Abstract
Dendritic exocytosis underpins a broad range of integrative and homeostatic synaptic functions. Emerging data highlight the essential role of SNAREs in trafficking and fusion of secretory organelles with release of peptides and neurotransmitters from dendrites. This Perspective analyzes recent evidence inferring axo-dendritic polarization of vesicular release machinery and pinpoints progress made with existing challenges in this rapidly progressing field of dendritic research. Interpreting the relation of new molecular data to physiological results on secretion from dendrites would greatly advance our understanding of this facet of neuronal mechanisms.
Keywords: dendritic release, neuronal polarization, retrograde transmission
Classically, a single neuron receives multimodal information as local electro-chemical signals through synaptic inputs converging onto its soma and dendrites. After integration, these confined events are translated into action potentials at the axon initial segment, which rapidly propagate to nerve terminals and trigger there quantal release of neurotransmitters. Recent electrophysiological and imaging data, however, dispute this rather naïve view of neurons, highlighting unforeseen traits and dynamics of dendritic integration and communication mechanisms between nerve cells. It emerges that neurons, in addition to transferring signals through canonical chemical synapses at the axon terminals, also interact via mixed electro-chemical and electrical synapses at somatic and dendritic juxtapositions, as well as through diffuse nonsynaptic volume transmission signaling (1–4). Significant evidence has also accrued suggesting essential roles for dendritic exocytosis in these and numerous other integrative and homeostatic processes. These include release of transmitters, neurotrophins, and modulatory peptides from dendrites, along with activity-dependent remodeling of synaptic connections via regulated expression of receptors and ion channels (5–7). Although a great body of data advocate the involvement of SNAREs in trafficking and vesicular fusion at dendrites, nonvesicular release of retrograde messengers (e.g., endocannabinoids, arachidonic acid, nitric oxide, and carbon monoxide) from this location with their autocrine and paracrine effects on target and precursor neurons has additionally been documented (8–10). This Perspective reviews and analyzes key research streams on SNARE-dependent retrosynaptic signaling in mammalian central neurons and highlights the outstanding issues and challenges in this rapidly progressing topic. Despite the well-defined significance of SNAREs for trafficking and exocytosis at dendrites in compliance with the view of highly conserved fusion machinery among different secretory pathways, new findings indicate their notable specialization, supporting the premise of molecular asymmetry as one of the key affiliates of neuronal polarization.
SNARE Hypothesis and Secretory Vesicle Fusion at Synapses
Membrane fusion constitutes one of the fundamental biological processes governing targeting and merging of intracellular organelles. Analysis of its mechanisms led to the discovery of SNAREs as highly conserved key nanoengines of various intracellular trafficking steps (11, 12). According to the contemporary model of membrane fusion, SNAREs, along with several regulatory proteins, which are localized in opposing membranes, attach and propel the merger of membranes, using the free energy that is released during the formation of a four-helix SNARE bundle. Although not undisputed, during the last 2 decades this model attracted scientific scrutiny from numerous angles and gained overwhelming support from functional, molecular, and genetic studies (13–15).
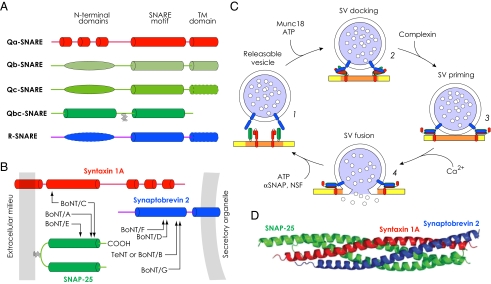
In eukaryotic cells, the SNARE protein family is represented by nine subfamilies, with a total of 36 members identified in humans (16). Originally, a strict separation was assumed between these proteins residing on the “donor” and “acceptor” membrane compartments, which led to their classification as vesicle membrane or target membrane SNAREs (v- and t-SNAREs) (17). This categorization, however, proved to be inept when homotypic fusion events were considered and especially in cases in which certain SNAREs function in several transport steps, with varying partners. The more recent and widely accepted classification is, therefore, based on reactive motifs contributed by each partner toward the formation of ultrastable coiled-coil SNARE complexes during membrane fusion, with its central hydrophilic layer containing three conserved glutamines (Q) and one arginine (R) (18). Accordingly, the contributing molecules are grouped into Qa-, Qb-, Qc-, Qbc-, and R-SNAREs (19, 20) (Fig. 1A). In neurons, SNAREs driving secretion are represented by a vesicle-associated membrane protein (VAMP, known also as synaptobrevin, R-SNARE), plasma membrane anchored-attached syntaxin (Qa-SNARE), and SNAP (comprising two SNARE motifs, Qbc-SNARE) variants (21). Advantageously for research, these proteins constitute natural targets for Clostridial neurotoxins (CNTs, both botulinum and tetanus toxins), which recognize and proteolytically inactivate SNAREs with astounding efficiency and specificity, causing blockade of synaptic transmission (22–24) (Fig. 1B). Unstructured in monomeric state, SNAREs when associated form highly stable ternary helical complexes (syntaxin and VAMP contributing one, whereas SNAP-25 provides two α-helices) before vesicular release (13, 18). This multireaction process, which was explicitly demonstrated at both the ensemble and single molecular levels, is initiated by the opening of syntaxin and binding of Munc18 to the syntaxin Habc N-terminal domain (25, 26), a step that promotes loose and reversible interaction between SNARE motifs of syntaxin with SNAP-25 and VAMP, leading to anchoring of the synaptic vesicle at the release site (Fig. 1C). Upon binding of complexin, another cytosolic regulatory protein, the transient SNARE core scaffold gets primed for rapid activation by the Ca2+ sensor synaptotagmin (27); the latter enforces the dissociation of complexin from the SNARE complex and puts into effect the zippering of four α-helical parallel bundles, resulting in vesicle fusion with the plasma membrane and neurosecretion (28) (Fig. 1 C and D). The bulk of recent data emerging from research on dendritic exocytosis, in general consistent with the model outlined above, suggests subtle variations in the machinery and mechanisms regulating vesicular release.
Fig. 1.
SNAREs and vesicular secretion from neurons. (A) Types of SNAREs with their gross structures schematized. The C-terminal of SNAREs corresponds to the transmembrane (TM) domains; the central portion contains the SNARE motif, and the N-terminal of Qa-SNAREs (syntaxins in neurons) possesses antiparallel three-helix bundles. In Qbc SNAREs (SNAP-23 and SNAP-25 in neurons), duplicated SNARE motifs are connected by a linker that is frequently palmitoylated (zig-zag lines) and anchors the protein to the membrane. During vesicular fusion at nerve terminals Qbc SNAREs (SNAP-23/25 variants) contribute two α-helices to the four-helical core complex, with the other two provided by Qa (syntaxin) and R (VAMP) SNAREs. Dashed borders highlight domains that are missing in some subfamily members. Modified with permission from ref. 18. (B) SNAREs constitute molecular targets of Clostridial neurotoxins, which recognize and cleave these fusogenic proteins at specific scissile bonds. Neuronal SNAREs serve as substrate for the protease light chains of tetanus toxin and botulinum neurotoxins: arrows indicate the cleavage sites of individual SNAREs by the different neurotoxins (BoNT, A-G serotypes of botulinum neurotoxin; TeNT, tetanus toxin). Plasma and vesicle membranes are indicated in gray (Left and Right, respectively). (C) Schematic of the SNARE-dependent synaptic vesicle cycle. Conformational shift from a closed to an open state of the N-terminal motif of syntaxin (Qa SNARE, red) conditioned by SM protein Munc18 initiates the loose interaction of syntaxin with SNAP-25 (Qbc SNARE, green) and VAMP (R-SNARE, blue), leading to ATP-dependent docking of SVs at the active zone (1, 2). This complex gets primed by complexin and Munc18 for activation by synaptotagmin and Ca2+ (3). Upon Ca2+ influx, synaptotagmin dissociates the complexin from the fusion complex and reacts with membrane phospholipids and the SNAREs, driving membrane fusion (4) and release of vesicular contents into the synaptic cleft. This step is followed by dissociation of the SNARE complex by NSF using the hydrolysis of ATP as energy source, liberating SNAREs for further reuse. (D) A crystal structure of SNARE core complex with four parallel α-helices associated in the ternary complex. The C-terminal of the helices, which points toward the vesicle and surface membranes, are oriented to the right. Modified with permission from ref. 136.
Dendritic Secretory Organelles
In somato-dendrites and axon terminals of nerve cells, secretory organelles are represented primarily by small synaptic vesicles (SSVs), filled with low molecular weight transmitters, and large dense core vesicles (LDCVs), containing peptides, modulators, biological amines, or neurotrophins (29, 30) (Fig. 2A). Although both LDCVs and SSVs are generated from the Golgi, they exhibit significant differences in their life cycles and destiny. In LDCVs the cargo proteins and peptides form aggregates, resulting in condensation of the granule matrix and membrane budding with the formation of early secretory granules, which mature by fusion with other granules while being transported through the trans-Golgi networks toward their release sites. After fusion with the plasmalema, vesicle membrane components of LDCVs are retrieved and recycled to the Golgi, where they get refilled with new cargo, thereby generating vesicles de novo in each cycle. SSVs also bud from Golgi as immature vesicles but have morphology and protein composition different from those of mature SSVs. These also reach release sites through active transport and there undergo maturation. However, in contrast to LDCVs, SSVs are refilled and extensively recycled locally before their degradation (31, 32).
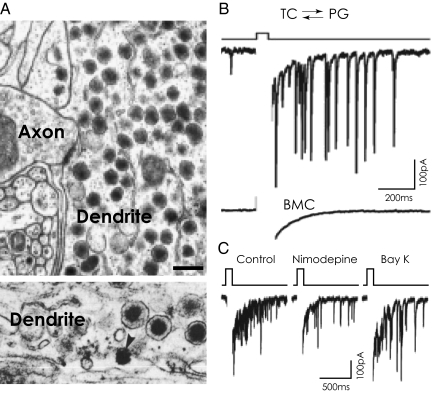
Fig. 2.
(A) Electron micrographs of rat hypothalamic area showing a dendritic shaft packed with LDCVs. The presence of synaptic contacts with axon terminal identifies the process as a dendrite. Arrowhead (Lower) indicates released dendritic secretory granule content into the interstitial space. Modified with permission from refs. 36 and 137. (Scale bar, 1.0 μm.) (B) Tufted cell (TC) dendro-dendritic inhibition mediated by periglomerular (PG) neurons. In the presence of tetrodotoxin, a voltage step is followed by a long-lasting barrage of inhibitory postsynaptic currents that were abolished by the GABAA receptor antagonist bicuculine (BMC). (C) L-type Ca2+ currents, which are normally not coupled to exocytosis, are required for TC–PG dendro-dendritic inhibition. Dendro-dendritic inhibition is reduced by nimodepine (L-type Ca2+ channel blocker) and recovered by activation of the same channel with Bay K. Modified with permission from ref. 34.
Release of both “classic” transmitters and peptides from dendrites seems to be primarily mediated via regulated exocytosis, except under basal conditions their constitutive release has been documented (33–35) (Fig. 2B). Tannic acid fixation of dendrites within the supraoptic nucleus revealed the morphology of LDCVs, with evidence of “omega” fusion profiles at the plasma membrane, clearly indicating the vesicular secretion of peptides from dendrites (36) (Fig. 2A). In several other neuron types, smaller dendritic vesicular elements have been observed, which may correspond to SSVs (31, 36). Despite the existence of discrete populations of secretory organelles (rapid releasable and reserve pools) in dendrites and their activity-dependent segregation have been proposed long ago (37), mechanisms of their targeting to the various pools and plasma membrane are essentially unknown. Unlike axon terminals, which rely on a somatic supply of LDCVs, substantial data suggest local mRNA translation and peptides synthesis in dendrites, with neurotransmitter secretion endoplasmic reticular (ER) sites visualized throughout the dendritic shafts of hippocampal and hypothalamic neurons (31, 38, 39). The lack of functional data on the principles and organization of dendritic endomembranes renders current understanding of postsynaptic membrane dynamics descriptive and incomplete (40). The significance of locally made vs. somatically produced neurotransmitters and peptides for dendritic secretion is currently also a matter of debate and intense research. Equally, it remains unclear why only a fraction of dendrites contain Golgi secretory outspots, raising the fundamental question of the significance of local translation in the absence of secretory pathways therein. Interestingly, the presence of smooth ER-derived organelles associated with small vesicular structures raises the possibility that some exocytotic vesicles can be developed directly from dendritic ER elements, bypassing the Golgi apparatus (38, 41). Although this straight route of secretory organelles to plasma membrane in dendrites has so far received little functional evidence, with targeting proteins remaining essentially unknown, if true it would represent a unique, noncanonical intracellular vesicle flow pathway that could explain how membrane proteins and peptides get locally trafficked in dendrites lacking Golgi outposts (42). In addition to secretory vesicles, several other exocytotic organelles, including early endosomes, recycling endosomes, late endosomes, and lysosomes, have been identified in dendrites of various neuron types, extensively reviewed recently (42, 43).
Secretion from Dendrites of Central Neurons Relies on SNAREs
There is considerable evidence for the involvement of SNAREs in secretion of both SSVs and LDCVs from dendrites of central neurons. The bulk of the data on SNARE-dependence of LDCV release comes from nigral dopaminergic cells and hypothalamic neurons. Dopamine has been observed in a variety of secretory organelles, including LDCV, SSVs, and tuberovesicular structures resembling smooth endoplasmic reticular stores (44). The advantageous anatomical arrangements of dopaminergic neurons with segregated dendrites, in substantia nigra pars reticulate, from somatic and axonal compartments not only made it possible to perform selective quantitative analysis of the dendritic release but provides a model for real-time monitoring of this physiological process in vivo (45, 46). Nevertheless, identification of subcellular storage compartments and precise molecular events leading to dendritic secretion of dopamine remained a subject of longstanding controversy (44, 47 –49) until a dual, quantal-reverse carrier-mediated secretion hypothesis was proposed (45). According to this model, dopamine can be released through both vesicular and carrier-mediated secretory mechanisms. Importantly, potent inhibition of dopamine secretion from somato-dendritic compartments of these neurons by CNTs in vitro and in vivo confirmed the involvement of SNAREs (50, 51). These functional data received support from more recent immunocytochemical studies, which highlighted the presence of the main CNTs-sensitive SNARE variants, including syntaxin3, VAMP2, and SNAP-25, in dendrites of nigral dopaminergic neurons (52).
Similar experiments also provided conclusive evidence for dendritic vesicular secretion from hypothalamic neurons. In dendrites of magnocellular neurons, for instance, a steep Ca2+-dependence of oxytocin release with clear structural attributes of vesicular storage and secretion from LDCVs has been firmly established (36, 53). The presence of SNAP-25 and syntaxin were also verified at this location (54). Importantly, probing dendritic oxytocin release with nanomolar concentrations of tetanus toxin revealed an essential role for VAMP in the secretion of this peptide-hormone (55). With earlier studies demonstrating the presence of α-SNAP mRNA and possible local translation of SNAP-25 in dendrites of hypothalamic neurons (56, 57), blockade of oxytocin release by CNTs substantiated the role of SNAREs as key mediators of dendritic peptide secretion here. Moreover, the data from several other brain areas, including hippocampus (58, 59), olfactory bulb (60), cerebellum (61), and neocortex (62, 63), are consistent with the requirement of SNARE proteins in dendritic neurosecretion and suggest the pervasion of this mechanism throughout the brain. It should be highlighted, however, that most of the data on SNARE-dependent dendritic secretion rely exclusively on CNT-induced inhibition of synaptic transmission, which is indirect evidence for SNARE involvement. In light of significant functional overlap and redundancy between different SNARE variants (64, 65) and recently recognized reliance of constitutive and regulated release on different SNAREs (64, 66 –68), the data described above should be interpreted with a great degree of caution and warrant further in-depth research.
Evidence for Specialization of Exocytotic Machinery for Dendritic Secretion
In biological systems, membrane fusion must be carefully controlled to exclude vesicle delivery to incorrect acceptor compartments that may prove detrimental. Therefore, intracellular pathways of secretory organelles follow highly regulated routes, to ensure targeted delivery to their fusion end-points. These journeys seem to be controlled primarily by Ras-related GTPases, Rab proteins instrumental in ensuring the specificity of contacts between fusion partners (18, 20). Although specialization of certain SNARE variants for individual fusion reactions and for guiding vesicular traffic has been recognized, the extent to which SNAREs contribute to these regulated flows of exocytotic organelles and their routing to different compartments remains a subject of great interest and ongoing debate; this is primarily due to the capacity of some SNAREs to assemble promiscuously with different variants (69, 70). For instance, in yeast ER–Golgi traffic, Sec22 and Ykt6 can substitute for each other as R-SNAREs. Similarly, in mammalian cells, SNAP-25 and SNAP-23, as well as VAMP2 and cellubrevin, are capable of replacing each other to varying degrees in regulated exocytosis (64, 71). Nevertheless, specific SNAREs have been assigned to most of the fusion steps and organelle types (18), with illustrative examples of such specialization in neuronal dendrites represented by syntaxin 4 and SNAP-23 (72, 73). Highly enriched in dendritic spines, these SNAREs are known to mediate dendrite-specific fusion reactions, reviewed in detail recently (42).
Adaptation of biochemical and physiological mechanisms of secretion from axon terminals and dendrites has also been proposed on the basis of data obtained from other models adopted for studying the targeting of secretory vesicles to these two poles of neurons (74, 75). In hypothalamic neurons, for example, dendritic LDCVs seem to differ in their content from those destined for release from axon terminals and have been proposed to rely on distinct exocytotic mechanisms (76). In the same way, in hippocampal neurons the membrane-associated SNAP-25 explicitly shown in axons was hardly detectable in dendrites, even though its presence has been demonstrated in the cytoplasm of both the soma and large dendrites of these cells (77). Evidence from nigral dopaminergic neurons suggests functional polarization of VAMP isoforms, with VAMP7 (TI-VAMP) [but not botulinum neurotoxin type B (BoNT/B)-sensitive VAMP variants] contributing to regulated vesicular secretion from dendrites (50, 53) despite the abundance of VAMP2 in both compartments. Intriguingly, the controversy over the presence of nonfunctional SNAREs in dendrites of hippocampal neurons has been partially resolved by the identification of VAMP2 on the pool of vesicles that bypass this neuronal compartment on the transcytotic journey to final functional destination at axon terminals (78). It remains to be established whether VAMP2 redundant in dendrites of nigral dopaminergic neurons (52) can be involved with LDCVs targeted for release at axon terminals.
Ca2+ Triggering of Dendritic Vesicular Secretion
At a canonical synapse, secretory vesicles in preparation for fusion dock at release sites and get primed for Ca2+-induced fusion with the plasma membrane (65) (Fig. 1C). Upon arrival of action potentials, voltage-gated Ca2+ channels open and allow influx of a pulse of Ca2+, thereby raising the intracellular [Ca2+] so that synchronous vesicular release gets triggered. Although there is convincing evidence to support the existence of such regulated Ca2+-induced dendritic secretion (79, 80), both the source and routes of Ca2+ entry, as well as the Ca2+ sensitivity of fusion machinery in dendrites, seem to differ significantly from those at axon terminals. Unlike release at axon endings that relies on action potential-induced nondecremented depolarization, Ca2+ elevations in dendrites can be graded and may be driven by back propagating action potentials or prolonged dendritic Ca2+ spikes. Additionally, they can be mediated through activation of Ca2+-permeable receptor-channel complexes or by release of Ca2+ from internal stores (37, 81, 82). Interestingly, a number of investigations suggest that different voltage-gated Ca2+ channels operate at these two poles of neurons, with L- and R-types dominating in dendrites, in contrast to axon terminals that depend primarily on P/Q- and N-type channels to mediate Ca2+ influx (83, 84) (Fig. 2B and see below). An illustrative example of such polarization is provided by dentate gyrus granule cells, where blockade of L-type Ca2+ current inhibits the dendritic release of dynorphin at the perforant pathway synapses but has no effect on secretion of the same substance from the axon terminals of these neurons (85). Differences have also been documented in the kinetics of free cytosolic [Ca2+], with fast dendritic Ca2+ transients displaying more diffuse and protracted characteristics. The latter seem to depend more heavily on activation of store-operating Ca2+ channels, with a metabotropic mechanism contributing significantly to the process (82, 86). In concert with slower kinetics of dendritic Ca2+ spikes, a greater component of diffuse efflux of [Ca2+] from internal stores would contribute appreciably toward global [Ca2+] elevations, which in turn should favor diffuse release of mediators or secretion of peptide and hormone-like substances from dendrites (87–89). Interestingly, although the relation between [Ca2+] and secretion of both classic transmitters and peptides (62, 90) seems to be steeper at axon terminals, neurosecretion from dendrites exhibits greater sensitivity to smaller elevations in [Ca2+] and exhibits higher tolerance to reduction of extracellular [Ca2+] (84, 91, 92). Hence, in contrast to micromolar [Ca2+] being required for release of classic transmitters at axon terminals (93, 94), the absolute minimum [Ca2+] elevations required for dendritic release range between 50 and 200 nM (62, 95). These concentrations match closely the intracellular [Ca2+] required for spontaneous exocytosis of neurotransmitters at nerve endings without notable effects on evoked release properties (67, 96). The higher efficiency of Ca2+ in dendrites has been speculated to be due to distinct synaptotagmin variants or contribution of an additional high-affinity Ca2+ sensor linking voltage-activated Ca2+ influx to the dendritic secretory machinery (92). This notion received support from experiments showing that despite the fact that the NMDA receptor-channel complex at dendrites in olfactory bulb granule cells is not directly coupled to vesicular release, small Ca2+ elevations mediated through NMDA receptor activation can be sufficient to trigger dendritic secretion of GABA (79). It is noteworthy that synaptotagmin 12 (97) and double C2 domain 2b (doc2b) (98) seem to ensure greater [Ca2+] sensitivity of the fusion machinery mediating spontaneous release at nerve terminals and, perhaps, could contribute toward dendritic secretion. Intriguingly, the direct contribution of synaptotagmin 10 to insulin-like growth factor 1 (IGF-1) release from somato-dendrites of olfactory bulb neurons has been identified recently (99). Localized on IGF-1 containing vesicles, Ca2+ binding by synaptotagmin 10 causes these vesicles to undergo exocytosis with spatial and temporal characteristics distinct from Ca2+-dependent exocytosis controlled by synaptotagmin 1 (99). These and perhaps other yet-unidentified sensor proteins also contribute toward greater responsiveness of dendritic secretory machinery to small elevations of cytoplasmic [Ca2+]. Thus, the higher responsiveness of dendritic fusion machinery to local and global Ca2+ elevations and the activation of graded vesicular release of transmitters or peptides are consistent with the specialization of dendrites for diffuse neurosecretion with retrograde modulator signaling. A range of diffuse metabotropic effects caused by dendritic release provides direct experimental support for this inference.
Sites for Dendritic Secretion: Active Zone vs. Ectopic Release?
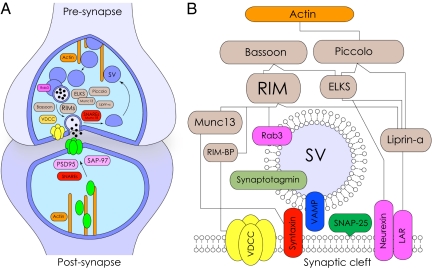
Requirement of active zone-like specializations for exocytosis from dendrites is one of the long-standing research queries of neurobiology. These restricted protein-rich areas of presynaptic plasma membrane provide a molecular scaffold for docking and priming of synaptic vesicles, with their controlled fusion and release of neurotransmitters triggered by action potential-induced Ca2+ elevations (Fig. 3 A and B). In nerve cells, vesicular secretion at active zones and distant from membrane release specializations (ectopic release) is well documented (100). Although the dendro-dendritic juxtapositions, with closely associated secretory organelles, were among the first synaptic elements recognized in the brain at an ultrastructural level (101), there is so far no evidence for the presence of electron-dense active zone tufts in dendrites. Notably, together with the presence of dense uniform vesicular pools, reminiscent of specialized release sites at canonical synapses (101–105), diffusely distributed secretory organelles have also been demonstrated in dendrites of central neurons, without preference for aggregations at membrane juxtapositions (36, 106, 107). Manifestly, vesicular clustering at axon terminal is typically associated with an electron-dense matrix, referred to as presynaptic grids (108), which have not been identified at dendrites of central neurons (31, 36, 106). Likewise, the molecular composition and architecture of dendro-dendritic synapses remains poorly studied (42), despite the latter being well recognized anatomically and physiologically. The presence of postsynaptic scaffolding molecules, such as PSD-93 and PSD-95, at dendro-dendritic juxtapositions of granule/mitral cells suggests their homology with conventional postsynaptic elements (109). However, no data are available on Rab3a interacting molecules (RIMs), proteins rich in E, L, K, and S (ELKS), Velis, Liprin-α or Munc13, Munc18, membrane-associated SNAREs, and other hallmark active zone proteins here and at other potential dendro-dendritic junctions. Likewise, in magnocellular hypothalamic neurons, Ca2+-dependent relocation of LDCVs from the deeper cytoplasm to the submembrane compartments has been demonstrated, yet with no ultrastructural evidence for release site specializations nearby (107). In the absence of active zones, the sensitivity of dendritic LDCV neurosecretion to CNTs (107, 110) indicates ectopic SNARE-dependent (v-SNAREs) release of peptides from the dendrites of these neurons, similar to that described in adrenal chromaffin cells (111, 112). Data obtained from nigral dopaminergic cells indicate that although their axon terminals in the striatum are enriched with vesicular aggregates and active zone type specializations with closely positioned vesicular pools (44, 113), their dendrites in the substantia nigra are devoid of such aggregations or any release specializations (114, 115). Note that as in hypothalamic neurons, dopamine secretion at both corpus striatum and substantia nigra is strongly inhibited by CNTs, which is consistent with its reliance on SNAREs (50). Interestingly, the ectopic dopamine release from dendrites of nigral neurons agrees with diffuse immunoreactivity of SNAP-25 therein (52, 54), contrasting with the punctuated presence of this SNARE variant at presynaptic axon terminals of other central neurons (116, 117). Finally, analysis of the distribution of vesicular glutamate transporters-3 (VGluT3), an SSV protein expressed preferentially in dendrites of cortical neurons (118, 119), also revealed its dispersed immunoreactivity at both subsynaptic areas as well as along the primary and secondary apical and basal dendrites (119), with no indication of active zone-like specializations therein. Thus, although the distribution of secretory organelles in dendrites of some central neurons suggests exocytotic specializations, the bulk of the data are consistent with their diffuse distribution with fusion outside of active zones, posing the fundamental question of regulatory mechanisms and the physiological significance of extrasynaptic retrograde signaling. Unquestionably, recent advances in optical imaging combined with computer models simulating transmission between neurons, which made possible analysis of presynaptic dynamics at the level of the individual vesicle (100, 120), should greatly assist future studies in these and numerous other fascinating questions raised by dendritic research.
Fig. 3.
Molecular architecture of the active zone. (A) Schematic of a canonical synapse with presynaptic release site enriched with secretory vesicles (SV, blue), active zone proteins (light brown), SNAREs/Ca2+ sensor (red), scaffolding/cytoskeletal proteins (orange), and voltage-gated Ca2+ channels (yellow). The adjacent postsynaptic receptive element comprises PSD95, SAP-97 (pink), receptors (green), scaffolding/cytoskeletal proteins (orange), and SNAREs (red). (B) Protein interaction network targeting secretory vesicles and driving their regulated fusion at the active zone. In cooperation with Rab3-GTPase and anchoring/cytoskeletal proteins [leucocyte common antigen-related protein (LAR), neurexins, actin], active zone proteins that include members of five protein families (RIMs, Bassoon and Piccolo, ELKS, and liprins-α) assist docking and priming of synaptic vesicles for SNARE-mediated fusion. VDCC, voltage-dependent Ca2+ channel.
Functional Polarization of Neurons and SNAREs
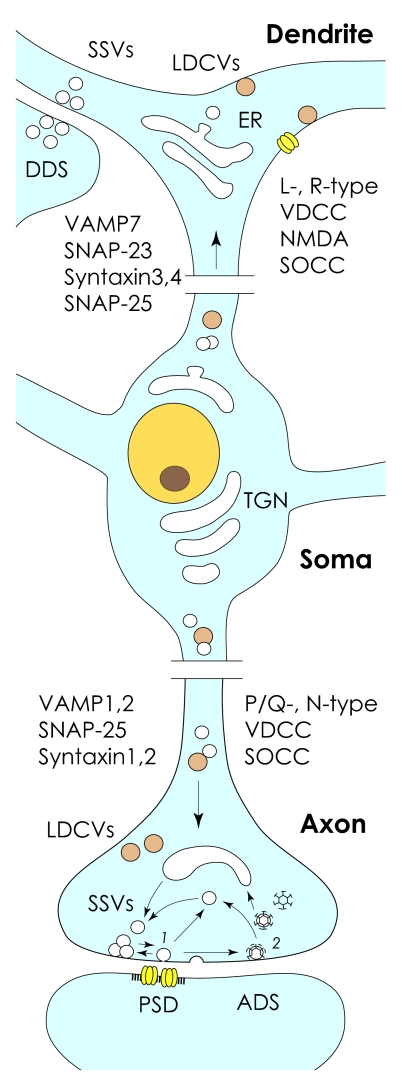
The highly asymmetric morphology of nerve cells led Ramon-y-Cajal to put forward the principle of functional polarization of neurons, which postulated unidirectional flow of information from dendrites to axons. Together with the anatomical, physiological, and biochemical discreteness of nerve cells, the polarization principle rests as one of the cornerstones of neuronal doctrine, affirming nerve cells as basic structural and functional modules of the brain (121). He explained that “for us, the cause of…polarization…is uniquely in the relationship which exists between the neurons…where the seat is the entry of excitation” (122). Indeed, as established later, these entry sites correspond to chemical synapses, specialized for vectorial transmission of signals via vesicular release of neurotransmitters (123, 124), a process blocked by CNTs. Accumulating data indicate congruity of SNARE-dependent exocytotic mechanisms and machinery at axon terminals and dendrites. However, as in other polarized cells (125), some SNARE inhibitors have revealed differential effects at these two neuronal poles, implying axo-dendritic specialization of membrane fusion nanoengines and release mechanisms (Fig. 4). Interestingly, although fusigenic asymmetry in epithelial cells seems to be established primarily by differential distribution of individual t-SNAREs (126, 127) as well as their regulation by different variants of Sec/Munc SM proteins (128, 129), in neurons both t- and v-SNAREs seem to contribute to such polarization (50, 73). Though explicit, these original data require further experimental validation with the use of advanced optical tools for single-molecule studies combined with genetic approaches and electrophysiology. Ad interim, it is tempting to speculate that polarization of secretory machinery and mechanisms facilitated the remarkable extension of dendritic functions during evolution far beyond mere information transmission and wiring of individual neurons into functional networks. This would provide dendrites with astounding flexibility and the capacity for integrating and processing tremendous volumes of information (130, 131). Indeed, along with the significant retrograde influence on synaptic inputs and dendritic cable properties through regulation of voltage-gated K+ and other currents, dendritic secretion endows nerve cells with the capacity to support a broad array of integrative and homeostatic functions (132–134). Thus, in addition to shared traits and functions with those recognized for axon terminals, SNARE-dependent dendritic secretion seems to be specialized for functions unique to this neuronal compartment.
Fig. 4.
Polarized exocytotic machinery of central neurons: a schematic. With both LDCVs and SSVs (light brown and white circles, respectively) identified in dendrites and axon terminals, Ca2+ elevations that triggers dendritic secretion are mediated by activation of L- and R-type voltage-dependent Ca2+ channels (VDCC), NMDA receptor-channel complex, and store-operating Ca2+ channels (SOCC). This contrasts with axon terminals, where Ca2+ elevations are mediated by P/Q-, N-type VDCC as well as SOCC mechanisms. Principal SNAREs identified at dendrites of neurons are VAMP7, SNAP-23, syntaxin3 and -4, and SNAP-25; at axon terminals SNAREs are represented by VAMP1 and -2, SNAP-25, and syntaxin1 and 2. PSD, postsynaptic density. DDS, dendro-dendritic synapse; ADS, axo-dendritic synapse. Note that neurosecretion at axon terminal can be caused by reversible hemifusion (“kiss-and-stay,” “kiss-and-run”) (1) or complete collapse of synaptic vesicles with the plasmalemma (2). Recycling of synaptic vesicles after their complete fusion involves clathrin-dependent endocytosis (coated vesicles).
Concluding Remarks and Future Directions
Disentangling the precise means used by nerve cells for information processing and transmission would afford important clues to one of the most enduring mysteries of our times—the human brain. It also is likely to unveil recipes for potential remedies to normalize brain functions that have been disrupted by numerous disorders and diseases. Emerging data suggest that release of mediators and peptides from dendrites exhibiting instructive and homeostatic effects offers neurons enormous integrative power and diversity. Indeed, with the capacity for graded tuning of afferent inputs through release of modulators and transmitters, dendrites effectively filter and sort inputs and adjust their strength. Although recent research greatly advanced our understanding of dendritic physiology, it has also raised a number of fundamental questions with important implications for the SNARE hypothesis and neuronal doctrine. Recognition of SNAREs and other regulatory proteins specialized for retrograde signaling at dendrites queries the canonical synapse-based view of redundancy of some SNARE proteins and predicts decorous sects for these molecules in the “functional catalog” of neurons. Likewise, vesicular secretion from dendrites in the absence of active zones along with apparently higher [Ca2+] sensitivity of dendritic exocytosis call for further refinement of the current canonical synapse-based neurotransmission hypothesis. Ironically, the emerging view on asymmetric secretory mechanisms at axo-dendritic poles of neurons, though complementing Ramon-y-Cajal's doctrine of dynamic polarization of neurons, also revitalizes the reticularist view of neuronal assemblies, led by Ramon-y-Cajal's key opponent Camillo Golgi, who championed the intimate reciprocal interaction between nerve cells (135). Clearly, along with the deep physiological division between dendrites and axons, the data outlined above strongly suggest their common functional and evolutionary missions—channeling and modulation of information flow between neurons. Notwithstanding all successes and failures, the stakes remain high for defining the meaning and significance of axo-dendritic polarization of release mechanisms for information processing at the level of the single neuron and overall integrative brain functions.
Acknowledgments
We thank Drs. Gary Lawrence, Valerie B. O'Leary, and Neal Lemon for insightful discussions and comments on this manuscript. We apologize for not citing all relevant articles on the topic because of space constraints. This work was supported by the Program for Research in Third Level Institutions Cycle 4 grant from the Irish Higher Educational Authority for the Neuroscience section of “Targeted-driven therapeutics and theranostics.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Brain Res Rev. 2010;64:137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 3.Tamás G, Buhl EH, Lörincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda T. Structural organization of the gap junction network in the cerebral cortex. Neuroscientist. 2007;13:199–207. doi: 10.1177/1073858406296760. [DOI] [PubMed] [Google Scholar]
- 5.Fitzsimonds RM, Poo MM. Retrograde signaling in the development and modification of synapses. Physiol Rev. 1998;78:143–170. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- 6.Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newpher TM, Ehlers MD. Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 2009;19:218–227. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Bolshakov VY, Siegelbaum SA. Hippocampal long-term depression: Arachidonic acid as a potential retrograde messenger. Neuropharmacology. 1995;34:1581–1587. doi: 10.1016/0028-3908(95)00127-r. [DOI] [PubMed] [Google Scholar]
- 9.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 10.Makara JK, et al. Involvement of nitric oxide in depolarization-induced suppression of inhibition in hippocampal pyramidal cells during activation of cholinergic receptors. J Neurosci. 2007;27:10211–10222. doi: 10.1523/JNEUROSCI.2104-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 12.Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 13.Brunger AT, Weninger K, Bowen M, Chu S. Single-molecule studies of the neuronal SNARE fusion machinery. Annu Rev Biochem. 2009;78:903–928. doi: 10.1146/annurev.biochem.77.070306.103621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens S, McMahon HT. Mechanisms of membrane fusion: Disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 15.Sørensen JB. Conflicting views on the membrane fusion machinery and the fusion pore. Annu Rev Cell Dev Biol. 2009;25:513–537. doi: 10.1146/annurev.cellbio.24.110707.175239. [DOI] [PubMed] [Google Scholar]
- 16.Hong W. SNAREs and traffic. Biochim Biophys Acta. 2005;1744:120–144. doi: 10.1016/j.bbamcr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 18.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 19.Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- 20.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudhof TC, Scheller RH. Mechanism and regulation of neurotransmitter release. In: Cowan WM, Sudhof TC, Stevens CF, editors. Synapses. Baltimore, MD: Johns Hopkins Univ Press; 2001. pp. 176–216. [Google Scholar]
- 22.Dolly JO, Lawrence GW, Meng J, Wang J, Ovsepian SV. Neuro-exocytosis: Botulinum toxins as inhibitory probes and versatile therapeutics. Curr Opin Pharmacol. 2009;9:326–335. doi: 10.1016/j.coph.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Montal M. Botulinum neurotoxin: A marvel of protein design. Annu Rev Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. [DOI] [PubMed] [Google Scholar]
- 24.Schiavo G, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 25.Carr CM, Rizo J. At the junction of SNARE and SM protein function. Curr Opin Cell Biol. 2010;22:488–495. doi: 10.1016/j.ceb.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deák F, et al. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon HT, Missler M, Li C, Südhof TC. Complexins: Cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 28.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lledo PM. Exocytosis in excitable cells: A conserved molecular machinery from yeast to neuron. Eur J Endocrinol. 1997;137:1–9. doi: 10.1530/eje.0.1370001. [DOI] [PubMed] [Google Scholar]
- 30.Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326:583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- 31.Apps DK, Cousin MA, Duncan RR, Wiegand UK, Simmons MJ. In: The Lifecycle of Secretory Vesicles: Implications for Dendritic Transmitter Release. Ludwig M, editor. Edinburgh, United Kingdom: Springer; 2005. [Google Scholar]
- 32.Tooze SA, Martens GJ, Huttner WB. Secretory granule biogenesis: Rafting to the SNARE. Trends Cell Biol. 2001;11:116–122. doi: 10.1016/s0962-8924(00)01907-3. [DOI] [PubMed] [Google Scholar]
- 33.Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- 34.Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: Signaling mechanisms of an olfactory microcircuit. Nat Neurosci. 2005;8:354–364. doi: 10.1038/nn1403. [DOI] [PubMed] [Google Scholar]
- 35.Murthy VN, Sejnowski TJ, Stevens CF. Dynamics of dendritic calcium transients evoked by quantal release at excitatory hippocampal synapses. Proc Natl Acad Sci USA. 2000;97:901–906. doi: 10.1073/pnas.97.2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 37.Tobin VA, et al. Thapsigargin-induced mobilization of dendritic dense-cored vesicles in rat supraoptic neurons. Eur J Neurosci. 2004;19:2909–2912. doi: 10.1111/j.1460-9568.2004.03388.x. [DOI] [PubMed] [Google Scholar]
- 38.Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma D, Morris JF. Local protein synthesis in magnocellular dendrites. Basic elements and their response to hyperosmotic stimuli. Adv Exp Med Biol. 1998;449:55–57. [PubMed] [Google Scholar]
- 40.Hanus C, Ehlers MD. In: Structural and Functional Organization of the Synapse. Hell J, Ehlers MD, editors. New York: Springer; 2008. pp. 205–250. [Google Scholar]
- 41.Gray EG, Guillery RW. A note on the dendritic spine apparatus. J Anat. 1963;97:389–392. [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 44.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuello A. In: Dendritic Neurotransmitter Release. Ludwig M, editor. New York: Springer; 2005. pp. 1–11. [Google Scholar]
- 46.Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- 47.Cuello AC, Kelly JS. Electron microscopic autoradiographic localization of [3H]-dopamine in the dendrites of the dopaminergic neurones of the rat substantia nigra in vivo [proceedings] Br J Pharmacol. 1977;59:527P–528P. [PMC free article] [PubMed] [Google Scholar]
- 48.Mercer L, del Fiacco M, Cuello AC. The smooth endoplasmic reticulum as a possible storage site for dendritic dopamine in substantia nigra neurones. Experientia. 1979;35:101–103. doi: 10.1007/BF01917903. [DOI] [PubMed] [Google Scholar]
- 49.Sotelo C. The fine structural localization of norepinephrine-3 H in the substantia nigra and area postrema of the rat. An autoradiographic study. J Ultrastruct Res. 1971;36:824–841. doi: 10.1016/s0022-5320(71)90033-5. [DOI] [PubMed] [Google Scholar]
- 50.Bergquist F, Niazi HS, Nissbrandt H. Evidence for different exocytosis pathways in dendritic and terminal dopamine release in vivo. Brain Res. 2002;950:245–253. doi: 10.1016/s0006-8993(02)03047-0. [DOI] [PubMed] [Google Scholar]
- 51.Fortin GD, Desrosiers CC, Yamaguchi N, Trudeau LE. Basal somatodendritic dopamine release requires snare proteins. J Neurochem. 2006;96:1740–1749. doi: 10.1111/j.1471-4159.2006.03699.x. [DOI] [PubMed] [Google Scholar]
- 52.Witkovsky P, Patel JC, Lee CR, Rice ME. Immunocytochemical identification of proteins involved in dopamine release from the somatodendritic compartment of nigral dopaminergic neurons. Neuroscience. 2009;164:488–496. doi: 10.1016/j.neuroscience.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergquist F, Ludwig M. Dendritic transmitter release: A comparison of two model systems. J Neuroendocrinol. 2008;20:677–686. doi: 10.1111/j.1365-2826.2008.01714.x. [DOI] [PubMed] [Google Scholar]
- 54.Schwab Y, et al. Calcium-dependent translocation of synaptotagmin to the plasma membrane in the dendrites of developing neurones. Brain Res Mol Brain Res. 2001;96:1–13. doi: 10.1016/s0169-328x(01)00244-3. [DOI] [PubMed] [Google Scholar]
- 55.de Kock CP, et al. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobsson G, Meister B. Molecular components of the exocytotic machinery in the rat pituitary gland. Endocrinology. 1996;137:5344–5356. doi: 10.1210/endo.137.12.8940356. [DOI] [PubMed] [Google Scholar]
- 57.Navone F, et al. Microvesicles of the neurohypophysis are biochemically related to small synaptic vesicles of presynaptic nerve terminals. J Cell Biol. 1989;109:3425–3433. doi: 10.1083/jcb.109.6.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maletic-Savatic M, Koothan T, Malinow R. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part II: Mediation by calcium/calmodulin-dependent protein kinase II. J Neurosci. 1998;18:6814–6821. doi: 10.1523/JNEUROSCI.18-17-06814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maletic-Savatic M, Malinow R. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part I: Trans-Golgi network-derived organelles undergo regulated exocytosis. J Neurosci. 1998;18:6803–6813. doi: 10.1523/JNEUROSCI.18-17-06803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsutani S, Yamamoto N. Postnatal development of dendritic spines on olfactory bulb granule cells in rats. J Comp Neurol. 2004;473:553–561. doi: 10.1002/cne.20107. [DOI] [PubMed] [Google Scholar]
- 61.Duguid IC, Pankratov Y, Moss GW, Smart TG. Somatodendritic release of glutamate regulates synaptic inhibition in cerebellar Purkinje cells via autocrine mGluR1 activation. J Neurosci. 2007;27:12464–12474. doi: 10.1523/JNEUROSCI.0178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zilberter Y, Kaiser KM, Sakmann B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24:979–988. doi: 10.1016/s0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]
- 63.Zilberter Y, Harkany T, Holmgren CD. Dendritic release of retrograde messengers controls synaptic transmission in local neocortical networks. Neuroscientist. 2005;11:334–344. doi: 10.1177/1073858405275827. [DOI] [PubMed] [Google Scholar]
- 64.Sørensen JB, et al. Differential control of the releasable vesicle pools by SNAP-25 splice variants and SNAP-23. Cell. 2003;114:75–86. doi: 10.1016/s0092-8674(03)00477-x. [DOI] [PubMed] [Google Scholar]
- 65.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 66.Bronk P, et al. Differential effects of SNAP-25 deletion on Ca2+ -dependent and Ca2+ -independent neurotransmission. J Neurophysiol. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- 67.Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol. 2011;21:275–282. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Washbourne P, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 69.Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J Biol Chem. 1999;274:15440–15446. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]
- 70.Yang B, et al. SNARE interactions are not selective. Implications for membrane fusion specificity. J Biol Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- 71.Borisovska M, et al. v-SNAREs control exocytosis of vesicles from priming to fusion. EMBO J. 2005;24:2114–2126. doi: 10.1038/sj.emboj.7600696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suh YH, et al. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat Neurosci. 2010;13:338–343. doi: 10.1038/nn.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- 75.Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- 76.Landry M, Vila-Porcile E, Hokfelt T, Calas A. Differential routing of coexisting neuropeptides in vasopressin neurons. Eur J Neurosci. 2003;17:579–589. [PubMed] [Google Scholar]
- 77.Tao-Cheng JH, Du J, McBain CJ. Snap-25 is polarized to axons and abundant along the axolemma: An immunogold study of intact neurons. J Neurocytol. 2000;29:67–77. doi: 10.1023/a:1007168231323. [DOI] [PubMed] [Google Scholar]
- 78.Sampo B, Kaech S, Kunz S, Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 2003;37:611–624. doi: 10.1016/s0896-6273(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 79.Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: Dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 80.Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 81.Higley MJ, Sabatini BL. Calcium signaling in dendrites and spines: Practical and functional considerations. Neuron. 2008;59:902–913. doi: 10.1016/j.neuron.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 82.Ludwig M, et al. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- 83.Bergquist F, Nissbrandt H. Influence of R-type (Cav2.3) and t-type (Cav3.1-3.3) antagonists on nigral somatodendritic dopamine release measured by microdialysis. Neuroscience. 2003;120:757–764. doi: 10.1016/s0306-4522(03)00385-3. [DOI] [PubMed] [Google Scholar]
- 84.Bergquist F, Jonason J, Pileblad E, Nissbrandt H. Effects of local administration of L-, N-, and P/Q-type calcium channel blockers on spontaneous dopamine release in the striatum and the substantia nigra: A microdialysis study in rat. J Neurochem. 1998;70:1532–1540. doi: 10.1046/j.1471-4159.1998.70041532.x. [DOI] [PubMed] [Google Scholar]
- 85.Simmons ML, Terman GW, Gibbs SM, Chavkin C. L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron. 1995;14:1265–1272. doi: 10.1016/0896-6273(95)90273-2. [DOI] [PubMed] [Google Scholar]
- 86.Ludwig M, Leng G. Conditional priming of dendritic neuropeptide release. In: Ludwig M, editor. Dendritic Neurotransmitter Release. New York: Springer; 2005. pp. 209–219. [Google Scholar]
- 87.Michael DJ, Cai H, Xiong W, Ouyang J, Chow RH. Mechanisms of peptide hormone secretion. Trends Endocrinol Metab. 2006;17:408–415. doi: 10.1016/j.tem.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 88.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 89.Pang ZP, Südhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 91.Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 92.Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: Substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neher E. A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse. Pflugers Arch. 2006;453:261–268. doi: 10.1007/s00424-006-0143-9. [DOI] [PubMed] [Google Scholar]
- 94.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 95.Kaiser KM, Lübke J, Zilberter Y, Sakmann B. Postsynaptic calcium influx at single synaptic contacts between pyramidal neurons and bitufted interneurons in layer 2/3 of rat neocortex is enhanced by backpropagating action potentials. J Neurosci. 2004;24:1319–1329. doi: 10.1523/JNEUROSCI.2852-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun J, et al. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maximov A, Shin OH, Liu X, Südhof TC. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J Cell Biol. 2007;176:113–124. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Groffen AJ, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao P, Maximov A, Südhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell. 2011;145:300–311. doi: 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsui K, Jahr CE. Exocytosis unbound. Curr Opin Neurobiol. 2006;16:305–311. doi: 10.1016/j.conb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 101.Rall W, Shepherd GM, Reese TS, Brightman MW. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966;14:44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- 102.Famiglietti EV., Jr Dendro-dendritic synapses in the lateral geniculate nucleus of the cat. Brain Res. 1970;20:181–191. doi: 10.1016/0006-8993(70)90287-8. [DOI] [PubMed] [Google Scholar]
- 103.Hirata Y. Some observations on the fine structure of the synapses in the olfactory bulb of the mouse, with particular reference to the atypical synaptic configurations. Arch Histol Jpn. 1964;24:293–302. doi: 10.1679/aohc1950.24.293. [DOI] [PubMed] [Google Scholar]
- 104.Price JL, Powell TP. The morphology of the granule cells of the olfactory bulb. J Cell Sci. 1970;7:91–123. doi: 10.1242/jcs.7.1.91. [DOI] [PubMed] [Google Scholar]
- 105.Price JL, Powell TP. The synaptology of the granule cells of the olfactory bulb. J Cell Sci. 1970;7:125–155. doi: 10.1242/jcs.7.1.125. [DOI] [PubMed] [Google Scholar]
- 106.Morris J. In: Dendritic Neurotransmitter Release. Ludwig M, editor. New York: Springer; 2005. pp. 15–35. [Google Scholar]
- 107.Tobin VA, Ludwig M. The actin filament and dendritic peptide release. Biochem Soc Trans. 2007;35:1243–1246. doi: 10.1042/BST0351243. [DOI] [PubMed] [Google Scholar]
- 108.Schoch S, Gundelfinger ED. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006;326:379–391. doi: 10.1007/s00441-006-0244-y. [DOI] [PubMed] [Google Scholar]
- 109.Sassoé-Pognetto M, et al. Organization of postsynaptic density proteins and glutamate receptors in axodendritic and dendrodendritic synapses of the rat olfactory bulb. J Comp Neurol. 2003;463:237–248. doi: 10.1002/cne.10745. [DOI] [PubMed] [Google Scholar]
- 110.Morris JF, Pow DV. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. Anat Rec. 1991;231:437–445. doi: 10.1002/ar.1092310406. [DOI] [PubMed] [Google Scholar]
- 111.Stevens DR, Schirra C, Becherer U, Rettig J. Vesicle pools: Lessons from adrenal chromaffin cells. Front Synaptic Neurosci. 2011;3:2. doi: 10.3389/fnsyn.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- 113.Pickel VM, Heras A. Ultrastructural localization of calbindin-D28k and GABA in the matrix compartment of the rat caudate-putamen nuclei. Neuroscience. 1996;71:167–178. doi: 10.1016/0306-4522(95)00441-6. [DOI] [PubMed] [Google Scholar]
- 114.Groves PM, Linder JC. Dendro-dendritic synapses in substantia nigra: Descriptions based on analysis of serial sections. Exp Brain Res. 1983;49:209–217. doi: 10.1007/BF00238581. [DOI] [PubMed] [Google Scholar]
- 115.Wassef M, Berod A, Sotelo C. Dopaminergic dendrites in the pars reticulata of the rat substantia nigra and their striatal input. Combined immunocytochemical localization of tyrosine hydroxylase and anterograde degeneration. Neuroscience. 1981;6:2125–2139. doi: 10.1016/0306-4522(81)90003-8. [DOI] [PubMed] [Google Scholar]
- 116.Nagy G, et al. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:417–429. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 117.Tafoya LC, et al. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J Neurosci. 2006;26:7826–7838. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Freund TF, Gulyás AI. GABAergic interneurons containing calbindin D28K or somatostatin are major targets of GABAergic basal forebrain afferents in the rat neocortex. J Comp Neurol. 1991;314:187–199. doi: 10.1002/cne.903140117. [DOI] [PubMed] [Google Scholar]
- 119.Harkany T, et al. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: Involvement of vesicular glutamate transporter 3. J Neurosci. 2004;24:4978–4988. doi: 10.1523/JNEUROSCI.4884-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Coggan JS, et al. Evidence for ectopic neurotransmission at a neuronal synapse. Science. 2005;309:446–451. doi: 10.1126/science.1108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cajal Ry. Neuron Theory or Reticular Theory: Objective Evidence of the Anatomical Unity of Nerve Cells. Madrid: Consejo Superior de Investigaciones Cientificas; 1954. [Google Scholar]
- 122.Cajal Ry. Histologie du Systeme Nerveux de l'Homme et les Vertebrates. Paris: Maloine; 1909. [Google Scholar]
- 123.Fatt P, Katz B. The electric activity of the motor end-plate. Proc R Soc Lond B Biol Sci. 1952;140:183–186. doi: 10.1098/rspb.1952.0055. [DOI] [PubMed] [Google Scholar]
- 124.Palade G, Palay S. Electron microscopical observation of interneuronal and neuromuscular synapse. Anat Rec. 1954;118:335–336. [Google Scholar]
- 125.Yeaman C. In: Protein Trafficking in the Exocytotic Pathway of Polarized Epithelial Cells. Bean AJ, editor. London: Elsevier Academic Press; 2007. [Google Scholar]
- 126.Low SH, et al. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Quiñones B, Riento K, Olkkonen VM, Hardy S, Bennett MK. Syntaxin 2 splice variants exhibit differential expression patterns, biochemical properties and subcellular localizations. J Cell Sci. 1999;112:4291–4304. doi: 10.1242/jcs.112.23.4291. [DOI] [PubMed] [Google Scholar]
- 128.Riento K, et al. Interaction of Munc-18-2 with syntaxin 3 controls the association of apical SNAREs in epithelial cells. J Cell Sci. 1998;111:2681–2688. doi: 10.1242/jcs.111.17.2681. [DOI] [PubMed] [Google Scholar]
- 129.Riento K, et al. A sec1-related vesicle-transport protein that is expressed predominantly in epithelial cells. Eur J Biochem. 1996;239:638–646. doi: 10.1111/j.1432-1033.1996.0638u.x. [DOI] [PubMed] [Google Scholar]
- 130.Häusser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- 131.Harris KD. Neural signatures of cell assembly organization. Nat Rev Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- 132.Johnston D, et al. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol. 2000;525:75–81. doi: 10.1111/j.1469-7793.2000.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shah MM, Hammond RS, Hoffman DA. Dendritic ion channel trafficking and plasticity. Trends Neurosci. 2010;33:307–316. doi: 10.1016/j.tins.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ludwig M, Pittman QJ. Talking back: Dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 135.Cowan WM, Kandel ER. A brief history of synapse and synaptic transmission. In: Cowan WM, Sudhof T, Stevens CF, editors. Synapse. Baltimore, MD: John Hopkins Univ Press; 2001. pp. 1–89. [Google Scholar]
- 136.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 137.Morris JF, Pow DV. Capturing and quantifying the exocytotic event. J Exp Biol. 1988;139:81–103. doi: 10.1242/jeb.139.1.81. [DOI] [PubMed] [Google Scholar]