Abstract
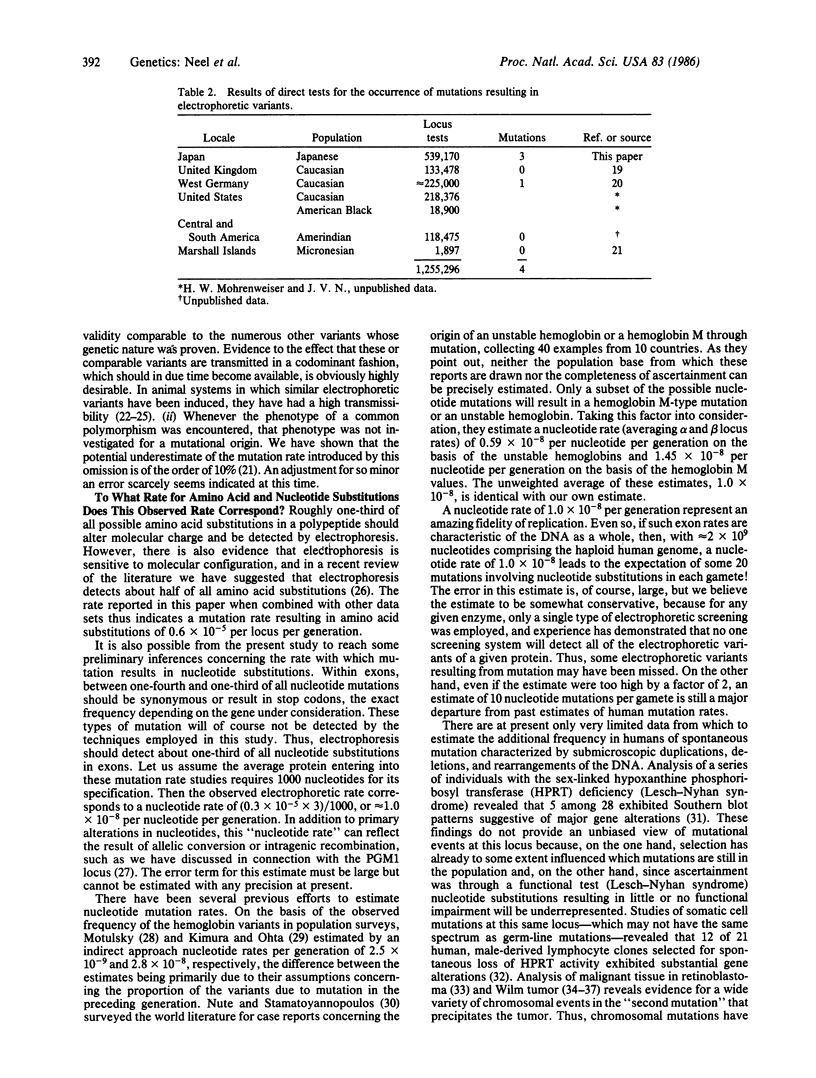
Studies of a Japanese population, involving a total of 539,170 locus tests distributed over 36 polypeptides, yielded three presumptive spontaneous mutations altering the electrophoretic mobility of the polypeptide. This corresponds to a mutation rate of 0.6 X 10(-5) per locus per generation. The a priori probability that undetected discrepancies between legal and biological parentage might in our test system result in an apparent electrophoretic mutation in this population is calculated to be only 0.3 X 10(-7) per locus per generation. Since electrophoresis only detects about half of the amino acid substitutions due to mutations of nucleotides, the corrected rate for mutations causing amino acid substitutions in polypeptides is 1.2 X 10(-5) per locus per generation. With allowance for synonymous mutations and those resulting in stop codons, the total mutation rate for nucleotide changes in the exons encoding a polypeptide becomes approximately equal to 1.8 X 10(-5) per locus per generation. When the present observations are combined with all of the other available data concerning mutation resulting in electrophoretic variants, the electrophoretic rate drops to 0.3 X 10(-5) per locus per generation, the total locus rate drops to roughly 1.0 X 10(-5), and the nucleotide rate drops to 1 X 10(-8). Even with this lower estimate, given approximately equal to 2 X 10(9) nucleotides in the haploid genome and an average of 10(3) exon nucleotides per polypeptide encoded, the implication, if these exon rates can be generalized, is of approximately equal to 20 nucleotide mutations per gamete per generation. This estimate of the frequency of "point" mutations does not include small duplications, rearrangements, or deletions resulting from unequal crossing-over, transcription errors, etc.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Shaw M., Schull W. J. Exclusion of paternity: the current state of the art. Am J Hum Genet. 1974 Jul;26(4):477–488. [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Giblett E. R. Genetic variation of soluble glutamic-oxaloacetic transaminase in man. Am J Hum Genet. 1971 Jul;23(4):419–424. [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Giblett E. R. Polymorphism of soluble glutamic-pyruvic transaminase: a new genetic marker in man. Science. 1971 Jul 9;173(3992):148–149. doi: 10.1126/science.173.3992.148. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Inherited variants of human nucleoside phosphorylase. Ann Hum Genet. 1971 May;34(4):395–408. doi: 10.1111/j.1469-1809.1971.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Ferrell R. E., Ueda N., Satoh C., Tanis R. J., Neel J. V., Hamilton H. B., Inamizu T., Baba K. The frequency in Japanese of genetic variants of 22 proteins. I. Albumin, ceruloplasmin, haptoglobin, and transferrin. Ann Hum Genet. 1977 May;40(4):407–418. [PubMed] [Google Scholar]
- Fildes R. A., Harris H. Genetically determined variation of adenylate kinase in man. Nature. 1966 Jan 15;209(5020):261–263. doi: 10.1038/209261a0. [DOI] [PubMed] [Google Scholar]
- Harris H., Hopkinson D. A., Robson E. B. The incidence of rare alleles determining electrophoretic variants: data on 43 enzyme loci in man. Ann Hum Genet. 1974 Jan;37(3):237–253. doi: 10.1111/j.1469-1809.1974.tb01832.x. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Coppock J. S., Mühlemann M. F., Edwards Y. H. The detection and differentiation of the products of the human carbonic anhydrase loci, CAI and CAII using fluorogenic substrates. Ann Hum Genet. 1974 Oct;38(2):155–162. doi: 10.1111/j.1469-1809.1974.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Mestriner M. A., Cortner J., Harris H. Esterase D: a new human polymorphism. Ann Hum Genet. 1973 Oct;37(2):119–137. doi: 10.1111/j.1469-1809.1973.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Johnson F. M., Lewis S. E. Electrophoretically detected germinal mutations induced in the mouse by ethylnitrosourea. Proc Natl Acad Sci U S A. 1981 May;78(5):3138–3141. doi: 10.1073/pnas.78.5.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. M., Roberts G. T., Sharma R. K., Chasalow F., Zweidinger R., Morgan A., Hendren R. W., Lewis S. E. The detection of mutants in mice by electrophoresis: results of a model induction experiment with procarbazine. Genetics. 1981 Jan;97(1):113–124. doi: 10.1093/genetics/97.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Ohta T. Mutation and evolution at the molecular level. Genetics. 1973 Apr;73(Suppl):19–35. [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Leder P. The organization and expression of cloned globin genes. Harvey Lect. 1980;74:81–100. [PubMed] [Google Scholar]
- Mathai C. K., Ohno S., Beutler E. Sex-linkage of the glucose-6-phosphate dehydrogenase gene in Equidae. Nature. 1966 Apr 2;210(5031):115–116. doi: 10.1038/210115a0. [DOI] [PubMed] [Google Scholar]
- Neel J. V. A revised estimate of the amount of genetic variation in human proteins: implications for the distribution of DNA polymorphisms. Am J Hum Genet. 1984 Sep;36(5):1135–1148. [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Satoh C., Hamilton H. B., Otake M., Goriki K., Kageoka T., Fujita M., Neriishi S., Asakawa J. Search for mutations affecting protein structure in children of atomic bomb survivors: preliminary report. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4221–4225. doi: 10.1073/pnas.77.7.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V. The detection of increased mutation rates in human populations. Perspect Biol Med. 1971;14(4):522–537. doi: 10.1353/pbm.1971.0043. [DOI] [PubMed] [Google Scholar]
- Ohlsson M., Feder J., Cavalli-Sforza L. L., von Gabain A. Close linkage of alpha and beta interferons and infrequent duplication of beta interferon in humans. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4473–4476. doi: 10.1073/pnas.82.13.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- SPENCER N., HOPKINSON D. A., HARRIS H. PHOSPHOGLUCOMUTASE POLYMORPHISM IN MAN. Nature. 1964 Nov 21;204:742–745. doi: 10.1038/204742a0. [DOI] [PubMed] [Google Scholar]
- Satoh C., Ferrell R. E., Tanis R. J., Ueda N., Kishimoto S., Neel J. V., Hamilton H. B., Baba K. The frequency in Japanese of genetic variants of 22 proteins. III. Phosphoglucomutase-1, phosphoglucomutase-2, 6-phosphogluconate dehydrogenase, adenylate kinase, and adenosine deaminase. Ann Hum Genet. 1977 Oct;41(2):169–183. doi: 10.1111/j.1469-1809.1977.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Siebert G., Ritter H., Kömpf J. Substrate affinity in PGM1, PGM2, and PGM2 isozymes. Hum Genet. 1980;56(2):213–215. doi: 10.1007/BF00295697. [DOI] [PubMed] [Google Scholar]
- Soares E. R. TEM-induced gene mutations at enzyme loci in the mouse. Environ Mutagen. 1979;1(1):19–25. doi: 10.1002/em.2860010108. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Neel J. V., Satoh C., Nishizaki J., Masunari N. A phylogeny for the principal alleles of the human phosphoglucomutase-1 locus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6636–6640. doi: 10.1073/pnas.79.21.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis R. J., Ueda N., Satoh C., Ferrell R. E., Kishi-Moto S., Neel J. V., Hamilton H. B., Ohno N. The frequency in Japanese of genetic variants of 22 proteins. IV. Acid phosphatase, NADP-isocitrate dehydrogenase, peptidase A, peptidase B and phosphohexose isomerase. Ann Hum Genet. 1978 May;41(4):419–428. doi: 10.1111/j.1469-1809.1978.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Ueda N., Satoh C., Tanis R. J., Ferrell R. E., Kishimoto S., Neel J. V., Hamilton H. B., Baba K. The frequency in Japanese of genetic variants of 22 proteins II. Carbonic anhydrase I and II, lactate dehydrogenase, malate dehydrogenase, nucleoside phosphorylase, triose phosphate isomerase, haemoglobin A and haemoglobin A2. Ann Hum Genet. 1977 Jul;41(1):43–52. doi: 10.1111/j.1469-1809.1977.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Patel P. I., Chinault A. C., Stout J. T., Jackson L. G., Hildebrand B. M., Caskey C. T. Molecular evidence for new mutation at the hprt locus in Lesch-Nyhan patients. Nature. 1984 Aug 2;310(5976):412–414. doi: 10.1038/310412a0. [DOI] [PubMed] [Google Scholar]


