Abstract
We have isolated the entire gene for rat thyroglobulin, the precursor for thyroid hormone biosynthesis. The gene is at least 170,000 base pairs (bp) long; 9000 bp of coding information are distributed in 42 exons of homogeneous size (150-200 bp) except for two exons of 1100 and 620 bp. The sequences coding for two major thyroxine-forming sites are localized in exons 2 and 39. These two sequences do not show any homology either at the DNA or at the protein-sequence level, even though they code for sites highly specialized for the same function. Furthermore, both the 3' and the 5' end of the thyroglobulin structural gene appear to be made of repetitive units, which again do not show any homology. On the basis of these observations, we propose that the thyroglobulin gene arose by shuffling of at least two segments, with different evolutionary histories, each of which already contained introns.
Full text
PDF
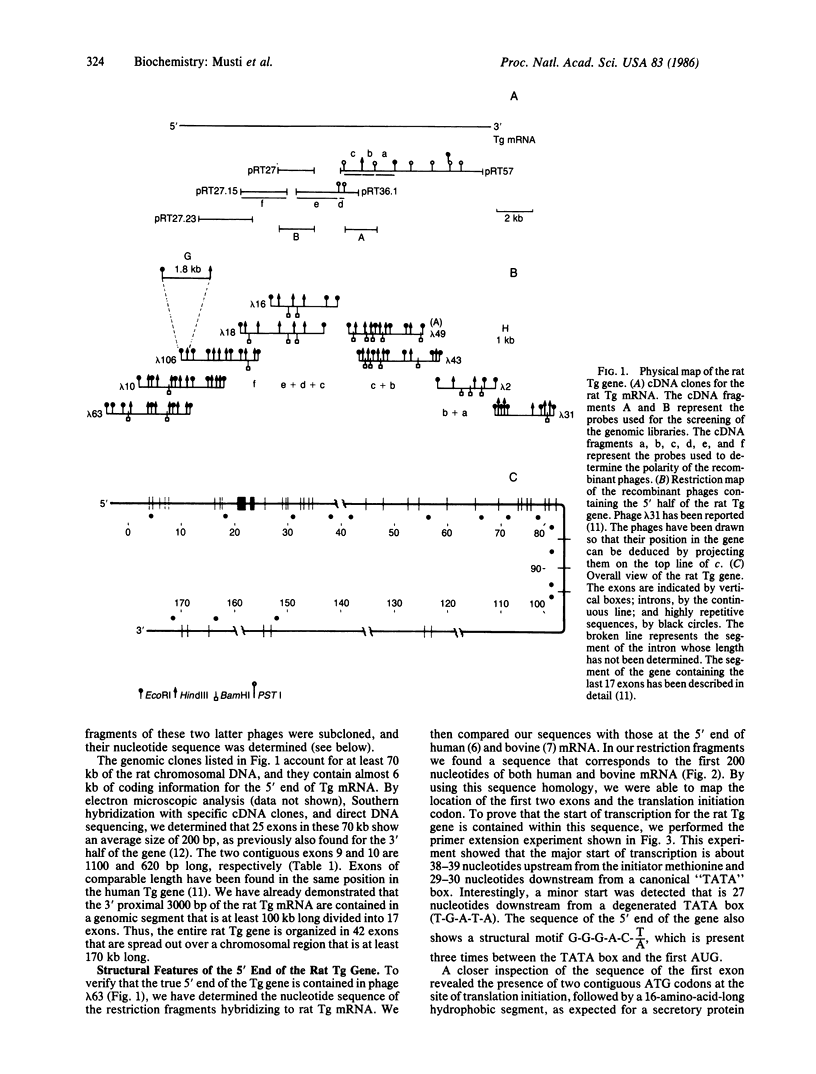
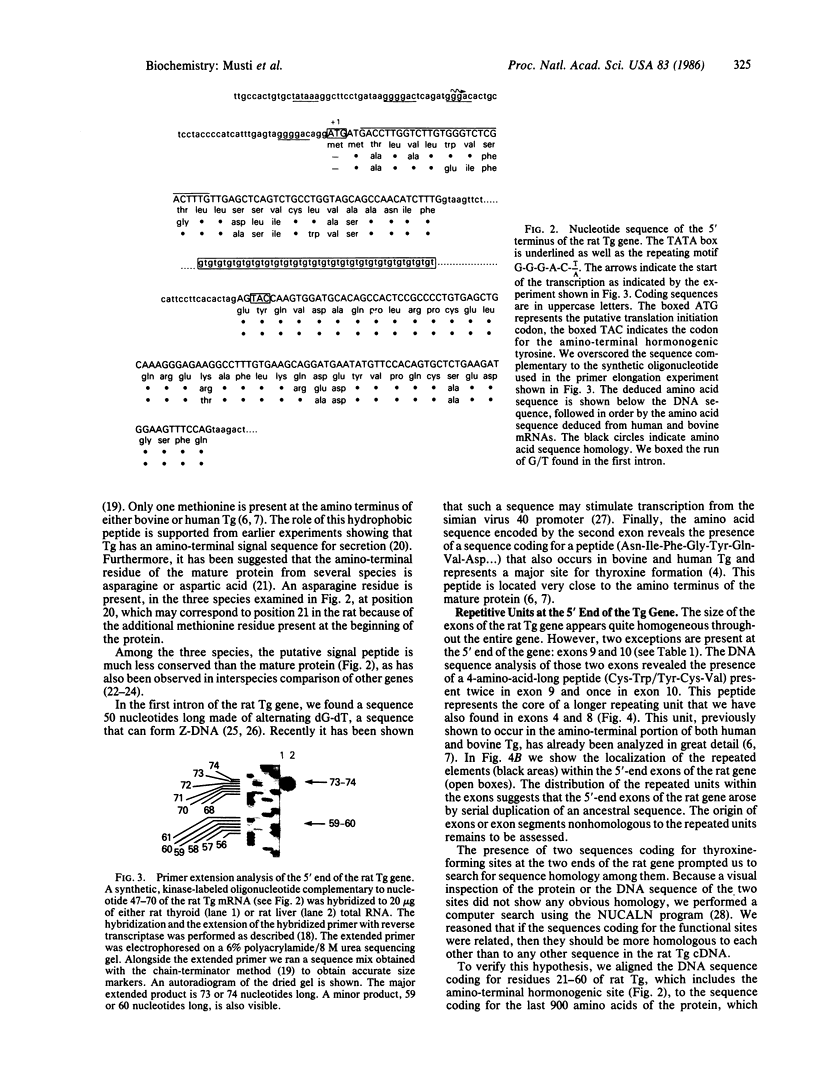
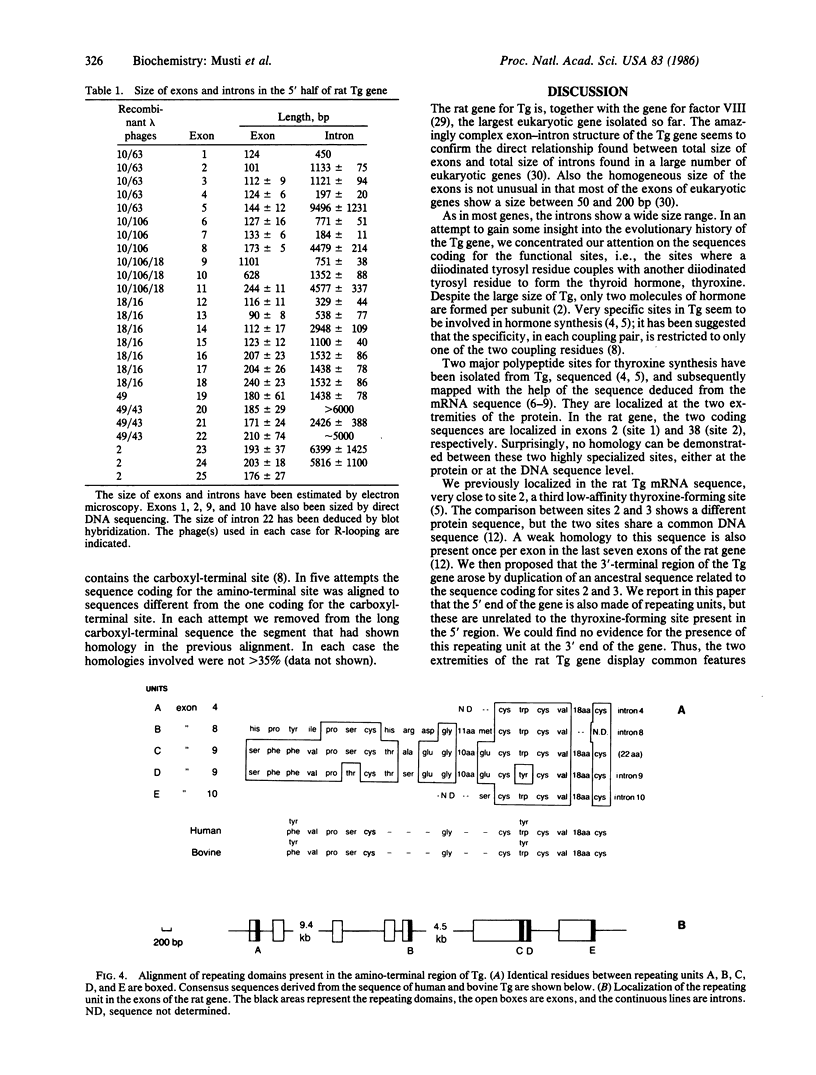

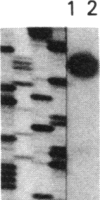
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvino C. G., Tassi V., Polistina C., Di Lauro R., Bonatti S. The segregation into microsomal vesicles and core-glycosylation in vitro of a 300-kDa rat thyroglobulin subunit. Eur J Biochem. 1982 Jun 15;125(1):15–19. doi: 10.1111/j.1432-1033.1982.tb06644.x. [DOI] [PubMed] [Google Scholar]
- Avvedimento V. E., Musti A. M., Obici S., Cocozza S., Di Lauro R. Structural organization of the 3' half of the rat thyroglobulin gene. Nucleic Acids Res. 1984 Apr 25;12(8):3461–3472. doi: 10.1093/nar/12.8.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Walter P., Chang C. N., Goldman B. M., Erickson A. H., Lingappa V. R. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- Christophe D., Pohl V., Van Heuverswijn B., De Martynoff G., Brocas H., Dumont J. E., Pasteels J. L., Vassart G. Isolation and characterization of a fragment of rat thyroglobulin gene. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1166–1175. doi: 10.1016/0006-291x(82)91092-0. [DOI] [PubMed] [Google Scholar]
- Di Lauro R., Obici S., Condliffe D., Ursini V. M., Musti A., Moscatelli C., Avvedimento V. E. The sequence of 967 amino acids at the carboxyl-end of rat thyroglobulin. Location and surroundings of two thyroxine-forming sites. Eur J Biochem. 1985 Apr 1;148(1):7–11. doi: 10.1111/j.1432-1033.1985.tb08799.x. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Genes-in-pieces revisited. Science. 1985 May 17;228(4701):823–824. doi: 10.1126/science.4001923. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Goralka T. M., Wion K. L., Chen E. Y., Eaton D. H., Vehar G. A., Capon D. J., Lawn R. M. Characterization of the human factor VIII gene. Nature. 1984 Nov 22;312(5992):326–330. doi: 10.1038/312326a0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Hobart P., Crawford R., Shen L., Pictet R., Rutter W. J. Cloning and sequence analysis of cDNAs encoding two distinct somatostatin precursors found in the endocrine pancreas of anglerfish. Nature. 1980 Nov 13;288(5787):137–141. doi: 10.1038/288137a0. [DOI] [PubMed] [Google Scholar]
- Lissitzky S. Thyroglobulin entering into molecular biology. J Endocrinol Invest. 1984 Feb;7(1):65–76. doi: 10.1007/BF03348380. [DOI] [PubMed] [Google Scholar]
- Malthiéry Y., Lissitzky S. Sequence of the 5'-end quarter of the human-thyroglobulin messenger ribonucleic acid and of its deduced amino-acid sequence. Eur J Biochem. 1985 Feb 15;147(1):53–58. doi: 10.1111/j.1432-1033.1985.tb08717.x. [DOI] [PubMed] [Google Scholar]
- Marriq C., Rolland M., Lissitzky S. Structure-function relationship in thyroglobulin: amino acid sequence of two different thyroxine-containing peptides from porcine thyroglobulin. EMBO J. 1982;1(4):397–401. doi: 10.1002/j.1460-2075.1982.tb01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken L., Massaer M., Simons M. J., Swillens S., Vassart G. Identification of hormonogenic domains in the carboxyl terminal region of bovine thyroglobulin. Biochem Biophys Res Commun. 1984 Dec 28;125(3):961–966. doi: 10.1016/0006-291x(84)91377-9. [DOI] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., De Martynoff G., Swillens S., Vassart G. Presence of hormonogenic and repetitive domains in the first 930 amino acids of bovine thyroglobulin as deduced from the cDNA sequence. Eur J Biochem. 1985 Feb 15;147(1):59–64. doi: 10.1111/j.1432-1033.1985.tb08718.x. [DOI] [PubMed] [Google Scholar]
- Naora H., Deacon N. J. Relationship between the total size of exons and introns in protein-coding genes of higher eukaryotes. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6196–6200. doi: 10.1073/pnas.79.20.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawitch A. B., Chernoff S. B., Litwer M. R., Rouse J. B., Hamilton J. W. Thyroglobulin structure-function. The amino acid sequence surrounding thyroxine. J Biol Chem. 1983 Feb 25;258(4):2079–2082. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. P., Rutter W. J. Sequence of the human somatostatin I gene. Science. 1984 Apr 13;224(4645):168–171. doi: 10.1126/science.6142531. [DOI] [PubMed] [Google Scholar]
- Spiro M. J. Studies on the protein portion of thyroglobulin. Amino acid compositions and terminal amino acids of several thyroglobulins. J Biol Chem. 1970 Nov 10;245(21):5820–5826. [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targovnik H. M., Pohl V., Christophe D., Cabrer B., Brocas H., Vassart G. Structural organization of the 5' region of the human thyroglobulin gene. Eur J Biochem. 1984 Jun 1;141(2):271–277. doi: 10.1111/j.1432-1033.1984.tb08188.x. [DOI] [PubMed] [Google Scholar]
- Uhler M., Herbert E. Complete amino acid sequence of mouse pro-opiomelanocortin derived from the nucleotide sequence of pro-opiomelanocortin cDNA. J Biol Chem. 1983 Jan 10;258(1):257–261. [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ommen G. J., Arnberg A. C., Baas F., Brocas H., Sterk A., Tegelaers W. H., Vassart G., de Vijlder J. J. The human thyroglobulin gene contains two 15-17 kb introns near its 3'-end. Nucleic Acids Res. 1983 Apr 25;11(8):2273–2285. doi: 10.1093/nar/11.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]