Abstract
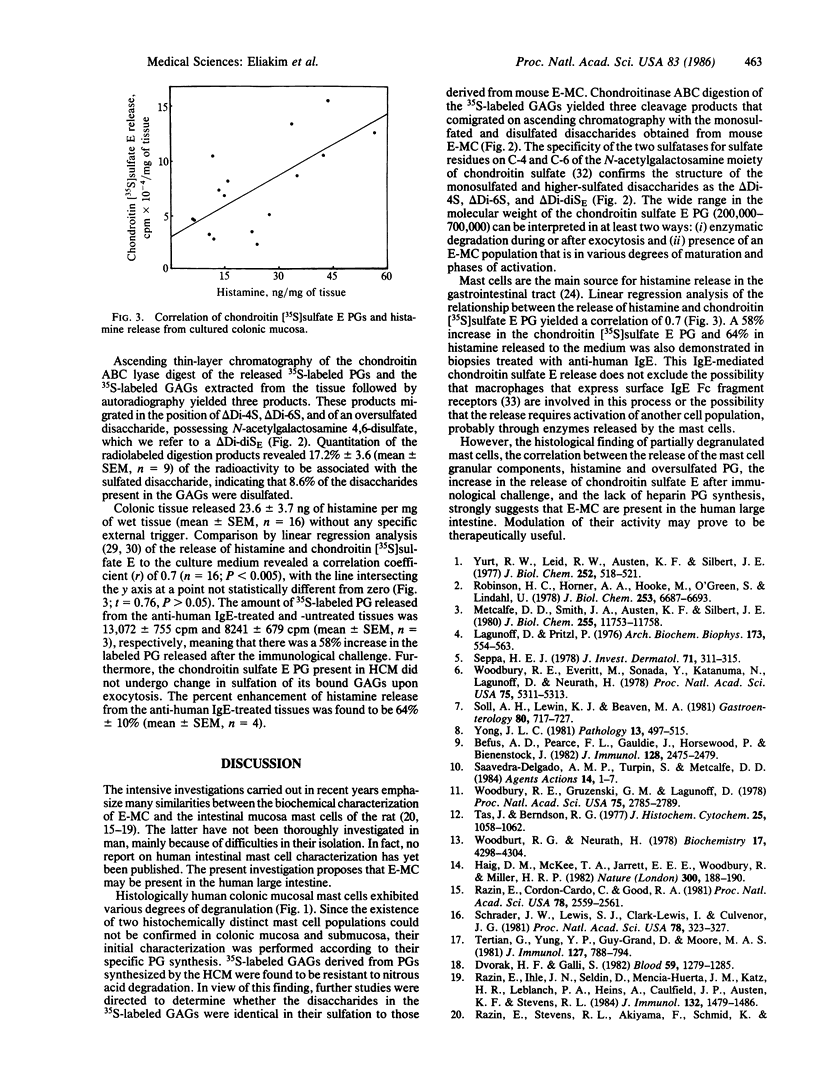
An association between the release of histamine and chondroitin sulfate E proteoglycan (PG) was demonstrated in human colonic mucosa (HCM). Colonic biopsy samples incorporated [35S]sulfate (2.7 X 10(6) +/- 188 X 10(3) cpm/mg of wet tissue; mean +/- SEM, n = 5) into PG, which was partially released into the culture medium during the incubation period. Ascending thin-layer chromatography of the released 35S-labeled PG after its digestion by chondroitin ABC lyase (chondroitinase, EC 4.2.2.4) followed by autoradiography yielded three products that migrated in the position of monosulfated disaccharides of N-acetylgalactosamine 4-sulfate and N-acetylgalactosamine 6-sulfate and of an oversulfated disaccharide possessing N-acetylgalactosamine 4,6-disulfate. Cultured colonic mucosa released 23.6 +/- 3.7 ng of histamine per mg of wet tissue (mean +/- SEM, n = 16) without any specific trigger. Comparison by linear regression analysis of the release of histamine and chondroitin [35S]sulfate E PG revealed a correlation coefficient (r) of 0.7 (n = 16; P less than 0.005). Histological examination of the colonic biopsies revealed the presence of many mast cells in various degrees of degranulation in the mucosa and submucosa, most of which were found in the submucosa. Incubation of the HCM biopsies in the presence of anti-human IgE revealed 58% +/- 12% (mean +/- SEM, n = 3) enhancement in the release of chondroitin [35S]sulfate E PG and 64% +/- 10% (mean +/- SEM, n = 4) of histamine release. The above correlation, the observation that most of the mast cells showed various degrees of degranulation, and the lack of heparin synthesis as opposed to the synthesis and immunological release of chondroitin sulfate E strongly suggest that the E mast cell exists in the human colon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anton A. H., Sayre D. F. A modified fluorometric procedure for tissue histamine and its distribution in various animals. J Pharmacol Exp Ther. 1969 Apr;166(2):285–290. [PubMed] [Google Scholar]
- Befus A. D., Pearce F. L., Gauldie J., Horsewood P., Bienenstock J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J Immunol. 1982 Jun;128(6):2475–2480. [PubMed] [Google Scholar]
- Binder V., Hvidberg E. Histamine content of rectal mucosa in ulcerative colitis. Gut. 1967 Feb;8(1):24–28. doi: 10.1136/gut.8.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Nabel G., Pyne K., Cantor H., Dvorak H. F., Galli S. J. Ultrastructural identification of the mouse basophil. Blood. 1982 Jun;59(6):1279–1285. [PubMed] [Google Scholar]
- Eastwood G. L., Trier J. S. Organ culture of human rectal mucosa. Gastroenterology. 1973 Mar;64(3):375–382. [PubMed] [Google Scholar]
- Haig D. M., McKee T. A., Jarrett E. E., Woodbury R., Miller H. R. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982 Nov 11;300(5888):188–190. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- Lagunoff D., Pritzl P. Characterization of rat mast cell granule proteins. Arch Biochem Biophys. 1976 Apr;173(2):554–563. doi: 10.1016/0003-9861(76)90292-7. [DOI] [PubMed] [Google Scholar]
- Metcalfe D. D., Smith J. A., Austen K. F., Silbert J. E. Polydispersity of rat mast cell heparin. Implications for proteoglycan assembly. J Biol Chem. 1980 Dec 25;255(24):11753–11758. [PubMed] [Google Scholar]
- Razin E., Cordon-Cardo C., Good R. A. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2559–2561. doi: 10.1073/pnas.78.4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Ihle J. N., Seldin D., Mencia-Huerta J. M., Katz H. R., LeBlanc P. A., Hein A., Caulfield J. P., Austen K. F., Stevens R. L. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984 Mar;132(3):1479–1486. [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Hamill A. L., Liu F. T., Katz D. H., Cohn Z. A. Secretion of leukotriene C and other arachidonic acid metabolites by macrophages challenged with immunoglobulin E immune complexes. J Exp Med. 1982 Oct 1;156(4):1077–1086. doi: 10.1084/jem.156.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Delgado A. M., Turpin S., Metcalfe D. D. Typical and atypical mast cells of the rat gastrointestinal system: distribution and correlation with tissue histamine. Agents Actions. 1984 Jan;14(1):1–7. doi: 10.1007/BF01966825. [DOI] [PubMed] [Google Scholar]
- Sack D. M., Peppercorn M. A. Drug therapy of inflammatory bowel disease. Pharmacotherapy. 1983 May-Jun;3(3):158–176. doi: 10.1002/j.1875-9114.1983.tb03245.x. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H. E. Rat skin main neutral protease: immunohistochemical localization. J Invest Dermatol. 1978 Nov;71(5):311–315. doi: 10.1111/1523-1747.ep12529791. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Lewin K. J., Beaven M. A. Isolation of histamine-containing cells from rat gastric mucosa: biochemical and morphologic differences from mast cells. Gastroenterology. 1981 Apr;80(4):717–727. [PubMed] [Google Scholar]
- Stevens R. L., Austen K. F. Effect of p-nitrophenyl-beta-D-xyloside on proteoglycan and glycosaminoglycan biosynthesis in rat serosal mast cell cultures. J Biol Chem. 1982 Jan 10;257(1):253–259. [PubMed] [Google Scholar]
- Stevens R. L., Razin E., Austen K. F., Hein A., Caulfield J. P., Seno N., Schmid K., Akiyama F. Synthesis of chondroitin sulfate E glycosaminoglycan onto p-nitrophenyl-beta-D-xyloside and its localization to the secretory granules of rat serosal mast cells and mouse bone marrow-derived mast cells. J Biol Chem. 1983 May 10;258(9):5977–5984. [PubMed] [Google Scholar]
- Tas J., Berndsen R. G. Does heparin occur in mucosal mast cells of the rat small intestine? J Histochem Cytochem. 1977 Sep;25(9):1058–1062. doi: 10.1177/25.9.71326. [DOI] [PubMed] [Google Scholar]
- Tertian G., Yung Y. P., Guy-Grand D., Moore M. A. Long-term in vitro culture of murine mast cells. I. Description of a growth factor-dependent culture technique. J Immunol. 1981 Aug;127(2):788–794. [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Everitt M., Sanada Y., Katunuma N., Lagunoff D., Neurath H. A major serine protease in rat skeletal muscle: evidence for its mast cell origin. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5311–5313. doi: 10.1073/pnas.75.11.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Gruzenski G. M., Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Neurath H. Purification of an atypical mast cell protease and its levels in developing rats. Biochemistry. 1978 Oct 3;17(20):4298–4304. doi: 10.1021/bi00613a029. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- Yong J. L. The mast cell: IV. An ultrastructural and autoradiographic study of the distribution and maturation of peritoneal mast cells in the rat. Pathology. 1981 Jul;13(3):497–515. doi: 10.3109/00313028109059067. [DOI] [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]