Abstract
Appropriate cleaning and disinfection procedures in zebrafish (Danio rerio) laboratories are crucial in preventing the spread of aquatic animal pathogens and minimizing the build-up of waste products and biologic matter. The procedures selected should accomplish these goals and incorporate the individual needs of various laboratories. In this study of a single zebrafish facility, we assessed the efficacy of 2 different cleaning and disinfection procedures for nets, tanks, and lids. ATP levels were used as a surrogate biomarker for microbial burden. We measured the number of relative light units (RLU), as an expression of the amount of ATP present, on items before and after disinfection and calculated the percentage reduction. We compared daily replacement of a commercial net disinfection product in J lab with weekly replacement in H lab and found a 96.6% reduction in RLU in H lab and a 91.2% reduction in J lab. These results indicate that either replacement schedule is effective. Evaluation of tanks and lids soaked in a bleach disinfection bath for 30 or 60 min revealed a 99.7% reduction in RLU at 30 min compared with 97.1% at 60 min. Therefore a 30-min soak in a bleach bath achieved a similar level of disinfection as did a 60-min soak. The current results demonstrate that these cleaning and disinfection methods are efficacious.
Abbreviation: SOP, standard operating procedure; RLU, relative light unit; RO, reverse osmosis
Cleaning is defined as the process of making free from dirt, foreign, and extraneous matter, whereas disinfection is the process of freeing from infection specifically by destroying harmful microorganisms.10 Maintaining good cleaning and disinfection procedures in a laboratory setting with zebrafish (Danio rerio) is crucial in preventing the spread of waterborne diseases, as well as reducing the build-up of waste products and biologic matter.4,6,5,7,12 To reach these goals, the disinfection procedures chosen must be effective and relevant to the individual needs of the laboratory.3 The cleaning and disinfection methods used by different zebrafish laboratories at our institution varied considerably. For instance, nets were either soaked in hot water for at least 10 min and dried11 or left to soak in a mixture of a commercial antimicrobial net disinfection product and reverse-osmosis–treated (RO) water for 1 h.12 Equipment such as tanks, baffles, lids, and feeding apparatuses and miscellaneous items used by animal care personnel, researchers, and laboratory personnel (carboys, air lines, squeeze bottles, and so forth) were disinfected by one of the previously mentioned methods in addition to use of a 1.98% or 5% bleach disinfection bath.12 In one facility, spawning traps and tanks were steam-autoclaved instead of soaking in bleach because these high-demand items required a more rapid turnaround due to their more frequent use compared with that of standard tanks used to house fish.12
In the current study, we evaluated 2 separate zebrafish rooms, H lab and J lab. The standard operating procedure (SOP) used in these labs for cleaning and disinfection was: 1) nets were rinsed with RO water, soaked for at least 1 h in a commercial net disinfection solution, rinsed with RO water, and air dried;12 2) Tanks, lids, feeding apparatuses, and miscellaneous items used by laboratory personnel and researchers were rinsed with RO water, soaked for at least 1 h in a 1.98% bleach disinfection bath, rinsed with RO water, and air dried.12 Important differences between the 2 rooms were J lab's larger size, greater number of tanks, and heavier traffic flow (Table 1). To reflect this difference, the SOP states that net disinfection solutions are replaced daily in J lab but weekly in H lab. The bleach disinfection baths in both rooms are exchanged weekly. The present study was conducted to validate the current cleaning and disinfection methods used by this facility.
Table 1.
Characteristics of H and J labs
| H lab | J lab | |
| Fish population | 13,914 | 19,586 |
| Tanks | 804 | 1103 |
| Racks | 24 | 29 |
The detection of ATP on environmental surfaces is indicative of the presence of organic matter including biofilm, feed residue, previous animal contact, and microorganisms.3,4 Organics present on surfaces provide a medium for microbial growth and can act as a barrier to cleaning or disinfection agents.4,9 To assess the efficacy of cleaning and disinfection methods, we used bioluminescent technology to detect residual ATP and recorded it as relative light units (RLU) by using a luminometer in conjunction with ATP testing swabs.3 ATP is present in the cells of all organisms and helps transport chemical energy within these cells.3,4,9 We selected this detection method because of its rapid turnover time in generating results, its ease of operation, and the low cost of the equipment and supplies needed relative to those of traditional culture or PCR detection methods.4,9 These current ATP detection systems have been validated as a surrogate testing method for replicate organism detection and counting plates; both of these methods have been used for several years to monitor the effectiveness of cleaning and disinfection procedures in hospitals, aerospace, veterinary surgery, and the food industry.1,2,4,8,9
In preliminary investigations, we measured RLU before and after cleaning and disinfection of different sized tanks (1, 3, and 9 L), lids, and nets. From these data, we calculated the percentage reduction in RLU values. An average of 98.5% reduction in RLU was seen for both cleaning and disinfection methods, with a maximum of 100% reduction and a minimum of 90% (data not shown). Setting a specific target RLU of nearly 100% reduction after cleaning and disinfection proved unreasonable for verifying effective cleaning and disinfection in a previous study.9 Reduction of RLU as a percentage proved to be a more efficient method in determining the best cleaning and disinfection procedure.9 Effective cleaning and disinfection quality was verified with RLU reductions of 70% to 100%.9 For the current study, we defined a 90% reduction in RLU as the target value for an acceptable level of disinfection. We hypothesized that: 1) the efficacy of commercial net disinfection solution in H lab would decrease over the week compared with that of J lab's, which is changed daily; 2) a 60-min soak in the bleach disinfection bath would continue to provide an average of 98.5% reduction in RLU over a week; and 3) a 30-min soak in the bleach disinfection bath would yield as high a percentage reduction in RLU as a 60-min soak.
Materials and Methods
All research conducted by the investigative groups using zebrafish housed in these facilities was approved by the IACUC of the University of Washington. The University of Washington has an assurance with the Office of Laboratory Animal Welfare and is AAALAC-accredited. All experiments were run in an active zebrafish animal housing facility.
Net disinfection solution.
Two experimental nets (Aquatic Eco-Systems, Apopka, FL) were tested prior to and after cleaning and disinfection according to the facility SOP. Nets were soiled by the routine procedure of moving fish from one tank to another. Nets were cleaned by high-pressure rinsing with RO water before disinfection by soaking (Figure 1) for 1 h in a commercial net-disinfection solution (active ingredients, benzalkonium chloride and methylene blue; Net Soak, Jungle Labs, Cincinnati, OH) prepared according to manufacturer recommendations (1 teaspoon [4.93 mL] product per 1 gal [3.8 L] RO water). Nets were then rinsed free of disinfectant with RO water.
Figure 1.
Opaque 5-gallon bucket used to hold nets and disinfection solution containing benzalkonium chloride and methylene blue. The lid is kept on the bucket when not in active use, to decrease light exposure and minimize evaporation.
The first experimental net was tested in the morning at no later than 1000; the second was tested at no later than 1500. A positive ‘dirty’ control and a negative ‘clean’ control were included in the study. The positive control was a net left soaking in a tank with live zebrafish for at least 1 h just prior to experimental evaluation, and the negative control was a net that had been cleaned and disinfected according to the facility SOP and that was clean, dry, and unused before testing. All nets were identified with written labels.
RLU were measured before disinfection and after the final rinsing step by using dry swabs (PocketSwab Plus, Charm Sciences, Lawrence, MA) and a luminometer (NovaLum, Charm Sciences; Figure 2). The surface sampling procedure followed the manufacturer's instructions, which indicated swabbing an area measuring 10 cm by 10 cm for no more than 5 s. The swab then was inserted into the meter for analysis, and an RLU value was displayed. H lab was tested on Sunday, Monday, Wednesday, and Friday for 1 wk. J lab was tested on 2 consecutive days, Sunday and Monday.
Figure 2.
Luminometer (NovaLum, Charm Sciences) with dry swab (PocketSwab, Charm Sciences).
Bleach bath.
One experimental 3-L lid and one 3-L tank were tested before and after cleaning and disinfection according to the facility SOP. J lab was chosen for this study, although both labs used the exact same methods. Tanks and lids were cleaned by using high-pressure RO water before being soaked (Figure 3) for 1 h in a 1.98% bleach disinfection bath (5.25% sodium hypochlorite, Clorox Bleach Regular, The Clorox Company, Oakland, CA; 1.5 L per 75.7 L [20 gal] RO water). Tanks and lids were rinsed free of disinfectant by using RO water. In addition, one 3-L lid and one 3-L tank were tested before and after a 30-min soak in the same bleach bath. All tests were run concurrently in the same bleach bath. All experimental soiled tanks had been in active use for 2 wk and generally had a visible film of either food or debris.
Figure 3.
Opaque bins used to hold the 1.98% bleach disinfection solution. The lid was kept on the bin when not in active use, to decrease light exposure and minimize evaporation.
Experimental testing began in the morning no later than 1000. A positive, ‘dirty’ control and a negative, ‘clean’ control were included in the study. The positive control was a visibly dirty (for example, debris and feed residue present), 3-L lid that had been used for 2 wk before being removed from a tank actively housing live zebrafish just prior to the experimental evaluation; the negative control was a 3-L tank that had been cleaned and disinfected according to the facility SOP and was clean, dry, and unused before testing. All experimental and control items were identified with written labels. The numbers of RLU before disinfection and after the final rinsing step were measured as described earlier. Testing was performed daily for 1 wk.
Statistical analysis.
Data were grouped according to the described disinfection method used by room and analyzed by using GraphPad Prism (GraphPad Software, La Jolla, CA) and Microsoft Office Excel 2007 (Microsoft, Redmond, WA). Linear regression models were used to report the best-fit value of the slope and intercept in comparing the percentage reduction data over time. Paired t tests were used to compare RLU values collected before and after cleaning and disinfection. A P value of less than 0.05 was considered statistically significant.
Results
Comparison of replacement frequency of commercial net disinfection solution.
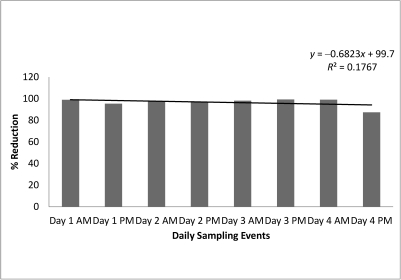
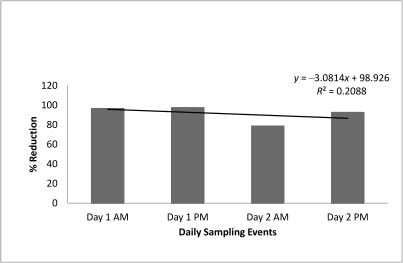
Weekly replacement of the net disinfection solution in H lab showed an average percentage reduction of 96.6% (Figure 4). Daily replacement of the net disinfection solution in J lab led to an average percentage reduction of 91.2% (Figure 5). Average RLU values before and after disinfection are summarized in Table 2 and include positive and negative control data. A one-tailed test comparing the numbers of RLU before and after a 60-min soak resulted in a P value of 0.0443 for J lab (significant) and a 0.00226 (very significant) for H lab.
Figure 4.
Percentage reduction in RLU seen in twice-daily samples (before disinfection with solution containing benzalkonium chloride and methylene blue and after final rinse). Day 1, Sunday; day 2, Monday; day 3, Wednesday; day 4, Friday.
Figure 5.
Percentage reduction in RLU seen in twice-daily samples (before disinfection with solution containing benzalkonium chloride and methylene blue and after final rinse). Day 1, Sunday; day 2, Monday.
Table 2.
Average RLU values before and after disinfection
| Before disinfection | After disinfection | |
| H lab nets | 1,620,705 | 35,203 |
| J lab nets | 2,387,599 | 132,506 |
| J lab lids | 2,732,254 | 12,531.07 |
| J lab tanks | 2,697,515 | 10,133.86 |
Comparison of 30- and 60-min soaking in bleach solution.
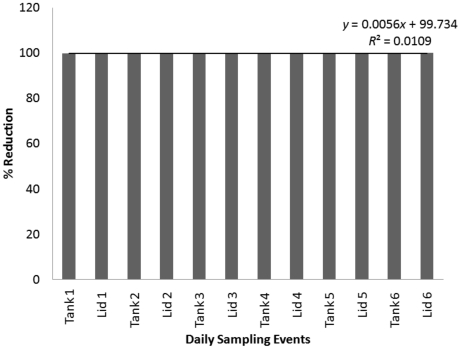
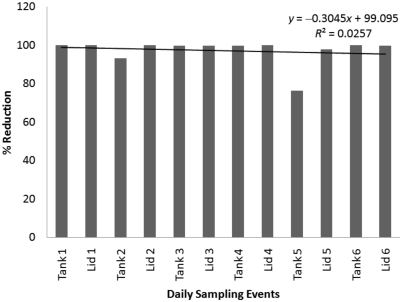
Disinfection by a 30-min soak in bleach (Figure 6) was tested daily for 1 wk and yielded an average percentage reduction of 99.7%. Disinfection by a 60-min soak in bleach (Figure 7) was tested daily for 1 wk and showed an average percentage reduction of 97.1%. Both of these mean values reflect the variability between the percentage reductions of individual samples over the respective time periods. Average RLU values before and after disinfection are summarized in Table 2 and include positive and negative control data.
Figure 6.
Percentage reduction in RLU seen in daily tests of one 3-L tank and one 3-L lid after 30 min in bleach solution. The numeral following ‘Tank’ or ‘Lid’ indicates the day on which the sample was obtained (day 1 through day 6, Sunday through Friday).
Figure 7.
Percentage reduction in RLU seen in daily tests of one 3-L tank and one 3-L lid after 60 min in bleach solution. The numeral following ‘Tank’ or ‘Lid’ indicates the day on which the sample was obtained (day 1 through day 6, Sunday through Friday).
One-tailed tests comparing the numbers of RLU before and after a 30- or 60-min soak resulted in P value of less than 0.0001 for both groups (extreme significance).
Discussion
The use of either a commercial net disinfection product or 1.98% bleach disinfection bath sufficiently disinfected common laboratory items in this zebrafish facility. Weekly replacement of the commercial net disinfection solution led to a higher percentage reduction in RLU from nets (96.6%) as compared with daily replacement (91.2%). A 30-min soak in a 1.98% bleach bath showed a slightly higher percentage reduction in RLU values of tanks and lids (99.7%) than did the required 60-min soak (97.1%). To our knowledge, the effectiveness of the cleaning and disinfection methods used by zebrafish facilities has not been evaluated previously. As a result, very little is known regarding the standards various facilities use to choose their cleaning and disinfection methods and the effectiveness of these procedures. In the current study, we analyzed 2 specific methods for disinfection of nets (commercial net disinfection product) and tanks and lids (1.98% bleach disinfection bath). Preliminary investigation confirmed the effectiveness of the 2 methods and resulted in an average 98.5% (range, 90% to 100%) reduction in RLU (ATP) present on individual items after disinfection. For the equipment sampled, the maximum value before disinfection was 38,207,030 RLU and the minimum value after disinfection was 0 RLU. Using these data, we considered a percentage reduction value of 90% or greater to be an effective indicator of adequate cleaning and disinfection of husbandry equipment.4 To further validate these results, these 2 methods, using a commercial net disinfection product or a 1.98% bleach disinfection bath, were assessed more closely in 2 different rooms.
The commercial net disinfection solutions are replaced at different intervals in the 2 rooms (weekly for H lab and daily for J lab). The daily replacement of the solution in J lab is in response to its higher traffic flow and greater number of fish and tanks compared with those in H lab (Table 1). This daily replacement strategy stems from the hypothesis that J lab nets would always be disinfected with the most effective concentration of the disinfection product. In comparison, H lab nets are placed in an older (and perhaps less effective) solution as days pass and usage increases. We hypothesized that the weekly replacement of the net disinfection solution in H lab would lead to less effective disinfection compared with that in J lab, and we assumed that with daily replacement of the solution in J lab, the data collected would be similar from day to day and therefore fewer samples could be taken. However, the percentage reduction in RLU was greater for H lab (96.6%) than J lab (91.2%). A one-tailed test comparing the numbers of RLU before and after a 60-min soak resulted in a P value of 0.0443 for J lab (significant) and a 0.00226 (very significant) for H lab. The results show that despite differences in the percentage reduction in RLU, both labs procedures can be considered effective in providing adequate disinfection. Differences in the results could be attributed to the facts that adding wet nets to the solution would dilute the solution and that small amounts of disinfectant are removed from the solution each time nets are removed. With a higher rate of usage, the more frequent addition of nets to and their removal from the commercial net disinfection solution in J lab potentially could reduce the final concentration of disinfectant and thus reduce its effectiveness. Finally, in the labeling of the stock bottles of this product, the vendor does not include a percentage value for the active disinfection ingredient, benzalkonium chloride. As a result, we were unable to determine or estimate the final concentration of this agent in the disinfectant solution used.
A commercial disinfection product was chosen over bleach, despite its higher disinfection efficacy, for use on nets for several reasons. Bleach is highly toxic at very low amounts to zebrafish (and other fish species).7 Bleach is used only on items that cannot absorb it, such as tanks, whereas nets potentially could absorb and retain this compound. As a result, the chance of fish coming into direct contact with bleach is minimal from exposed tanks but not nets. In addition, the nets used are made of a very fine nylon mesh, which would deteriorate quickly in bleach solution. Finally, the commercial disinfection product keeps nets soft, which is important for reducing skin trauma (for example, scale loss) during the netting and transfer process.
Both rooms replace the 1.98% bleach disinfection baths every Monday. For this experiment, we chose to study J lab because of its higher animal numbers, greater traffic flow, and higher rate of equipment turnover, even though both rooms follow the same methods of cleaning and disinfection. The tests for 30 and 60 min soaks were run concurrently in the same 1.98% bleach disinfection bath. The percentage reduction in RLU averaged 99.7% for a 30-min soak and 97.1% for a 60-min soak. One-tailed tests comparing the numbers of RLU before and after a 30-or 60-min soak resulted in P value of less than 0.0001 for both groups, indicating extreme significance. For this experiment, we chose 3-L tanks and lids because of the ease of sampling small areas and because they represented a large percentage of the tanks currently in use in both rooms. The results showed very low variability in the percentage reduction values calculated due to differences in soak time and that both soak times were effective in providing adequate disinfection. Therefore, a 30-min soak likely would be sufficient for each laboratory's needs. In addition, a 60-min contact time continued to deliver a high level of disinfection over 1 wk. Differences in the results could be due to increased agitation of the bleach bath solution during the placement of the items in the tank within the 30-min time period. Because these bleach baths typically are static (Figure 3), the process of adding materials to these containers mixes the solution and therefore could provide increased removal of residual organic debris from the equipment, thereby increasing the effectiveness of the disinfection.
In conclusion, the current study reports the effectiveness of 2 cleaning and disinfection methods, using a commercial net disinfection product and a 1.98% bleach disinfection bath, in which disinfectant concentration and frequency of solution replacement were the variables. The results support this laboratory's SOP requirements for high levels of disinfection and validate that these goals are indeed being met. However, this inquiry into the effectiveness of cleaning and disinfection practices for this zebrafish facility should be only the beginning. Many different methods used to clean and disinfect equipment in zebrafish facilities have yet to be evaluated. Important factors in deciding the appropriate cleaning and disinfection methods should consider several variables including, but not limited to, the type and frequency of equipment used, husbandry practices followed, number of zebrafish housed per enclosure or system, level of facility usage, system configuration and water quality, and type of research conducted.5,7 Comparisons between different methods likely would supply valuable data potentially leading to community-wide acceptable definition of what actually constitutes ‘effective cleaning and disinfection’ for zebrafish. This area of study could have important implications for the zebrafish community and researchers using other similar aquatic animal species.
Acknowledgments
We thank David White and John Huntington from the Department of Biological Structure for their support and the use of their facility resources for this project and Tia Lerud and Xiuwen Zheng from the Department of Biostatistics and Statistics for providing statistical consultation. Neither author has a competing financial interest in the selection or use of commercial products listed.
References
- 1.Cais-Sokolinska D, Pikul J. 2008. Evaluation of steel surface cleanliness level in dairies using the bioluminescence method. Bulletin of the Veterinary Institute in Pulawy 15:625–629 [Google Scholar]
- 2.Cais-Sokolinska D, Pikul J. 2008. Using the bioluminescence and microbiological contact methods in sustaining a proper hygienic level in food processing plants. Acta Scientarium Polonorum. Technologia Alimentaria 7:53–60 [Google Scholar]
- 3.Charm Sciences [Internet]. 2010. Charm Sciences-ATP FAQ's. [Cited September 2010]. Available at: http://www.charm.com.
- 4.Ednie D, Wilson R, Lang M. 1998. Comparison of 2 sanitation monitoring methods in an animal research facility. Contemp Top Lab Anim Sci 37:71–7412456174 [Google Scholar]
- 5.Institute for Laboratory Animal Research 2010. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 6.Kent M, Spitsbergen J, Matthews J, Fournie J, Westerfield M. [Internet]. 2007. ZIRC health services zebrafish disease manual. [Cited 01 September 2010]. Available at: http://zebrafish.org/zirc/health/diseaseManual.php.
- 7.Koerber A, Kalishman J. 2009. Preparing for a semiannual IACUC inspection of a satellite zebrafish (Danio rerio) facility. J Am Assoc Lab Anim Sci 48:65–75 [PMC free article] [PubMed]
- 8.Leon M, Albrecht J. 2007. Comparison of adenosine triphosphate (ATP) bioluminescence and aerobic plate counts (APC) on plastic cutting boards. Journal of Food Service 18:145–152 [Google Scholar]
- 9.McPherson J, Savarino J. 2007. Using bioluminescence technology to monitor kennel sanitizing procedures. Tech Talk 12:1–3 [Google Scholar]
- 10.Merriam-Webster's new collegiate dictionary, 11th ed, p 246, 363. 2008. Springfield (MA): Merriam-Webster [Google Scholar]
- 11.Parichy D. [Internet]. 2008. ParichyLab_protocols. [Cited 01 September 2010]. Available at: http://protist.biology.washington.edu/dparichy/ProtocolsPage.htm
- 12.White D. [Internet]. 2005. UW HSB zebrafish facilities. [Cited 01 September 2010]. Available at: http://sites.google.com/a/uw.edu/uw-hsb-zebrafish-facilities/