Abstract
Despite several side effects, glucocorticoids (GCs) have been widely used for 60 y to treat rheumatoid arthritis on the basis of their antiinflammatory effects. However, the cells targeted by GCs and the transcriptional mechanisms underlying their actions through the glucocorticoid receptor (GR) in steroid therapy remain poorly defined. Using cell type-specific GR-deficient mice subjected to antigen-induced arthritis (AIA) as a model of human rheumatoid arthritis, we show that GC action on T cells but not myeloid cells is critical for therapeutic intervention in AIA. Furthermore, the resistance of mice expressing a DNA binding-defective GR (GRdim) to GC treatment reveals that dimerization of the GR is indispensable for the antiinflammatory effects. In these mice, the GC-induced suppression of TH1 and TH17 cell-derived proinflammatory cytokines is impaired. Our finding that IL-17A−/− mice are resistant to GC therapy, whereas IFN-γ−/− mice respond as efficiently as WT mice implies that IL-17–producing T cells and not IFN-γ–producing T cells are the most important targets for an efficient GC therapy. The present study's identification of the critical cell type and the mode of GR action in steroid therapy of AIA significantly advances our understanding of steroid therapy and should lead to therapies with greater efficiency and fewer side effects.
Keywords: conditional knockout mice, activated T cells, corticosteroid therapy, chronic inflammation
Rheumatoid arthritis (RA) is a severe autoimmune disease characterized by massive inflammation of peripheral joints that subsequently leads to the progressive destruction of articular cartilage and bone (1). For more than 60 y, RA patients have been treated with glucocorticoids (GCs) (2) because of their unsurpassed antiinflammatory effects. Indeed, GCs remain an essential component of RA therapy (3) despite their severe side effects.
The glucocorticoid receptor (GR) is a nuclear receptor that resides in the cytoplasm in the absence of ligand. Upon hormone binding, the GR can interfere with signal transduction components in the cytoplasm, such as Jun N-terminal kinases or PI3 kinases. Nonetheless, the majority of GR molecules translocate into the nucleus (4), where they alter gene expression by acting as a transcription factor via two different modes of action: the GR can dimerize and bind to palindromic elements in the promoter of GC-regulated genes, or it can interact as a monomer with DNA-bound transcription factors such as NF-κB, activator protein 1 (AP-1), interferon regulatory factor 3 (IRF-3), and signal transducer and activator of transcription (STAT)-5 (5, 6).
Very few studies have addressed the mode of action required for GC antiinflammatory activities in animal inflammatory models (4, 7). GRdim mice carry a point mutation in the DNA-binding domain, thereby abrogating dimerization and the DNA-binding capacity of the GR but leaving the interaction with NF-κB and AP-1 intact (7). In steroid therapy of phorbol ester-induced irritative inflammation, dimerization of the GR is not required (8). However, in contact hypersensitivity, dimerization and binding of the GR to DNA are necessary for the antiinflammatory effects (9). The cell type required to execute the antiinflammatory activities also differs in various inflammatory models. For example, myeloid cells are the target cells for the antiinflammatory effects of GCs in contact hypersensitivity (9) and septic shock models (10), whereas peripheral T cells are the primary targets in experimental autoimmune encephalomyelitis (a rodent model of multiple sclerosis) (11). Thus, the critical cell type and mode of GR action involved in steroid therapy seem to depend on the type of inflammation.
To identify the target cell types and mechanisms of GCs in RA therapy, we used the antigen-induced arthritis (AIA) mouse model. AIA is characterized by severe inflammation in knee joints, and the pathomechanism critically depends on T cells (12); in particular, CD4+ TH1 (13) and IL-17–producing CD4+ cells (TH17 cells) have been shown to be crucial (14, 15). However, the AIA model is also characterized by a strong infiltration of neutrophils, macrophages, and dendritic cells (DCs) into the knee joint cavity. Finally, AIA proceeds to a chronic phase with hyperplasia of the synovial lining, pannus formation, infiltration of mononuclear cells, and subsequently severe destruction of cartilage and bone. Here we demonstrate that the GR in T cells, but not in myeloid cells, is critical for suppression of inflammation by GCs and that GR dimer-dependent gene regulation is pivotal for the antiinflammatory effects of GCs in T cells in AIA and, at least in part, in another RA model.
Results
GCs Inhibit the Acute Phase of AIA.
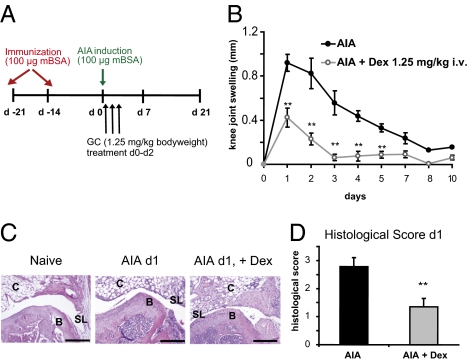
First, we established a therapeutic regimen of treating AIA with GCs (Materials and Methods and Fig. 1A). Treatment with the GR agonist dexamethasone (Dex; 1.25 mg/kg i.v.) resulted in reduced knee joint swelling compared with control-treated animals (Fig. 1B). The antiinflammatory effects of this GC dose were confirmed by histopathological analysis of knee joints 1 d after arthritis induction, the peak of the inflammatory response (Fig. 1C). In contrast to healthy naïve mice, knees from mice with AIA exhibited hyperplasia of synovial cells, strong exudate, and massive infiltration of polymorphonuclear cells in the arthritic joints (Fig. 1C), resembling the histopathological findings in human RA (16, 17). Importantly, in joints of Dex-treated animals, cellular infiltration in the articular and connective tissue and synovial inflammation were reduced (Fig. 1C), resulting in a significantly lower histopathological score (Fig. 1D). Thus, our protocol for treatment of AIA with Dex resembles the therapeutic effects of GCs in the acute phase of human RA and so represents a suitable model to investigate the relevant cell type involved in steroid therapy.
Fig. 1.
GC treatment suppresses AIA. (A) Treatment scheme of AIA induction and Dex application (Materials and Methods). (B) Effect of Dex treatment on AIA knee joint swelling at indicated time points. (C) Representative H&E stainings of naïve healthy knee joints and of arthritic PBS- and Dex-treated joints at day 1. B, bone of joint; C, connective tissue; SL, synovial layer. (Scale bars, 0.2 mm.) (D) Histological score of PBS- and Dex-treated arthritic knee joints at day 1 according to Tolk and Földi's grading of joint inflammation (Materials and Methods). In B and D, n = 8; **P < 0.01.
Immune Suppressive Actions of GCs Require the GR in T Cells.
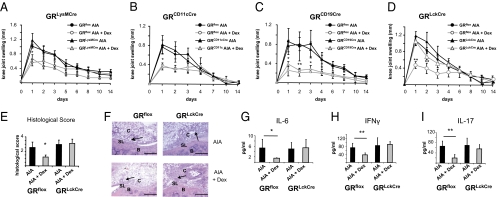
We first studied GRLysMCre mice lacking the GR in myeloid cells (9) because neutrophils and macrophages are early joint-infiltrating cells in the development of AIA. AIA in GRLysMCre mice resulted in a massive knee joint swelling comparable to that in GRflox control mice (Fig. 2A). Disease progression in the mutant animals was similar to that in GRflox mice, excluding a role of endogenous GCs in macrophages. Furthermore, AIA could be efficiently repressed by GC treatment in both genotypes (Fig. 2A). Thus, the GR in myeloid cells is not essential for the antiinflammatory effects of GCs in AIA.
Fig. 2.
GC-mediated suppression of AIA requires the GR in T cells. (A–D) Knee joint swelling of (A) GRLysMCre, (B) GRCD11cCre, (C) GRCD19Cre, and (D) GRLckCre mice and their respective littermate controls (GRflox) subjected to AIA and PBS or Dex treatment. (E) Histological score of arthritic knee joints of PBS- and Dex-treated GRflox and GRLckCre mice at day 1. (F) Representative H&E stainings of arthritic knee joints of PBS- and Dex-treated GRflox and GRLckCre mice at day 1 (arrows indicate infiltrations of inflammatory cells) (Scale bars, 0.2 mm.) (G–I) Serum levels of (G) IL-6, (H) IFN-γ, and (I) IL-17 in PBS- and Dex-treated arthritic WT and GRLckCre mice at day 1. In A–E and G–I, n = 5–6; *P < 0.05, **P < 0.01.
Because of the involvement of DCs in antigen presentation and their ability to produce cytokines, we analyzed GRCD11cCre mice. Generated by crossing GRflox mice with CD11cCre mice (18), the GR-encoding gene is almost completely ablated in CD11c+ DCs and partially, but not completely, in macrophages and T cells (Fig. S1 A and B). Both GRCD11cCre and GRflox mice developed severe knee joint swelling, and Dex could effectively suppress the symptoms of AIA (Fig. 2B), indicating that the GR in DCs is not critical for immune suppression of AIA.
Next we examined GRCD19Cre mice with a conditional deletion of the GR in B cells (Fig. S1 C and D) (19). GRCD19Cre mice showed a disease course similar to that in GRflox mice, and the clinical signs could be as efficiently suppressed by GC treatment as in control animals (Fig. 2C). This is in line with IgG serum analysis data from arthritic mice upon GC treatment, whereby Dex had no influence on the anti-mBSA (methylated BSA) IgG level (Fig. S2A).
Because AIA is a T cell-dependent inflammatory disease (20), we investigated the capacity of GCs to suppress AIA in GRLckCre mice. These mice display a very efficient recombination of the GR loxP allele in double- and single-positive thymocytes as well as in mature T cells (indicated by real-time PCR) (Fig. S1 E and F), and deletion of the GR does not affect their abundance (Fig. S3). Although the inflammatory response in the absence of GCs was not altered in GRLckCre mice, suppression of the inflammatory swelling response after Dex treatment was severely impaired (Fig. 2D). GCs also failed to reduce cellular infiltrates in knee joints (Fig. 2F), the overall histopathological score (Fig. 2E), and serum levels of the proinflammatory cytokines IL-6, IFN-γ, and IL-17 (Fig. 2 G–I). Importantly, Dex also failed to reduce the swelling response and IL-17 level in GRLckCre mice when applied after full establishment of the disease at day 1 (Fig. S4). Hence, these data demonstrate that T cells but not myeloid cells or B cells are the target cells for GC-mediated immune suppression of AIA.
Dimerized GR in T Cells Is Necessary for Antiinflammatory Effects.
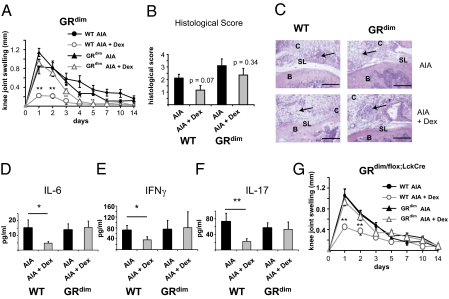
Using GRdim mice with an impaired GR dimerization, we next addressed whether GR dimerization is critical for the antiinflammatory effects of GCs or whether transrepression by the GR is sufficient. Arthritic GRdim mice exhibited a normal inflammatory response, but unlike their wild-type (WT) littermates, GC treatment completely failed to reduce knee joint swelling (Fig. 3A). Moreover, in comparison with controls, steroid therapy failed to reduce cellular infiltration and serum levels of IL-6, IFN-γ, and IL-17 in GRdim mice (Fig. 3 C–F and Fig. S5). The histopathological score was not significantly reduced by Dex in both genotypes, albeit in WT mice the difference between Dex- and PBS-treated animals was close to significance (Fig. 3B) (P = 0.07). Thus, the dimerized GR is required for the antiinflammatory effects of GCs in AIA.
Fig. 3.
GC-mediated suppression of AIA requires dimerization of the GR in T cells. (A) Clinical development of AIA in PBS- and Dex-treated WT mice and mice harboring a dimerization-deficient GR (GRdim) determined from measurements of knee joint swelling. (B) Histological score of knee joints of PBS- and Dex-treated arthritic WT and GRdim mice at day 1. (C) Representative H&E stainings of PBS- and Dex-treated arthritic knee joints of WT and GRdim mice (arrows indicate infiltrations of inflammatory cells) (Scale bars, 0.2 mm.) (D–F) Serum levels of (D) IL-6, (E) IFN-γ, and (F) IL-17 measured in PBS- and Dex-treated arthritic WT and GRdim mice at day 1. (G) Knee joint swelling of PBS- and Dex-treated arthritic GRdim/flox and GRdim/flox;LckCre mice, which lack the dimerized function of GR exclusively in T cells. In A, B, and D–G, n = 5–7; *P < 0.05, **P < 0.01.
Next, we investigated whether GR dimerization is also required for GC action in other models of arthritis. GRdim mice back-crossed to the DBA/1 background were subjected to glucose-6-phosphate isomerase-induced arthritis (G6PI-IA), a severe form of polyarthritis (21). Application of Dex starting at the onset of the disease (day 9) and continuing until day 15 efficiently suppressed the inflammatory score in WT mice, whereas it was only slightly reduced in GRdim mice in the acute phase of G6PI-IA (Fig. S6). However, at later phases from day 15 onward, a reduction of the swelling response could also be observed in Dex-treated GRdim mice, albeit at a lower efficiency (Fig. S6A). Taken together, these results demonstrate that the dimerization function of the GR is essential for suppressing acute inflammation in two arthritis models, indicating a general role of GR dimerization in steroid therapy of this disease.
Our analyses of GRdim and GRLckCre mice suggested that both GR dimerization and the presence of the GR in T cells are necessary for the antiinflammatory effects of GCs in arthritis therapy. To test whether the importance of GR dimerization is restricted to T cells, we crossed homozygous GRdim mice (genotype GRdim/dim) with GRLckCre mice (genotype GRflox/flox;LckCre). The resulting GRdim/flox;LckCre mice were heterozygous for the GRloxP and the GRdim allele and selectively expressed cre-recombinase in T cells. Thus, they carried a dimerization-deficient GR in T cells expressed from a hemizygous GR locus (Fig. S1G) but maintained a GR-loxP allele and consequently WT GR expression in all other cells. Both GRdim/flox;LckCre and GRdim/flox control mice developed severe arthritis, with a normal disease progression similar to that in WT mice (Fig. 3G compared with Fig. 3A). The induced knee joint swelling was efficiently suppressed by GCs in GRdim/flox mice only; Dex treatment of GRdim/flox;LckCre mice could not reduce the clinical signs (Fig. 3G). Thus, the dimerized GR in T cells is indispensable for the antiinflammatory effects of GCs in AIA therapy.
GCs Reduce T-Helper Cell Cytokines in Arthritic Mice but Do Not Induce Regulatory T Cells.
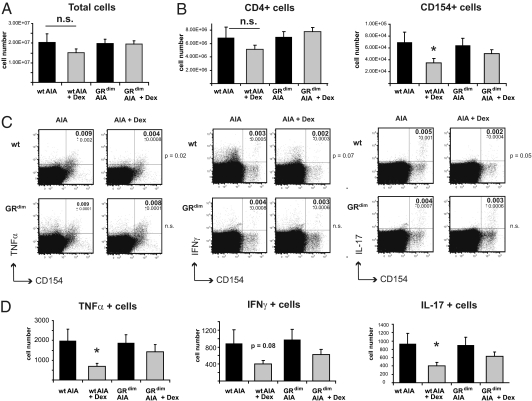
We next investigated how GCs regulate T cells in AIA. GC treatment did not significantly affect total cell number (Fig. 4A) or CD4+ cell number (Fig. 4B, Left) in the draining lymph nodes of WT and GRdim mice. Accordingly, apoptosis and the proliferation rate of CD4+ cells were unaltered under these conditions (Fig. S7). We then analyzed the capacity of T cells to respond to the antigen mBSA by flow cytometry of isolated draining lymph node cells from arthritic Dex-treated WT and GRdim mice (day 1) restimulated ex vivo for 6 h with mBSA (Fig. S8A). Because CD154 (CD40 ligand) is up-regulated after T cell receptor triggering (22), the fraction of CD154+ cells in response to mBSA treatment ex vivo represents the fraction of antigen-specific cells. The frequency of CD154+ cells in Dex-treated WT mice showed a tendency to be reduced (Fig. S8B). Upon calculation of the total number of activated CD154+ cells in the draining lymph nodes, a significant reduction was detected (Fig. 4B, Right). In contrast, there were no differences in the frequency and number of CD154+ cells between Dex- and PBS-treated GRdim mice.
Fig. 4.
GCs reduce TH1 and TH17 cell numbers in WT mice but not in GRdim mice. (A) Total cell numbers of draining lymph nodes derived from arthritic PBS- or Dex-treated WT and GRdim mice at day 1. (B) Cells were restimulated ex vivo for 6 h with mBSA, then stained with anti-CD4 and intracellularly with anti-CD154. The number of CD4+ cells (Left) and CD154+ cells (Right) was calculated using the frequency of CD4+ cells and CD154+ cells, respectively, from the total number of cells (shown in A). (C) Intracellular FACS staining of restimulated draining lymph node cells from B. Cells were stained with anti-TNFα (Left), anti–IFN-γ (Center), and anti–IL-17 (Right). Subsets of CD4+ cells are represented, and P values are indicated to the right of plots. (D) Intracellular FACS analysis of mBSA-restimulated lymph node cells determining the numbers of cytokine-producing cells in the total number of cells. In all four panels, n = 18; *P < 0.05; n.s., not significant.
As expected, a minor fraction of the CD4+ cells produced TNF-α, IFN-γ, or IL-17 upon restimulation with mBSA (Fig. 4C). The percentage of TNF-α–producing CD4+ cells was reduced in Dex-treated WT mice but not in GRdim mice (Fig. 4C). IFN-γ was not significantly reduced in either genotype, although there was a tendency for stronger reduction in WT cells (P = 0.08). These results indicate that the antigen-induced TH1 response is diminished in WT but not in GRdim mice. TH17 cells are known to play an important role in autoimmunity, particularly in RA (14, 15). The frequency of IL-17–producing CD4+ cells was twofold lower in the draining lymph nodes of GC-treated WT animals. However, GC treatment failed to reduce the frequency of these cells in GRdim mice (Fig. 4C). Although we did not observe any reduction of the percentage of TNF-α– and IL-17–producing cells within the fraction of CD154+ cells (Fig. S8C), their absolute numbers were diminished in Dex-treated WT mice. Importantly, this reduction was absent in GRdim mice (Fig. 4D). IFN-γ–producing cells were not significantly altered by Dex in both genotypes, albeit in WT mice the difference was close to significance (Fig. 4D).
Regulatory T cells are instrumental in preventing autoimmune diseases, including experimental AIA. Nonetheless, we could exclude an induction of regulatory T cells by GCs as a potential antiinflammatory mechanism, because the number of CD4+CD25+FoxP3+ T cells was not increased in Dex-treated mice with AIA (Fig. S9 A and B). Thus, a reduction of TH1 and TH17 cells likely contributes to the antiinflammatory effects of GCs in AIA.
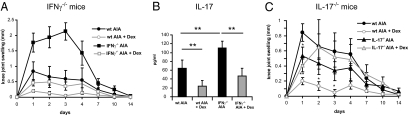
IL-17A−/− Mice but Not IFN-γ−/− Mice Are Resistant to GC Therapy.
Because the numbers of TH1 and TH17 cells were reduced after GC treatment of AIA, we examined to what extent the suppression of TH1 and TH17 cytokines contributes to the antiinflammatory effects. We tested IL-17A−/− and IFN-γ−/− mice with AIA for their response to GCs. As expected (23), IFN-γ−/− mice developed an exacerbated disease, indicated by a significantly higher knee joint swelling compared with controls (Fig. 5A), caused by elevated IL-17 levels (Fig. 5B). Despite the 2.5-fold stronger knee swelling in IFN-γ−/− mice, Dex treatment was still able to significantly reduce the arthritis (Fig. 5A) and to suppress IL-17 levels (Fig. 5B). We then tested whether it is the reduction of IL-17 that is crucial for the antiinflammatory effects. IL-17A−/− mice showed a reduced knee joint swelling in comparison with controls at day 1, but not at subsequent days (Fig. 5C). Intriguingly, GC application did not further reduce the clinical signs of arthritis in IL-17A−/− mice, which were elevated compared with Dex-treated WT mice (Fig. 5C). Thus, mice deficient for IL-17 are resistant to Dex treatment.
Fig. 5.
GC response of IFN-γ−/− and IL-17A−/− mice. (A) Clinical development of AIA in PBS- and Dex-treated WT mice and mice deficient for IFN-γ determined from measurements of knee joint swelling. (B) Serum level of IL-17 measured in PBS- and Dex-treated arthritic WT and IFN-γ−/− mice at day 1. (C) Clinical development of AIA in PBS- and Dex-treated WT mice and mice deficient for IL-17 determined from measurements of knee joint swelling. In all three panels, n = 5–7; **P < 0.01.
Taken together, our findings demonstrate that GC therapy is dependent on the dimerized GR in T cells, which seems to be necessary to reduce the number of IL-17–producing cells.
Discussion
The present study shows that GR dimerization in T cells and a reduction of IL-17–producing cells are critical for the antiinflammatory effects of GCs in AIA, a mouse model exhibiting characteristic features of RA (17).
Although macrophages and neutrophils are considered key players in the pathology of human RA (24–26) and AIA (27, 28), we did not observe an impaired effectiveness of GC treatment in arthritic mice lacking the GR in myeloid cells or lacking the GR in B cells. The latter finding is in line with our observation that IgG titers in WT mice were not altered after Dex treatment in AIA and in the G6PI-IA model (Fig. S2). Although it has been reported that GCs reduce IgG titers in healthy people (29), inhibition of B cell activity and autoantigen production do not contribute to the treatment of AIA by GCs. GCs potently affect DC maturation, activation, and migration and cytokine release (4). However, in the context of a therapeutic application of GCs in AIA, the GR in DCs plays a minor role; mice deficient for the GR in CD11c+ DCs exhibited a potent suppression of AIA by GCs.
Our finding that GRLckCre mice were completely resistant to GC-induced amelioration of AIA symptoms reveals that T cells are the most important targets of GCs in AIA. Interestingly, the partial recombination of the GRloxP allele in T cells in GRCD11cCre mice (Fig. S1 A and B) did not impair the therapeutic response to Dex. Only in GRLckCre mice that exhibit an almost 100% recombination of the GRloxP allele in T cells (Fig. S1F) we observed a complete resistance toward GCs.
Our study provides evidence that GR dimer-dependent processes, such as transcriptional transactivation, are involved in the suppression of T cell activation and that dimerization of the GR is essential for immune suppression in at least two arthritis models. GRdim mice were completely resistant to GCs after induction of AIA and partially resistant after G6PI-IA induction. Although GCs were able to exert a partial antiinflammatory response despite the absence of GR dimerization in the later progression phase, GR dimerization proved to be critical in the acute phase of G6PI-IA. These findings suggest that dimerization of the GR in general might be required for the treatment of arthritis by GCs, challenging concepts that selective GR agonists that avoid GR dimerization maintain antiinflammatory efficacy in the treatment of RA. For example, the selective GR agonist compound A (CpdA), believed to transrepress NF-κB activity but not to transactivate GR dimer-dependent target genes, exhibits efficient therapeutic potential in collagen-induced arthritis (30), suggesting that GR monomers are sufficient for the suppression of inflammation in RA. However, there is evidence that CpdA does not fully circumvent the involvement of GR dimerization in the suppression of RA: it can repress NF-κB activity in a GR-independent manner via attenuation of IκBα degradation and MAPK activation in RA synovial fibroblasts (31), and it can induce expression of the antiinflammatory-acting phosphatase dual specificity phosphatase 1 (32). This strongly suggests that CpdA is not fully dissociative with regard to DNA dimerization. Thus, our findings further underscore that selective GR modulators need to be fully characterized regarding their cell type-specific functions before conclusions on their usefulness can be drawn.
The findings presented here demonstrate that impairment of GR dimerization restricted to T cells is sufficient to ameliorate the immune suppressive effects of GCs, indicating that GR dimer-dependent inhibition of T cell function is the underlying cause of this effect. Inhibition of proinflammatory transcription factors [e.g., AP-1 (33)] by the GR monomer (34) and even nongenomic effects of the GR at the T cell receptor (TCR) complex (35) have been hypothesized to be involved in the suppression of T cells. In particular, activated antigen-specific T cells expressing CD40L were reduced by Dex in AIA, suggesting a central role of this regulatory mechanism. Other mechanisms such as reducing T-cell numbers by apoptosis or impaired proliferation, as well as an induction of regulatory T cells, were not observed and therefore are unlikely to be involved. The lower number of activated CD40+ T cells is in line with the well-established observation that GCs suppress T cell activation in general in terms of IL-2 expression (36). A reduction of cytokine expression in T cells, however, does not seem to contribute to the efficacy of GC therapy: we did not detect diminished TNF-α, IFN-γ or IL-17 levels within the fraction of activated T cells. This is corroborated by our finding that IL-17 produced in in vitro-generated WT TH17 cells was suppressed by only ≈20% after Dex treatment (Fig. S9 C and D).
Intriguingly, the frequency of antigen-specific TH1 and TH17 cells in draining lymph nodes was reduced in a GR dimerization-dependent manner. This is also true for TNF-α– and IL-17–producing cells that were reduced upon GC treatment in wild-type but not in GRdim mice and reflected in part by our observation that GC treatment of AIA did not reduce serum levels of IL-6, IFN-γ, and IL-17 in GRLckCre and GRdim mice. However, the major cause of the antiinflammatory effects of GCs in AIA is repression of IL-17– rather than IFN-γ–producing cells. IFN-γ−/− mice developed a significantly more severe arthritis; however, Dex application was still able to ameliorate the disease to a degree comparable to that in WT mice. The enhanced severity of IFN-γ−/− mice is due to elevated IL-17 levels (23), which can be reduced by Dex, resulting in a milder arthritis. Of note, we cannot exclude that TNF-α is a critical target for immunosuppression by GCs, because we did not analyze TNF-α–deficient mice. In contrast, IL-17A−/− mice displayed a milder AIA on day 1 but still mounted a robust inflammatory response on the following days. AIA in IL-17A−/− mice was not altered by Dex treatment, whereas it strongly reduced the swelling response in WT mice. Of note, we cannot entirely exclude a “compensatory” inflammatory response in IL-17A−/− mice that differs from the type of response in WT mice. Nonetheless, the unresponsiveness of IL-17A−/− mice to Dex treatment and the reduction of IL-17 levels in WT and IFN-γ−/− mice, but not in GR mutant mice, strongly indicate that IL-17 is a major target for immunosuppression by GCs.
GCs are capable of suppressing TH1 cytokines like IFN-γ and TNF-α by reducing STAT-4 activity through direct interaction, thereby inhibiting the TH1 lineage-specific transcription factor T-bet (37, 38). To date, only a few studies have reported suppressive effects of GCs on TH17 cells (39–41). The present study demonstrates this phenomenon in an arthritis model.
The identification of IL-17 as an essential target of steroid therapy in AIA suggests that interfering with TH17 cells using more specific compounds (42, 43) could be sufficient to treat arthritis and avoid other steroid-associated side effects. Indeed, beneficial outcomes in RA patients treated with an IL-17–neutralizing antibody have recently been reported (44).
Materials and Methods
Mice.
All animal experiments were performed in accordance with accepted standards of animal welfare and with permission of the responsible authorities of the Bundesland Thüringen in Germany. Origin and generation of used mouse strains is described in SI Materials and Methods.
AIA and G6PI-IA.
AIA was effectuated as previously described (23) using 8- to 12-wk-old mice (BALB/c or C57BL/6 background). G6PI-IA was established as described elsewhere (21). Details for histological and serum analysis are described in SI Materials and Methods.
Lymph Node Cell Analysis.
Cells from draining lymph nodes (inguinal and popliteal) were isolated 24 h after AIA induction and cultured with mBSA or 4α-phorbol 12-myristate 13-acetate and ionomycin, followed by brefeldin A treatment and subsequent analysis by flow cytometry. Details regarding proliferation and apoptosis determination are described in SI Materials and Methods.
TH17 in Vitro Differentiation.
Naïve CD4+TCRβ+CD62LhighCD44low cells were purified from spleens and lymph nodes of BALB/c mice by flow cytometry sorting and cultured in the presence of anti-CD3, anti-CD28 anti–IL-4 (11B11), and anti–IFN-γ (XMG.1) antibodies, as well as IL-6, TGF-β, IL-1β, and TNF-α. After 7 d, cells were restimulated with plate-bound anti-CD3 and anti-CD28 antibodies for 6 h with or without Dex, in the presence of brefeldin A. Details are described in SI Materials and Methods.
Statistics.
For all statistical analyses, a two-tailed Student t test was used. All data are presented as the mean ± SEM.
Supplementary Material
Acknowledgments
We thank Susanne Ostermay for technical help; Dominique Galendo, Anja Gruebl, Sara Holly, and Kristin Oehler for mouse husbandry; Biomodels Austria Vetmeduni Vienna for mouse delivery; and Dr. Yoichiro Iwakura for providing IL-17 KO mice. This work was supported by Deutsche Forschungsgemeinschaft Grants TU220/3 (to J.P.T.) and TU220/6 (to J.P.T.) and KA 755/6-1 (to T.K.) within the priority program SPP1468 “Osteoimmunology IMMUNOBONE”, the Boehringer Ingelheim Foundation (J.P.T.), and the Leibniz Graduate School for Age Research (U.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105857108/-/DCSupplemental.
References
- 1.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 2.Hench PS, Kendall EC, Slocumb CH, Polley HF. Effects of cortisone acetate and pituitray ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions. Arch Intern Med. 1950;85:545–566. doi: 10.1001/archinte.1950.00230100002001. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan J, Power L. Glucocorticoids: Action and new therapeutic insights in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:233–237. doi: 10.1097/BOR.0b013e3280d6471a. [DOI] [PubMed] [Google Scholar]
- 4.Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Tuckermann JP, Kleiman A, McPherson KG, Reichardt HM. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab Sci. 2005;42:71–104. doi: 10.1080/10408360590888983. [DOI] [PubMed] [Google Scholar]
- 6.Herrlich P. Cross-talk between glucocorticoid receptor and AP-1. Oncogene. 2001;20:2465–2475. doi: 10.1038/sj.onc.1204388. [DOI] [PubMed] [Google Scholar]
- 7.Reichardt HM, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 8.Reichardt HM, et al. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 2001;20:7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuckermann JP, et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest. 2007;117:1381–1390. doi: 10.1172/JCI28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood. 2007;109:4313–4319. doi: 10.1182/blood-2006-10-048215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wüst S, et al. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol. 2008;180:8434–8443. doi: 10.4049/jimmunol.180.12.8434. [DOI] [PubMed] [Google Scholar]
- 12.van den Berg WB. Lessons from animal models of arthritis over the past decade. Arthritis Res Ther. 2009;11:250. doi: 10.1186/ar2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolhain RJ, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenburg AM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–1969. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 14.Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: Lessons from animal models. Arthritis Res Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubberts E, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 16.Brackertz D, Mitchell GF, Mackay IR. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977;20:841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- 17.Kannan K, Ortmann RA, Kimpel D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology. 2005;12:167–181. doi: 10.1016/j.pathophys.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pohlers D, et al. Anti-CD4 monoclonal antibody treatment in acute and early chronic antigen-induced arthritis: Influence on T helper cell activation. Clin Exp Immunol. 2004;135:409–415. doi: 10.1111/j.1365-2249.2003.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert D, Maier B, Morawietz L, Krenn V, Kamradt T. Immunization with glucose-6-phosphate isomerase induces T cell-dependent peripheral polyarthritis in genetically unaltered mice. J Immunol. 2004;172:4503–4509. doi: 10.4049/jimmunol.172.7.4503. [DOI] [PubMed] [Google Scholar]
- 22.Frentsch M, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 23.Irmler IM, Gajda M, Bräuer R. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J Immunol. 2007;179:6228–6236. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 24.Nissler K, et al. Anti-CD4 monoclonal antibody treatment in acute and early chronic antigen induced arthritis: Influence on macrophage activation. Ann Rheum Dis. 2004;63:1470–1477. doi: 10.1136/ard.2003.013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinne RW, Stuhlmüller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res Ther. 2007;9:224. doi: 10.1186/ar2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillinger MH, Abramson SB. The neutrophil in rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21:691–714. [PubMed] [Google Scholar]
- 27.Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon J, et al. Systemic macrophage activation in locally-induced experimental arthritis. J Autoimmun. 2001;17:127–136. doi: 10.1006/jaut.2001.0534. [DOI] [PubMed] [Google Scholar]
- 29.Cupps TR, Edgar LC, Thomas CA, Fauci AS. Multiple mechanisms of B cell immunoregulation in man after administration of in vivo corticosteroids. J Immunol. 1984;132:170–175. [PubMed] [Google Scholar]
- 30.Dewint P, et al. A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. J Immunol. 2008;180:2608–2615. doi: 10.4049/jimmunol.180.4.2608. [DOI] [PubMed] [Google Scholar]
- 31.Gossye V, et al. Differential mechanism of NF-kappaB inhibition by two glucocorticoid receptor modulators in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009;60:3241–3250. doi: 10.1002/art.24963. [DOI] [PubMed] [Google Scholar]
- 32.Joanny E, et al. Anti-inflammatory effects of selective glucocorticoid receptor modulators (SGRMs) are partially dependent on upregulation of dual specificity phosphatase 1 (DUSP1) Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01574.x. 10.1111/j.1476-5381.2011.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vacca A, et al. Glucocorticoid receptor-mediated suppression of the interleukin 2 gene expression through impairment of the cooperativity between nuclear factor of activated T cells and AP-1 enhancer elements. J Exp Med. 1992;175:637–646. doi: 10.1084/jem.175.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonat C, et al. Antitumor promotion and antiinflammation: Down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 35.Löwenberg M, et al. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO Rep. 2006;7:1023–1029. doi: 10.1038/sj.embor.7400775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Northrop JP, Crabtree GR, Mattila PS. Negative regulation of interleukin 2 transcription by the glucocorticoid receptor. J Exp Med. 1992;175:1235–1245. doi: 10.1084/jem.175.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franchimont D, et al. Inhibition of Th1 immune response by glucocorticoids: Dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol. 2000;164:1768–1774. doi: 10.4049/jimmunol.164.4.1768. [DOI] [PubMed] [Google Scholar]
- 38.Liberman AC, Druker J, Refojo D, Holsboer F, Arzt E. Glucocorticoids inhibit GATA-3 phosphorylation and activity in T cells. FASEB J. 2009;23:1558–1571. doi: 10.1096/fj.08-121236. [DOI] [PubMed] [Google Scholar]
- 39.Momcilović M, et al. Methylprednisolone inhibits interleukin-17 and interferon-gamma expression by both naive and primed T cells. BMC Immunol. 2008;9:47. doi: 10.1186/1471-2172-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prause O, et al. IL-17-producing T lymphocytes in lung tissue and in the bronchoalveolar space after exposure to endotoxin from Escherichia coli in vivo—effects of anti-inflammatory pharmacotherapy. Pulm Pharmacol Ther. 2009;22:199–207. doi: 10.1016/j.pupt.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Miljković Z, Momcilović M, Miljković D, Mostarica-Stojković M. Methylprednisolone inhibits IFN-gamma and IL-17 expression and production by cells infiltrating central nervous system in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2009;6:37. doi: 10.1186/1742-2094-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huh JR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solt LA, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genovese MC, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.