Abstract
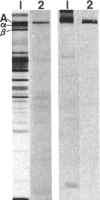
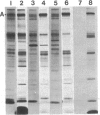
Ankyrin is a peripheral membrane protein that mediates the attachment of the erythrocyte membrane skeleton to the plasma membrane. We show that [3H]palmitic acid is incorporated into ankyrin in vivo. The majority of the 3H-labeled fatty acid is covalently bound to the polypeptide, as it cannot be removed by strong detergents or by chloroform/methanol extraction but is labile to alkaline hydrolysis. The binding of fatty acid occurs predominantly after the assembly of ankyrin onto the membrane skeleton, since it continues when protein synthesis is inhibited with emetine. Fatty acid acylation of ankyrin is constitutive in erythroid cells throughout chicken embryo development. It also occurs in mature avian and mammalian erythrocytes suggesting that the fatty acid bound to ankyrin turns over more rapidly than the polypeptide. Fatty acid acylation of assembled ankyrin may modulate the interaction of ankyrin with the plasma membrane. It may also provide a mechanism by which the membrane skeleton influences the organization of the lipid bilayer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Cohen P., Santikarn S., Williams D. H., Calder A. G., Smith A., Klee C. B. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982 Dec 27;150(2):314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Bruns G. A., Ingram V. M. The erythroid cells and haemoglobins of the chick embryo. Philos Trans R Soc Lond B Biol Sci. 1973 Oct 25;266(877):225–305. doi: 10.1098/rstb.1973.0050. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. Q., Bennett V. Brain ankyrin. A membrane-associated protein with binding sites for spectrin, tubulin, and the cytoplasmic domain of the erythrocyte anion channel. J Biol Chem. 1984 Nov 10;259(21):13550–13559. [PubMed] [Google Scholar]
- Hargreaves W. R., Giedd K. N., Verkleij A., Branton D. Reassociation of ankyrin with band 3 in erythrocyte membranes and in lipid vesicles. J Biol Chem. 1980 Dec 25;255(24):11965–11972. [PubMed] [Google Scholar]
- Keenan T. W., Heid H. W., Stadler J., Jarasch E. d., Franke W. W. Tight attachment of fatty acids to proteins associated with milk lipid globule membrane. Eur J Cell Biol. 1982 Feb;26(2):270–276. [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylated simian virus 40 large T-antigen: a new subclass associated with a detergent-resistant lamina of the plasma membrane. EMBO J. 1983;2(7):1151–1157. doi: 10.1002/j.1460-2075.1983.tb01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman D., Hsu D. J., Marchesi V. T. Evidence that spectrin binds to macromolecular complexes on the inner surface of the red cell membrane. J Cell Sci. 1980 Apr;42:1–22. doi: 10.1242/jcs.42.1.1. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Marinetti G. V., Cattieu K. Tightly (covalently) bound fatty acids in cell membrane proteins. Biochim Biophys Acta. 1982 Feb 23;685(2):109–116. doi: 10.1016/0005-2736(82)90086-4. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Goblin (ankyrin) in striated muscle: identification of the potential membrane receptor for erythroid spectrin in muscle cells. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3292–3296. doi: 10.1073/pnas.81.11.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. The patterns of expression of two ankyrin isoforms demonstrate distinct steps in the assembly of the membrane skeleton in neuronal morphogenesis. Cell. 1984 Dec;39(2 Pt 1):309–320. doi: 10.1016/0092-8674(84)90009-6. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981 May 25;256(10):4715–4718. [PubMed] [Google Scholar]
- Repasky E. A., Granger B. L., Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982 Jul;29(3):821–833. doi: 10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Fatty acid acylation of eukaryotic cell proteins. Methods Enzymol. 1983;96:795–801. doi: 10.1016/S0076-6879(83)96067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Bracha M., Schlesinger M. J. Evidence for covalent attachment of fatty acids to Sindbis virus glycoproteins. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1687–1691. doi: 10.1073/pnas.76.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F. Fatty acid binding: a new kind of posttranslational modification of membrane proteins. Curr Top Microbiol Immunol. 1983;102:101–129. doi: 10.1007/978-3-642-68906-2_3. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Intermediate filament systems are collapsed onto the nuclear surface after isolation of nuclei from tissue culture cells. Exp Cell Res. 1982 Mar;138(1):207–214. doi: 10.1016/0014-4827(82)90107-0. [DOI] [PubMed] [Google Scholar]