Abstract
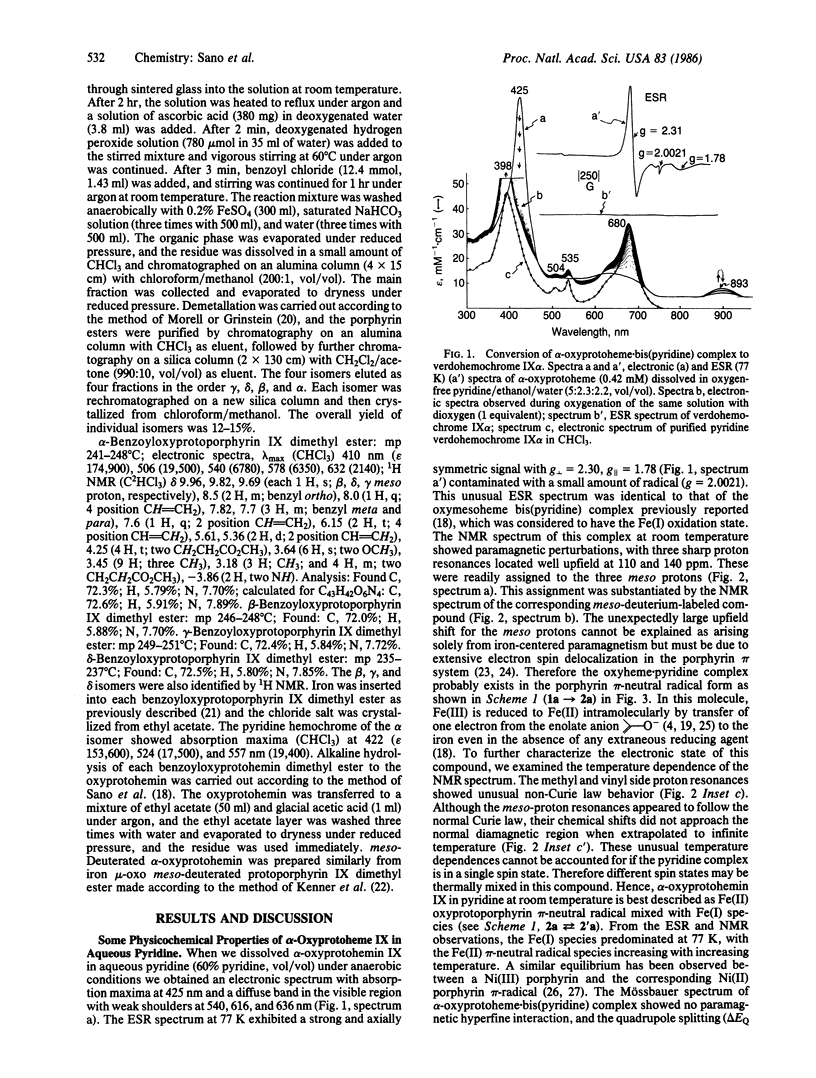
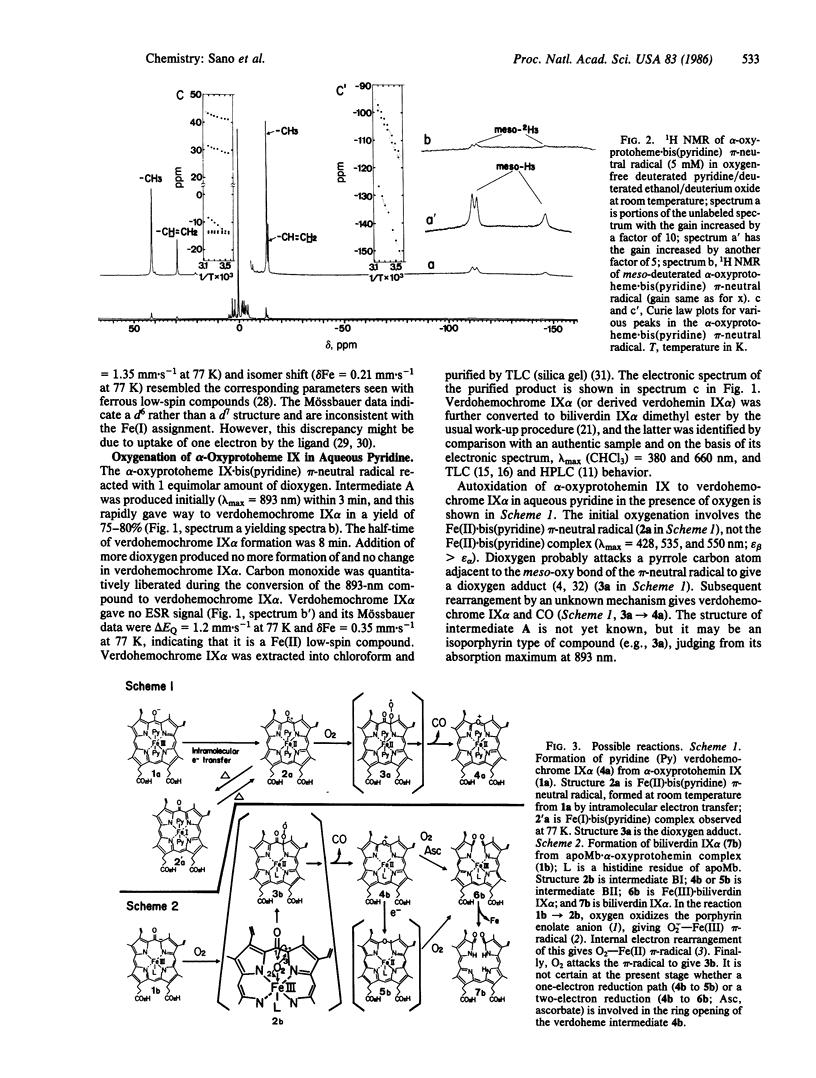
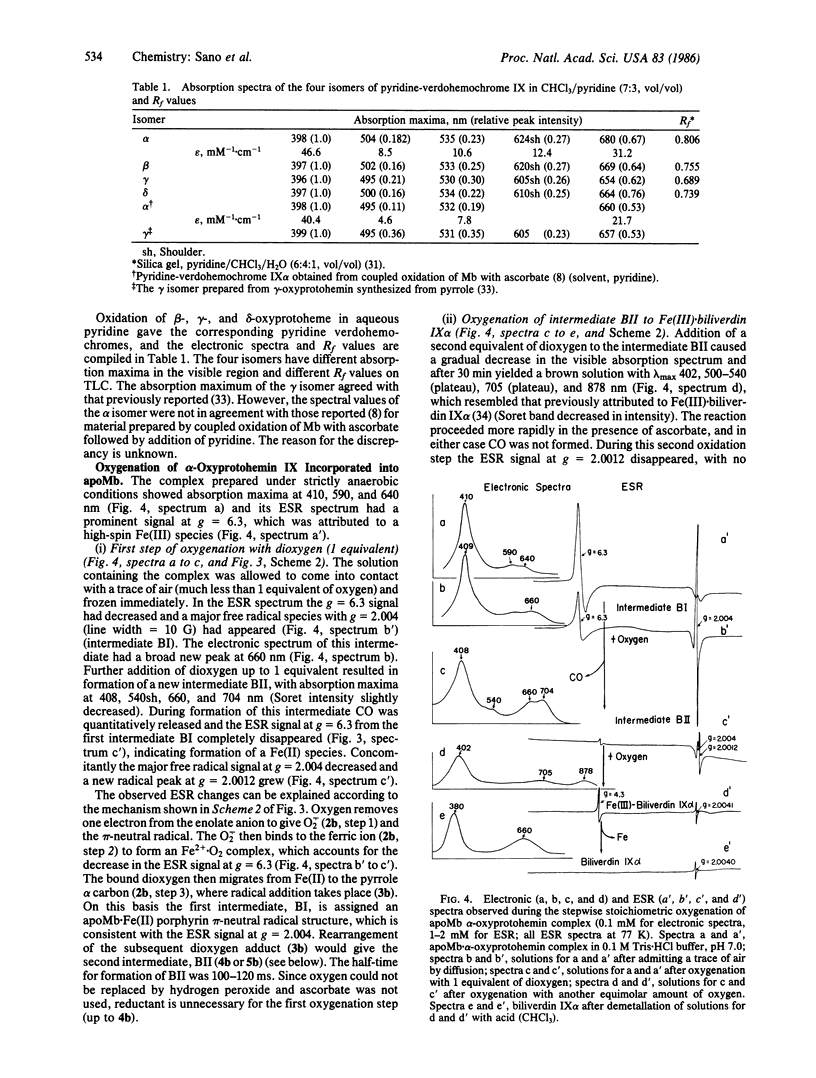
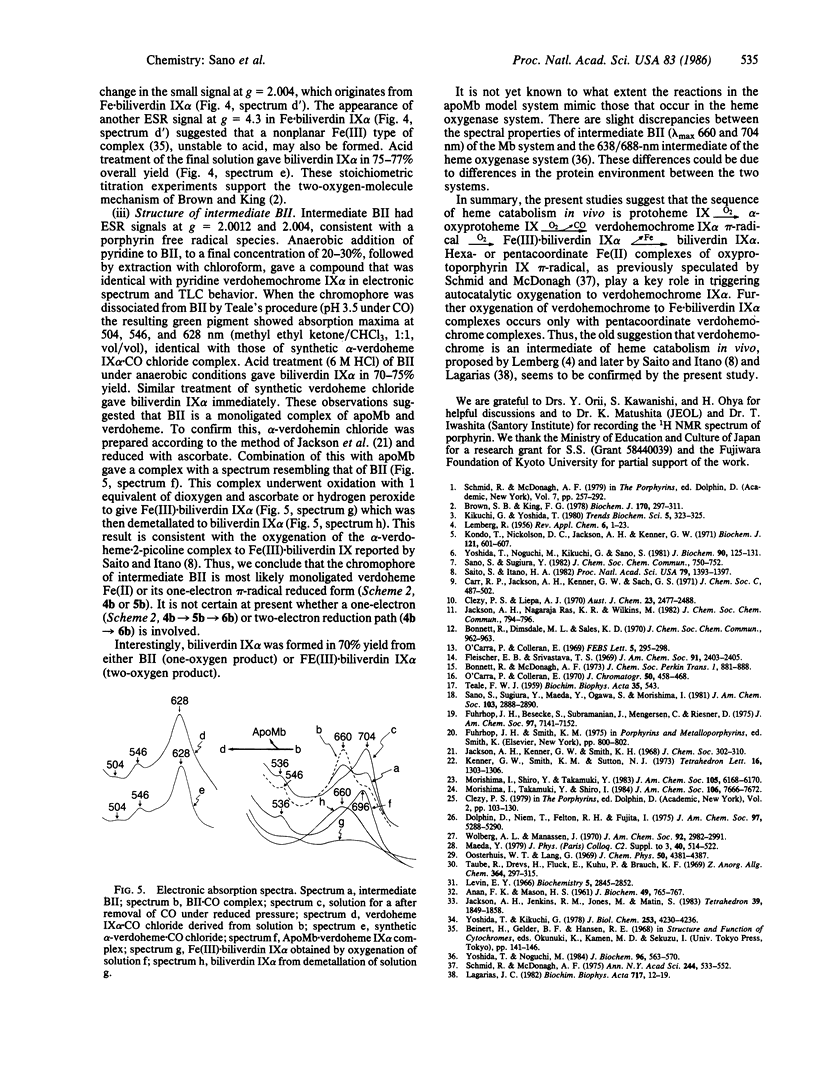
alpha-Oxyprotohemin IX, an early intermediate in heme catabolism, was synthesized and its autoxidation to biliverdin IX alpha was studied. In anaerobic aqueous pyridine, alpha-oxyprotohemin (hexacoordinated) underwent autoreduction to yield an Fe(II) alpha-oxyprotoporphyrin pi-neutral radical bis(pyridine) complex, which reacted with an equimolar amount of dioxygen to give pyridine.verdohemochrome IX alpha and CO in 75-80% yield via an intermediate with an absorption maximum at 893 nm. Verdohemochrome IX alpha did not react with further dioxygen. Reconstituted apomyoglobin.alpha-oxyprotohemin IX complex (pentacoordinated) reacted with an equimolar amount of dioxygen to form an Fe(II) oxyporphyrin pi-neutral radical intermediate, which rearranged to a green compound (lambda max 660 and 704 nm) with elision of CO. The green product, which is probably an apomyoglobin.verdoheme pi-radical complex, reacted with another equimolar amount of dioxygen to give Fe(III).biliverdin IX alpha. Demetallation of this gave biliverdin IX alpha in overall yield of 70-75%. These results indicate that the sequence of oxyheme autoxidation in the presence of apomyoglobin is alpha-oxyprotoheme IX O2----CO----verdohemochrome IX alpha pi-radical O2----Fe(III).biliverdin IX alpha. A similar mechanism may prevail in vivo. The hexa- and pentacoordinated Fe(II) pi-radical form of the oxyporphyrin is crucial in triggering the autoxidation of the complex to verdohemochrome IX alpha. Further oxygenation of verdohemochrome IX alpha to Fe(III).biliverdin IX alpha occurred only in the pentacoordinated apomyoglobin.verdoheme Fe(II) complex.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 'Carra P. O., Colleran E. HAEM catabolism and coupled oxidation of haemproteins. FEBS Lett. 1969 Nov 29;5(4):295–298. doi: 10.1016/0014-5793(69)80372-8. [DOI] [PubMed] [Google Scholar]
- ANAN F. K., MASON H. S. An 018 study of the hemoglobin degradation to biliverdin in the model reaction. J Biochem. 1961 Jun;49:765–767. doi: 10.1093/oxfordjournals.jbchem.a127369. [DOI] [PubMed] [Google Scholar]
- Bonnett R., McDonagh A. F. The meso-reactivity of porphyrins and related compounds. VI. Oxidative cleavage of the haem system. The four isomeric biliverdins of the IX series. J Chem Soc Perkin 1. 1973;9:881–888. doi: 10.1039/p19730000881. [DOI] [PubMed] [Google Scholar]
- Brown S. B., King R. F. The mechanism of haem catabolism. Bilirubin formation in living rats by [18O]oxygen labelling. Biochem J. 1978 Feb 15;170(2):297–311. doi: 10.1042/bj1700297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin D., Niem T., Felton R. H., Fujita I. Letter: Reversible intramolecular electron transfer in an oxidized nickel porphyrin. J Am Chem Soc. 1975 Sep 3;97(18):5288–5290. doi: 10.1021/ja00851a050. [DOI] [PubMed] [Google Scholar]
- Kondo T., Nicholson D. C., Jackson A. H., Kenner G. W. Isotopic studies of the conversion of oxophlorins and their ferrihaems into bile pigments in the rat. Biochem J. 1971 Feb;121(4):601–607. doi: 10.1042/bj1210601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarias J. C. The structure of verdohemochrome and its implications for the mechanism of heme catabolism. Biochim Biophys Acta. 1982 Jul 16;717(1):12–19. doi: 10.1016/0304-4165(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Levin E. Y. Studies on verdohemochrome. Biochemistry. 1966 Sep;5(9):2845–2852. doi: 10.1021/bi00873a010. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Colleran E. Separation and identification of biliverdin isomers and isomer analysis of phycobilins and bilirubin. J Chromatogr. 1970 Aug 12;50(3):458–468. doi: 10.1016/s0021-9673(00)97973-1. [DOI] [PubMed] [Google Scholar]
- Saito S., Itano H. A. Verdohemochrome IX alpha: preparation and oxidoreductive cleavage to biliverdin IX alpha. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1393–1397. doi: 10.1073/pnas.79.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R., McDonagh A. F. The enzymatic formation of bilirubin. Ann N Y Acad Sci. 1975 Apr 15;244:533–552. doi: 10.1111/j.1749-6632.1975.tb41553.x. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Wolberg A., Manassen J. Electrochemical and electron paramagnetic resonance studies of metalloporphyrins and their electrochemical oxidation products. J Am Chem Soc. 1970 May 20;92(10):2982–2991. doi: 10.1021/ja00713a010. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Features of the reaction of heme degradation catalyzed by the reconstituted microsomal heme oxygenase system. J Biol Chem. 1978 Jun 25;253(12):4230–4236. [PubMed] [Google Scholar]
- Yoshida T., Noguchi M. Features of intermediary steps around the 688-nm substance in the heme oxygenase reaction. J Biochem. 1984 Aug;96(2):563–570. doi: 10.1093/oxfordjournals.jbchem.a134868. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Noguchi M., Kikuchi G., Sano S. Degradation of mesoheme and hydroxymesoheme catalyzed by the heme oxygenase system: involvement of hydroxyheme in the sequence of heme catabolism. J Biochem. 1981 Jul;90(1):125–131. doi: 10.1093/oxfordjournals.jbchem.a133441. [DOI] [PubMed] [Google Scholar]


