the phase III study (1034) and the expanded-access program (1037).
Mechanism and Characteristics
It remains unclear what percentage of fatigue is cancer-related and what is sunitinib-associated, because both types of fatigue are very similar and often coexistent. Cancer-related fatigue is highly prevalent in mRCC patients, even those not receiving active anticancer therapy. To date, the mechanisms for both fatigue types (cancer-related fatigue and sunitinib-induced) are still poorly understood. Central fatigue in contrast to peripheral fatigue appears to play a major role in the loss of endurance in cancer patients [14]. Changes at the level of the muscle can cause peripheral fatigue, whereas central nervous system failure to drive or activate motoneurons adequately is considered central fatigue [15].
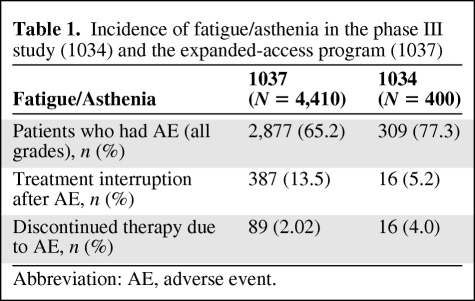
In studies, sunitinib-related fatigue was highly variable in both degree and duration. It appeared more common in men, particularly in young men, previously treated patients, and patients with repeated treatment interruptions. Typically, it occurred 2–3 weeks after treatment start, increased in intensity during weeks 3 and 4, and tended to improve during the 2-week off-treatment period. Although a recurrent problem, there did not appear to be an increase in intensity of fatigue/asthenia with increasing treatment cycles (Figure 1) but rather a decrease. Whether this phenomenon represents an adaptation and learning process by the patient or a true lower incidence remains unclear. However, fatigue/asthenia appeared to be an uncommon sole reason for dose modification (Table 1).
Table 1.
Incidence of fatigue/asthenia in the phase III study (1034) and the expanded-access program (1037)
Abbreviation: AE, adverse event.
Management Recommendations
A number of potential alternative causes for fatigue should be ruled out. Fatigue may be caused or exacerbated by underlying dehydration, hypothyroidism, hypercalcemia, anemia, or depression. It is important to ensure adequate fluid and nutritional intake. All patients should have thyroid function tests prior to and during sunitinib treatment and be treated according to standard medical practice (see also Hypothyroidism). Thyroid hormone replacement is recommended in mRCC patients with overt hypothyroidism (defined as an elevated serum thyroid-stimulating hormone (TSH) level and a low T4 level) and possible hypothyroidism-related symptoms [16]. Blood transfusions may be used at the discretion of the treating physician to correct anemia. Depression, a frequent finding in metastatic cancer patients, is known to impact upon the severity of fatigue. Fatigue improves in some patients who have received antidepressants or methylphenidate [17]. Heart failure can also be associated with fatigue.
Patient education about fatigue and its occurrence is very important. A warning that fatigue may occur, tools to manage fatigue when it presents, and a discussion of whom the patient will reach out to with issues and of whom will be their support, are helpful. Providing patients with pamphlets explaining side effects prior to initiating treatment is useful.
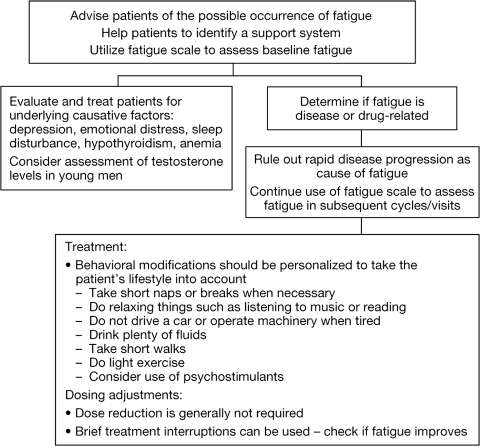
Currently, there are very few evidence-based interventions to treat fatigue. Significant fatigue/asthenia interfering with quality of life may be best managed by changes in dose and schedule as discussed above. Some general principles in the treatment of fatigue are shown in Figure 2. The minimum recommendations for exercise include resistance training or aerobic exercise three times a week for 30 minutes. Recent randomized trials demonstrate better response in patients using resistance training [18]. However, the current level of activity has to be taken into account when making exercise recommendations. Patients who are already very physically active may need advice on how to reduce their activity level. Psychostimulants have been tested and anecdotal evidence, as well as one review, suggested some benefit; however, a high placebo response was also seen. Further studies are needed to clarify the role of nutritional supplements such as L-carnitine, melatonin, and American ginseng in fatigue management [19–21].
Figure 2.
Recommendations for fatigue management.
Data from ongoing adjuvant trials in RCC will hopefully provide more clarity about the true incidence and degree of sunitinib-induced fatigue. Because these patients are categorized as disease-free, fatigue experienced by these patients would be more likely related to the effects of treatment rather than their disease. A clearer understanding of the molecular mechanisms causing sunitinib-related fatigue would allow more targeted treatment, which might enable better maintenance of drug levels throughout treatment.
Hypothyroidism
Incidence
One or more thyroid function test abnormalities developed in up to 85% of mRCC patients [16, 22–24]. There is a discrepancy between incidence rates reported in prospective trials and retrospective series, most likely due to infrequent testing for hypothyroidism, particularly in early studies, before hypothyroidism was recognized as a common side effect.
Thyroid dysfunction while receiving sunitinib can present as TSH elevation only with normal T4 levels (subclinical hypothyroidism) or TSH elevation and low T4 (overt hypothyroidism) that is more likely to be associated with clinical features of hypothyroidism. Brief episodes of temporary thyrotoxicosis due to thyroiditis, often followed by hypothyroidism, have also been described [25].
Mechanism and Characteristics
Sunitinib may cause hypothyroidism through its action on the VEGFR in the thyroid, but this is not likely to be the primary mechanism, as other VEGF inhibitors such as bevacizumab do not demonstrate similar effects. Although the exact mechanism by which this complication occurs remains unknown, the absence of visualized thyroid tissue preceded by TSH suppression reported in some patients suggests that sunitinib may induce a destructive thyroiditis through follicular cell apoptosis [16, 22]. Other possible mechanisms include endothelial dysfunction, regression of fenestrated capillaries, inhibition of iodine uptake, and reduced synthesis of thyroid hormone [24, 26, 27].
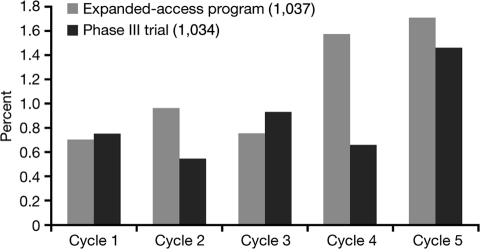
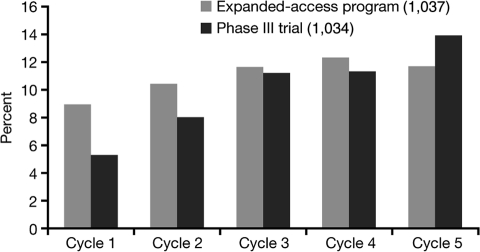
Hypothyroidism has been reported in patients receiving sunitinib as early as 1–2 weeks after initiation of therapy [27]. TSH tends to improve during the 2-week off-treatment period. In the sunitinib studies, incidence tended to increase over time (Figure 3), while severity did not seem to increase with cycles (data not shown). In retrospective series, up to 80% of patients with abnormal thyroid function tests developed symptoms consistent with hypothyroidism such as fatigue, anorexia, edema, fluid retention, or cold intolerance. Thyroid hormone replacement clinically benefited only about 40–50% of patients treated, suggesting other sunitinib-induced mechanisms for these side effects [16].
Figure 3.
Incidence of hypothyroidism by cycle in the phase III study (1034) and the expanded-access program (1037).
Interestingly, progression-free as well as overall survival have been suggested to be longer in patients who experience hypothyroidism compared with euthyroid patients (10.3 versus 3.6 months) [27]. It is not yet clear if this is a true direct effect or an indirect effect of sunitinib in patients with hypothyroidism. A positive correlation between hypothyroidism and improved clinical outcome has also been observed in breast cancer, brain cancer, and head and neck cancers. Although the development of subclinical hypothyroidism in patients treated with sunitinib appears to be associated with a better therapeutic outcome, there is no clinical data indicating that treatment of overt hypothyroidism worsens the outcome [28, 29].
Management Recommendations
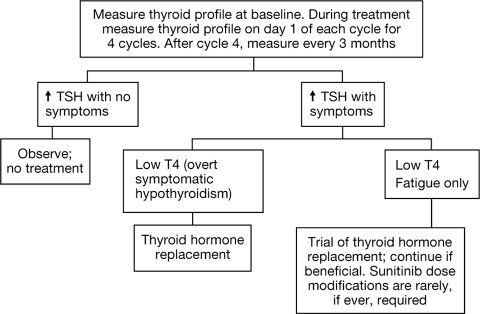
Regular surveillance of thyroid function is warranted in patients receiving sunitinib (Figure 4) [22]. Patients developing overt hypothyroidism should be treated with thyroid hormone replacement therapy. Typical levothyroxine doses should allow normalization of TSH concentrations and resolution of symptoms. Those with asymptomatic subclinical hypothyroidism can be followed without levothyroxine therapy and treated when and if overt hypothyroidism develops (Figure 4). Treatment interruptions (∼5% of patients in clinical trials) or even treatment discontinuation (<1% of patients in clinical trials) or dose modifications for thyroid dysfunction are generally not necessary.
Figure 4.
Recommendations for thyroid dysfunction management.
Skin Toxicity
Skin toxicity typically occurs after 3–4 weeks of treatment [30]. Observed skin changes include HFS, hair color changes, skin rash, dry skin, skin discoloration, acral erythema, and subungual splinter hemorrhages. HFS appears to be the most significant of these toxicities, whereas the other skin toxicities appear well manageable. Pre-existing skin conditions should be evaluated and treated prior to sunitinib therapy.
Hand-Foot Syndrome/Acral Erythema
Incidence
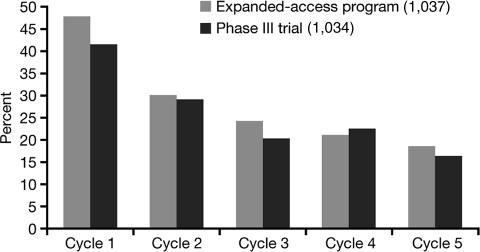
In the sunitinib studies, ∼30% of patients developed HFS (Figure 5). Hand-foot changes may present as painful symmetrical erythematous and edematous areas on the palms and soles, commonly preceded or accompanied by paraesthesias, tingling, or numbness. Desquamation can occur in severe cases. Painful hyperkeratotic areas on pressure points surrounded by rings of erythematous and oedematous lesions as well as painful bullous lesions, blisters, or skin cracking may be noted. These most often occur in areas of flexure or pressure. Pre-existing sole hyperkeratosis seems to confer a predisposition for painful sole involvement and functional consequences. Sunitinib-induced HFS may or may not interfere with function.
Figure 5.
Incidence of hand-foot syndrome by cycle in the phase III study (1034) and the expanded-access program (1037).
Although this syndrome may sometimes clinically resemble the more classic chemotherapy-induced HFS or palmar-plantar erythrodysaesthesia that can arise with 5-FU, cytarabine, capecitabine, or doxorubicin, most patients with sunitinib-induced HFS have more localized and hyperkeratotic lesions that are distinct from classic chemotherapy-induced HFS.
Mechanism and Characteristics
HFS often develops within the first 2–4 weeks of sunitinib administration [31]. The exact pathogenesis of this type of HFS is still unknown. Changes can been seen in the epidermal and dermal layers and followed throughout the course of HFS [32, 33]. The most consistent histologic changes are dermal vascular modifications with slight endothelial changes in grade 1–2 HFS and more pronounced vascular alterations with extensive and linear layers of keratinocyte necrosis and intraepidermal cleavage in grade 3 HFS and peribullous lesions [30, 34]. Because of the erythematous and tender nature of the HFS lesions, some postulate that an inflammatory infiltrate must be present; however, histologic findings indicate a mild lymphoid infiltrate usually devoid of eosinophils and rather prominent dilated capillaries (telangiectasias) [32, 34]. Others postulate that secretion of the TKI into the eccrine glands results in direct toxicity to the skin, as is the case in doxorubicin-associated HFS [32, 35, 36]. However, there is no direct evidence that TKIs are secreted by the eccrine glands, and the lack of cytotoxic, metaplastic, or structural changes of the eccrine units weigh against skin damage secondary to local sweat secretion [34].
Sunitinib induces endothelial cell apoptosis in vitro and in animal tumor models, and pathologic changes observed in this study suggest that dermal vessel alteration and apoptosis might be due to direct anti-VEGFR and/or anti-PDGFR effects of sunitinib on dermal endothelial cells [37]. Blockade of VEGFR and PDGFR by sunitinib promotes tumor vessel regression by interfering with endothelial cell survival and repair mechanisms [38]. When endothelial survival mechanisms are inhibited in palmoplantar high-pressure areas subjected to repeated trauma through walking, hand washing, and other daily activities, such as palms and soles, these areas may be unable to repair and thereby acquire the reactive characteristics of HFS [39, 40].
The dose-dependent relationship between sunitinib and HFS also suggests a direct toxic effect of sunitinib in HFS pathogenesis [40]. Because an overlap in targets for sorafenib and sunitinib lies in VEGFR and PDGFR inhibition, HFS appears to be an indirect effect of the inhibition of these proangiogenic pathways [33, 40, 41]. The combined inhibition of these receptors appears to be essential because PDGFR (imatinib) or VEGF (bevacizumab) inhibition alone does not result in a similar rate of HFS [42].
Management Recommendations
Management strategies for HFS are shown in Figure 6. Palliative intervention includes moisturizers, foot and hand care products (e.g., gel pad inserts, cotton gloves, and clobetasol propionate cream), and medication for pain management. Simple petroleum jelly based ointments (e.g., Vaseline, Aquaphor) are excellent moisturizers and can be applied liberally and often from the beginning of therapy. Patients should decrease pressure on affected areas, staying off feet when possible and avoiding friction/pressure to hands. During treatment, shock-absorbing shoe inserts may be used to relieve painful pressure points and supportive footwear with proper insoles and wicking socks to draw moisture from the plantar surface seem to be helpful [40].
Figure 6.
Recommendations for management of hand-foot syndrome.
Treatment of ≥grade 2 HFS usually includes the above discussed measures as well as dose interruptions, schedule alterations, and, if necessary, dose reductions as discussed previously. As pain generally abates quickly (usually within 2–3 days but may take 5 days or longer for higher grades) and the healing begins promptly on removal of the drug, brief (2–5 days) dose interruptions may provide substantial benefit while allowing for sustained long-term sunitinib therapy. Topical morphine, such as 10 mg combined with a petroleum jelly–based ointment (e.g., Aquaphor), can be used for patients experiencing severe pain. On the basis of anecdotal evidence, steroid creams can also be considered, although well-conducted studies are lacking. HFS is not an inflammatory response, but steroid creams may prevent secondary inflammatory processes from taking place. Creams can be applied three times a day and used with gloves overnight as recommended for moisturizers.
For some patients, topical skin adhesives (medical-grade superglue) applied to cracks and painful areas are an option. Although it is expensive and generally requires frequent application, it can help patients continue work or other activities of daily living. Grade 3 HFS almost always requires dose interruption and frequently subsequent reduction and/or schedule modifications.
If a patient believed to have HFS does not respond to dose interruption or dose reduction, then other diagnoses must be considered and, if necessary, treated, including fungal infection or overgrowth, dyshidrotic eczema, allergic contact dermatitis, and irritant dermatitis.
Skin Rash and Other Skin Toxicity
Generalized erythema, maculopapular or seborrheic dermatitis-like rashes have been reported in approximately 20%–25% of patients, with the vast majority being NCI CTCAE grade 1–2 [1–3, 30, 39]. Skin rashes associated with sunitinib treatment rarely require dose reduction, and symptoms tend to decrease over time.
Patients should be advised to use moisturizing skin creams frequently, in particular after showers and before bedtime. Urea-containing lotions may be helpful, in particular if the skin is very dry. Anti-itch formulas and anti-dandruff shampoos can be used if itch or scalp discomfort is present [40]. A recent study suggested a benefit in controlling tyrosine kinase-associated skin rash by using a colloidal oatmeal lotion [43]. Patients should avoid hot showers, use sun protection, and wear loose-fitting cotton clothes. Topical therapies, for example, steroid creams, may be used for severe cases.
Yellow discoloration of the skin due to the yellow color of the active drug and metabolite, as well as hair depigmentation, most likely caused by blockade of c-KIT signaling and other receptors, can occur. These effects are reversible; however, a bilirubin level may have to be determined to distinguish jaundice due to liver damage or biliary obstruction from sunitinib-induced skin discoloration [30]. Sunitinib-induced hair depigmentation is thought to be caused by blockade of c-KIT signaling, which is vitally important for both melanocyte proliferation/differentiation and proper pigment production [44, 45].
Oral Toxicity
Incidence
Oral changes, including sensitivity and taste changes, dry mouth, and oral mucosal sensitivity (often referred to as stomatitis/mucositis), occur with varying frequency, in ∼60% of patients. Most toxicities are ≤NCI CTCAE grade 2, and dose adjustments (<10%) or treatment discontinuation (<1%) are seldom necessary. Oral toxicities generally occur 7–14 days after the start of therapy, appear to increase throughout therapy, and improve during the 2-week off-treatment period. In the sunitinib studies, oral toxicities recurred in subsequent cycles, but the incidence appeared to decrease over these cycles (Figure 7).
Figure 7.
Incidence of oral toxicity by cycle in the phase III study (1034) and the expanded-access program (1037).
Mechanism and Characteristics
The oral reactions seen during sunitinib treatment differ from those seen in chemotherapy-induced mucositis, which is characterized by local tissue damage and an inflammatory reaction. Chemotherapy-related oral mucositis typically clusters with myelosuppression and mucositis throughout the gastrointestinal tract, causing diarrhea, nausea, and vomiting. Sunitinib-induced oral toxicity, in contrast, appears to be primarily a “functional” irritation of the mucosa. Patients report a general sensitivity in the mouth, which feels sore, or they have alterations in taste, but clinical findings are largely normal and patients do not experience the typical physical signs of a mucositis/stomatitis caused by chemotherapy (“functional stomatitis”). A single case exhibiting severe morphologic changes including bullous, painful, ulcerative tongue lesions and lichenoid and necrotizing palatal changes has been described [46]. However, anecdotally, none of the members of the interdisciplinary panel had ever seen such a reaction in their clinical practices.
Very few data are available to describe the reactions seen with sunitinib, and the exact mechanism of sunitinib-induced oral toxicities remains unknown. VEGF has been found to be a component of normal human saliva, suggesting that salivary VEGF may play a role in regulating physiologic and pathologic angiogenic and other vascular responses in salivary and mucosal tissues [47]. The VEGF and the MAP-kinase pathway may be involved in mucosal integrity/defense and repair; however, problems with integrity have not been observed, suggesting this is more likely neuropathically mediated.
Management Recommendations
Treatment for oral side effects is symptomatic only and consists mainly of a modified diet, nutritional consultation, and magic mouthwash (Table 2). Good oral care should be maintained throughout sunitinib therapy [48]. Magic mouthwash (Table 2) can be used for symptomatic relief. Oral toxicity can usually be managed symptomatically and rarely requires dose adjustment or treatment interruptions.
Table 2.
Recommendations for management of oral toxicities
Diarrhea
Incidence
Diarrhea occurs in ∼50% of patients, but grade 3/4 toxicity is rare and observed in only 3%–5% of cases. Some degree of diarrhea is often the main toxicity remaining when other common toxicities have been controlled with dose/schedule changes. In contrast to chemotherapy-induced diarrhea, which is usually continuous, sunitinib-induced diarrhea can occur irregularly with days of diarrhea mixed in with days of normal bowel movements.
Mechanisms and Characteristics
The underlying pathogenesis for sunitinib-induced diarrhea is not known. Bowel mucosa changes consistent with ischemic colitis have been reported after treatment with other VEGF interacting agents, in particular bevacizumab [49].
Management Recommendations
Dose reductions are rarely necessary for grades 1 and 2 toxicity, which can be managed by oral hydration and oral antidiarrheal agents, such as loperamide, as needed. Treatment should be interrupted for grade 3 or 4 diarrhea until diarrhea is grade ≤1 or has returned to baseline. Usually, the diarrhea resolves quickly in the 2-week off-treatment period between cycles. Dose/schedule changes, as discussed previously, are frequently required to control diarrhea in subsequent cycles.
Patients can be advised to temporarily discontinue use of stool softeners and some fiber supplements as well as magnesium-containing antacids, drink plenty of liquids (but in small amounts at a time, avoiding drinking fluids with meals and for 1 hour after), eat and drink often in small amounts, and avoid spicy foods, fatty foods, caffeine, and high-fiber foods.
Other Gastrointestinal Side Effects
A number of other gastrointestinal side effects, including taste changes, dry mouth, nausea, vomiting, and indigestion, occur with varying frequency (10%–30%). Dose adjustments or interruptions are seldom necessary. Anorexia is found in about 10–20% of patients but rarely exceeds grade 2. Although anorexia rarely require dose modifications, underlying causes should always be investigated, in particular a potential relationship to coexisting hypothyroidism and other gastrointestinal toxicities. Patient education regarding nutrition and consultation with a dietician is recommended.
The emetogenic potential of sunitinib is low. Less than 5% of patients experience grade 3 or 4 vomiting, and only 10%–20% experience grade 1–2 [1, 7]. Nausea appears to occur more frequently, and grade 3–4 nausea is rare. Common antiemetics can be used to relieve or prevent nausea and vomiting. However, particular care should be used when combining sunitinib with antidopamineric agents, such as domperidone, or 5HT3 antagonists, such as granisetron, ondansetron, and dolasetron, because they have been associated with QT/QTc interval prolongation and/or torsade de pointes (see Cardiac Toxicity) [50]. H2-blockers are recommended for the treatment of heartburn and indigestion.
Hematotoxicity
Incidence
Sunitinib induces neutropenia and thrombocytopenia in ∼20% of non-Asian patients. Only 5%–8% of patients develop grade 3/4 neutropenia or thrombocytopenia, and very few cases of neutropenic fever have yet been reported. Blood counts usually recover quickly within the 2-week treatment break. Ethnic background appears to impact on the incidence of hematotoxicity. Recent data suggest a significantly higher incidence of myelotoxicity, in particular neutropenia and thrombocytopenia, in Asian patient populations [51].
Mechanisms and Characteristics
Sunitinib most likely induces myelotoxicity through inhibition of c-KIT. c-KIT is well known for its role in hematopoiesis and melanocyte differentiation [52].
Management Recommendations
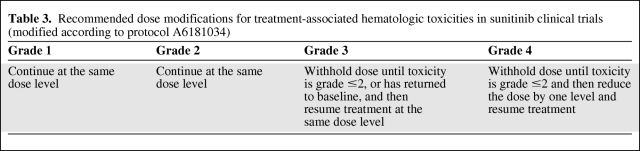
A substantially higher incidence of grade 3/4 neutropenia and thrombocytopenia has been observed in Asian patients and may require closer observation in these patients. Recurring grade 3/4 neutropenia or thrombocytopenia persisting for at least 5 days and/or neutropenic fever/bleeding signs may require dose/schedule changes (Table 3). Patients with asymptomatic grade 3 or 4 neutropenia at the end of the 4-week treatment period and rapid recovery in the 2-week off-treatment period may not require a dose reduction, but a schedule change may be considered.
Table 3.
Recommended dose modifications for treatment-associated hematologic toxicities in sunitinib clinical trials (modified according to protocol A6181034)
Because of the usually rapid recovery of blood counts during the 2-week off-treatment period, hematologic growth factors are rarely indicated. Grade 3/4 anemia usually does not require dose modification. Because of the recent discussion about the potential risks and toxicities, the use of erythropoietin-stimulating agents should be cautioned.
Conclusions
Sunitinib has demonstrated significant efficacy in the first-line treatment of mRCC. However, the unique toxicities associated with targeted therapies pose a new challenge for the healthcare team. It has become clear that effective toxicity management is a key requirement for achieving the maximum benefit for the patient, because continuous therapy and dose intensity are important and dose reductions should be avoided whenever possible.
Most sunitinib toxicities are typically mild to moderate in intensity and are generally manageable with standard medical interventions, without treatment discontinuation or permanent dose reduction. However, the accumulation of several lower-grade side effects can represent a substantial challenge and often require dose/schedule changes and, in some cases, treatment termination.
Hypertension, hypothyroidism, myelosuppression, and most gastrointestinal side effects only occasionally necessitate treatment interruption and permanent dose reduction or even treatment termination, whereas HFS, fatigue, stomatitis, and diarrhea more frequently require dose reductions and even treatment discontinuation.
Patient education about potentially bothersome side effects is an important part of toxicity prevention and treatment. Effective communication within the health care team and with the patients is key to successful toxicity management in patients with mRCC.
Little is known about the mechanisms leading to these side effects, which makes causal treatment of side effects impossible. Their exploration remains a priority to improve management. Thus, symptomatic management is the only treatment for most side effects. A number of general recommendations for cancer treatment-related side effects have been published [53–55].
The impact of pharmacogenomics on the incidence and severity of side effects is poorly understood. Recent evidence has suggested that heterogeneity in toxicity and efficacy among patients receiving anti-VEGF therapy could be at least partially explained by genomic variability, including single-nucleotide polymorphisms, providing a possible explanation for the differences in toxicity frequencies between Asians and non-Asians in this analysis. Female gender, age, and low body surface area have also been reported to predict for severe side effects. A better understanding of genetic and non-genetic determinants of sunitinib toxicity should help to optimize drug treatment in individual patients. The prospective exploration of alternative schedules is needed to minimize toxicity while maintaining efficacy.
Acknowledgment
The meeting in Boston on November 19, 2009, at which the data presentation, interpretation, and discussion took place, was supported by Pfizer Inc.
Author Contributions
Conception/Design: Christian Kollmannsberger, Georg Bjarnason, Patrick Burnett, Patricia Creel, Mellar Davis, Nancy Dawson, Darren Feldman, Suzanne George, Jerome Hershman, Thomas Lechner, Amy Potter, Eric Raymond, Nathaniel Treister, Laura Wood, Shenhong Wu, Ronald Bukowski
Provision of study material or patients: Christian Kollmannsberger, Georg Bjarnason, Patrick Burnett, Patricia Creel, Mellar Davis, Nancy Dawson, Darren Feldman, Suzanne George, Jerome Hershman, Thomas Lechner, AmyPotter, Eric Raymond, Nathaniel Treister, Laura Wood, Shenhong Wu,Ronald Bukowski
Collection and/or assembly of data: Christian Kollmannsberger, Georg Bjarnason, Patrick Burnett, Patricia Creel, Mellar Davis, Nancy Dawson, Darren Feldman, Suzanne George, Jerome Hershman, Thomas Lechner, Amy Potter, Eric Raymond, Laura Wood, Shenhong Wu, Ronald Bukowski
Data analysis and interpretation: Christian Kollmannsberger, Georg Bjarnason, Patrick Burnett, Patricia Creel, Mellar Davis, Nancy Dawson, Darren Feldman, Suzanne George, Jerome Hershman, Thomas Lechner, Amy Potter, Eric Raymond, Nathaniel Treister, Laura Wood, Shenhong Wu, Ronald Bukowski
Manuscript writing: Christian Kollmannsberger, Georg Bjarnason, Patrick Burnett, Patricia Creel, Mellar Davis, Nancy Dawson, Darren Feldman, Suzanne George, Jerome Hershman, Thomas Lechner, Amy Potter, Eric Raymond, Nathaniel Treister, Laura Wood, Shenhong Wu, Ronald Bukowski
Final approval of manuscript: Christian Kollmannsberger, Georg Bjarnason, Patrick Burnett, Patricia Creel, Mellar Davis, Nancy Dawson, Darren Feldman, Suzanne George, Jerome Hershman, Thomas Lechner, Amy Potter, Eric Raymond, Nathaniel Treister, Laura Wood, Shenhong Wu, Ronald Bukowski
The authors take full responsibility for the content of the paper but thank Caroline Masterman at ACUMED (Tytherington, U.K.) supported by Pfizer, Inc., for her editorial assistance with the figures and text formatting.
References
- 1.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol. 2005;23:1028–1043. doi: 10.1200/JCO.2005.01.186. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24:5601–5608. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 6.Houk BE, Bello CL, Poland B, et al. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2009;66:357–371. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 7.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 8.CTEP NCI. Common Terminology Criteria for Adverse Events v3.0. May 22, 2003. Available at http://ctep.cancer.gov/reporting/ctc.html.
- 9.Rosen LS, Mulay M, Long J, et al. Phase I trial of SU011248, a novel tyrosine kinase inhibitor in advanced solid tumors. Proc Am Soc Clin Oncol. 2003;22 abstract 765. [Google Scholar]
- 10.Maki RG, Fletcher JA, Heinrich MC, et al. Results from a continuation trial of SU11248 in patients (pts) with imatinib (IM)-resistant gastrointestinal stromal tumor (GIST) Proc Am Soc Clin Oncol. 2005;23 abstract 9011. [Google Scholar]
- 11.Atkinson BJ, Tannir NM, Jonasch E. Schedule modifications and treatment outcomes for sunitinib-related adverse events. American Society of Medical Oncology 2010 Genitourinary Cancers Symposium; 2010. abstract 357. [Google Scholar]
- 12.Motzer RJ, Basch E. Targeted drugs for metastatic renal cell carcinoma. Lancet. 2007;370:2071–2073. doi: 10.1016/S0140-6736(07)61874-1. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Hudes GR, Curti BD, et al. Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J Clin Oncol. 2007;25:3958–3964. doi: 10.1200/JCO.2006.10.5916. [DOI] [PubMed] [Google Scholar]
- 14.Yavuzsen T, Davis MP, Ranganathan VK, et al. Cancer-related fatigue: central or peripheral? J Pain Symptom Manage. 2009;38:587–596. doi: 10.1016/j.jpainsymman.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 16.Rini BI, Tamaskar I, Shaheen P, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99:81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 17.Minton O, Stone P, Richardson A, et al. Drug therapy for the management of cancer related fatigue. Cochrane Database Syst Rev. 2008:CD006704. doi: 10.1002/14651858.CD006704.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev. 2010;36:185–194. doi: 10.1016/j.ctrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Gramignano G, Lusso MR, Madeddu C, et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition. 2006;22:136–145. doi: 10.1016/j.nut.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Barceló EJ, Mediavilla MD, Tan DX, et al. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem. 2010;17:2070–2095. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- 21.Barton DL, Soori GS, Bauer BA, et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer. 2010;18:179–187. doi: 10.1007/s00520-009-0642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai J, Yassa L, Marqusee E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660–664. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
- 23.Wolter P, Dumez H, Schoffski P. Sunitinib and hypothyroidism. N Engl J Med. 2007;356:1580. doi: 10.1056/NEJMc070327. [DOI] [PubMed] [Google Scholar]
- 24.Wong E, Rosen LS, Mulay M, et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid. 2007;17:351–355. doi: 10.1089/thy.2006.0308. [DOI] [PubMed] [Google Scholar]
- 25.Grossmann M, Premaratne E, Desai J, et al. Thyrotoxicosis during sunitinib treatment for renal cell carcinoma. Clin Endocrinol (Oxf) 2008;69:669–672. doi: 10.1111/j.1365-2265.2008.03253.x. [DOI] [PubMed] [Google Scholar]
- 26.Mannavola D, Coco P, Vannucchi G, et al. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab. 2007;92:3531–3534. doi: 10.1210/jc.2007-0586. [DOI] [PubMed] [Google Scholar]
- 27.Wolter P, Stefan C, Decallonne B, et al. The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br J Cancer. 2008;99:448–454. doi: 10.1038/sj.bjc.6604497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. 2011;117:534–544. doi: 10.1002/cncr.25422. [DOI] [PubMed] [Google Scholar]
- 29.Baldazzi V, Tassi R, Lapini A, et al. The impact of sunitinib-induced hypothyroidism on progression-free survival of metastatic renal cancer patients: a prospective single-center study. Urol Oncol. 2010 Sep 28; doi: 10.1016/j.urolonc.2010.07.015. [E-pub ahead of print], doi: 10.1016;j.urolonc.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 31.Porta C, Paglino C, Imarisio I, et al. Uncovering Pandora's vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med. 2007;7:127–134. doi: 10.1007/s10238-007-0145-8. [DOI] [PubMed] [Google Scholar]
- 32.Beldner M, Jacobson M, Burges GE, et al. Localized palmar-plantar epidermal hyperplasia: a previously undefined dermatologic toxicity to sorafenib. The Oncologist. 2007;12:1178–1182. doi: 10.1634/theoncologist.12-10-1178. [DOI] [PubMed] [Google Scholar]
- 33.Suwattee P, Chow S, Berg BC, et al. Sunitinib: a cause of bullous palmoplantar erythrodysesthesia, periungual erythema, and mucositis. Arch Dermatol. 2008;144:123–125. doi: 10.1001/archderm.144.1.123. [DOI] [PubMed] [Google Scholar]
- 34.Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–1961. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 35.Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2008;158:592–596. doi: 10.1111/j.1365-2133.2007.08357.x. [DOI] [PubMed] [Google Scholar]
- 36.Jacobi U, Waibler E, Schulze P, et al. Release of doxorubicin in sweat: first step to induce the palmar-plantar erythrodysesthesia syndrome? Ann Oncol. 2005;16:1210–1211. doi: 10.1093/annonc/mdi204. [DOI] [PubMed] [Google Scholar]
- 37.Rouffiac V, Bouquet C, Lassau N, et al. Validation of a new method for quantifying in vivo murine tumor necrosis by sonography. Invest Radiol. 2004;39:350–356. doi: 10.1097/01.rli.0000124457.99229.bb. [DOI] [PubMed] [Google Scholar]
- 38.Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 39.Tsai KY, Yang CH, Kuo TT, et al. Hand-foot syndrome and seborrheic dermatitis-like rash induced by sunitinib in a patient with advanced renal cell carcinoma. J Clin Oncol. 2006;24:5786–5788. doi: 10.1200/JCO.2006.08.6868. [DOI] [PubMed] [Google Scholar]
- 40.Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 41.Sheen YS, Huang CL, Chu CY. Eccrine squamous syringometaplasia associated with sunitinib therapy. J Eur Acad Dermatol Venereol. 2007;21:1136–1137. doi: 10.1111/j.1468-3083.2006.02123.x. [DOI] [PubMed] [Google Scholar]
- 42.Thompson DS, Greco FA, Spigel DR, et al. Bevacizumab, erlotinib, and imatinib in the treatment of patients with advanced renal cell carcinoma: update of a multicenter phase II trial. Proc Am Soc Clin Oncol. 2006;24 abstract 4594. [Google Scholar]
- 43.Alexandrescu DT, Vaillant JG, Dasanu CA. Effect of treatment with a colloidal oatmeal lotion on the acneform eruption induced by epidermal growth factor receptor and multiple tyrosine-kinase inhibitors. Clin Exp Dermatol. 2007;32:71–74. doi: 10.1111/j.1365-2230.2006.02285.x. [DOI] [PubMed] [Google Scholar]
- 44.Botchkareva NV, Khlgatian M, Longley BJ, et al. SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J. 2001;15:645–658. doi: 10.1096/fj.00-0368com. [DOI] [PubMed] [Google Scholar]
- 45.Hemesath TJ, Price ER, Takemoto C, et al. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature. 1998;391:298–301. doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- 46.Mignogna MD, Fortuna G, Leuci S, et al. Sunitinib adverse event: oral bullous and lichenoid mucositis. Ann Pharmacother. 2009;43:546–547. doi: 10.1345/aph.1L592. [DOI] [PubMed] [Google Scholar]
- 47.Taichman NS, Cruchley AT, Fletcher LM, et al. Vascular endothelial growth factor in normal human salivary glands and saliva: a possible role in the maintenance of mucosal homeostasis. Lab Invest. 1998;78:869–875. [PubMed] [Google Scholar]
- 48.Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100:2026–2046. doi: 10.1002/cncr.20163. [DOI] [PubMed] [Google Scholar]
- 49.Lordick F, Geinitz H, Theisen J, et al. Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: report of three cases. Int J Radiat Oncol Biol Phys. 2006;64:1295–1298. doi: 10.1016/j.ijrobp.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Navari RM, Koeller JM. Electrocardiographic and cardiovascular effects of the 5-hydroxytryptamine3 receptor antagonists. Ann Pharmacother. 2003;37:1276–1286. doi: 10.1345/aph.1C510. [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Chung H-C, Mainwaring P, et al. An Asian subpopulation analysis of the safety and efficacy of sunitinib in metastatic renal cell carcinoma. Eur J Cancer Suppl. 2009;7:428. [Google Scholar]
- 52.Lammie A, Drobnjak M, Gerald W, et al. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–1425. doi: 10.1177/42.11.7523489. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell SA, Beck SL, Hood LE, et al. Putting evidence into practice: evidence-based interventions for fatigue during and following cancer and its treatment. Clin J Oncol Nurs. 2007;11:99–113. doi: 10.1188/07.CJON.99-113. [DOI] [PubMed] [Google Scholar]
- 54.Muehlbauer PM, Thorpe D, Davis A, et al. Putting evidence into practice: evidence-based interventions to prevent, manage, and treat chemotherapy- and radiotherapy-induced diarrhea. Clin J Oncol Nurs. 2009;13:336–341. doi: 10.1188/09.CJON.336-341. [DOI] [PubMed] [Google Scholar]
- 55.Adams LA, Shepard N, Caruso RA, et al. Putting evidence into practice: evidence-based interventions to prevent and manage anorexia. Clin J Oncol Nurs. 2009;13:95–102. doi: 10.1188/09.CJON.95-102. [DOI] [PubMed] [Google Scholar]