Abstract
Yeast phosphatidylinositol-transfer protein (Sec14p) is essential for Golgi secretory function and cell viability. This requirement of Sec14p is relieved by genetic inactivation of the cytidine diphosphate-choline pathway for phosphatidycholine (PtdCho) biosynthesis. Standard phenotypic analyses indicate that inactivation of the phosphatidylethanolamine (PtdEtn) pathway for PtdCho biosynthesis, however, does not rescue the growth and secretory defects associated with Sec14p deficiency. We now report inhibition of choline uptake from the media reveals an efficient “bypass Sec14p” phenotype associated with PtdEtn-methylation pathway defects. We further show that the bypass Sec14p phenotype associated with PtdEtn-methylation pathway defects resembles other bypass Sec14p mutations in its dependence on phospholipase D activity. Finally, we find that increased dosage of enzymes that catalyze phospholipase D-independent turnover of PtdCho, via mechanisms that do not result in a direct production of phosphatidic acid or diacylglycerol, effect a partial rescue of sec14-1ts-associated growth defects. Taken together, these data support the idea that PtdCho is intrinsically toxic to yeast Golgi secretory function.
INTRODUCTION
Sec14p represents the major phosphatidylinositol/phosphatidycholine (PtdIns/PtdCho) transfer protein in yeast (Bankaitis et al., 1989, 1990). Analyses of mutations that allow yeast to survive in the absence of the normally essential Sec14p demonstrate that Sec14p integrates phospholipid metabolism with the phospholipid requirements of Golgi secretory function (Cleves et al., 1991b; Kearns et al., 1998). Our early hypotheses concerning the essential role of Sec14p in stimulating yeast Golgi secretory processes posited a Sec14p-mediated modulation of an intrinsic toxicity of PtdCho to Golgi function (Cleves et al., 1991a,b; McGee et al., 1994). One key finding that led to this hypothesis was that genetic inactivation of the cytidine diphosphate (CDP)-choline pathway for PtdCho biosynthesis effects “bypass Sec14p,” whereas defects in the PtdEtn-methylation pathway do not. These effects were observed irrespective of the choline content of the medium upon which the bypass Sec14p mutants were selected and scored at 37°C (Cleves et al., 1991b). Although issues of differential localization of these two pathways were suggested to account for this puzzling specificity, the concept was also raised that metabolic flux through the CDP-choline pathway exerts its toxic effects by consuming a critical metabolite (McGee et al., 1994). Demonstrations that the PtdCho-bound form of Sec14p down-regulates CDP-choline pathway activity emphasizes the antagonistic relationship between the CDP-choline pathway and Golgi secretory function (McGee et al., 1994; Skinner et al., 1995; Phillips et al., 1999). This body of evidence led to our proposal that DAG is a key stimulator of Golgi secretory function (Kearns et al., 1997, 1998).
We now report that defects in PtdCho biosynthesis via the PtdEtn-methylation pathway can also effect bypass Sec14p. We demonstrate that sec14-1ts mutants engage in a cycle of PtdCho turnover and recapture of excreted choline that activates salvage of the released choline via the CDP-choline pathway. This cycle obscures the bypass Sec14p effects that are associated with PtdEtn-methylation pathway dysfunction. Conditions of either a genetic or environmental nature that prevent active choline salvage endow Sec14p-independent growth to yeast mutants deficient in PtdEtn-methylation pathway activity. The bypass Sec14p associated with PtdEtn-methylation pathway defects resembles all other known pathways for bypass Sec14p in that it depends on an active phospholipase D (PLD) (Sreenivas et al., 1998; Xie et al., 1998). Finally, we demonstrate that increased rates of PtdCho degradation via pathways that are independent of PLD and do not result in phosphatidic acid (PtdOH) or diacylglycerol (DAG) formation, also exert a partial suppression of sec14-1ts-associated growth defects.
Taken together, these data support the concept that PtdCho is intrinsically toxic to yeast Golgi function, and that a primary function of Sec14p is to maintain a Golgi PtdCho composition that is permissive for efficient transport of proteins from this organelle. This conclusion supports our early proposals that elevated PtdCho in Golgi membranes is incompatible with Golgi secretory function (Cleves et al., 1991a,b).
MATERIALS AND METHODS
Yeast Strains, Plasmids, and Genetic Techniques
Yeast strains used in this study are listed in Table 1. Media and standard genetic techniques have been described (Ito et al., 1983; Rothstein, 1983; Sherman et al., 1983). Plasmid shuffle assays for complementation of sec14Δ were performed with yeast strain CTY1461 (Table 1) as described (Lopez et al., 1994; Phillips et al., 1999). The plasmids used in this study are listed in Table 2. Further details are available from us upon request.
Table 1.
Yeast strains
| Strain | Genotype | Origin |
|---|---|---|
| CTY182 | MATa, ura3-52, Δhis3-200, lys2-801am | Bankaitis et al., (1989) |
| CTY1-1A | MATa, ura3-52, Δhis3-200, lys2-801am, sec14-1ts | Bankaitis et al., (1989) |
| CTY2-1C | MATα, ade2-101, sec14-1ts | Cleves et al., (1989) |
| CTY102 | MATa, ura3-52, Δhis3-200, lys2-801am, sec14-1ts, pct1-2 | Cleves et al., (1991b) |
| CTY950 | MATa, CTY1-1A/YEplac195 | This study |
| CTY1374 | MATa, CTY1-1A, cho2 | This study |
| CTY1375 | MATa, CTY1-1A, opi3 | This study |
| CTY1376 | MATa, CTY1-1A, cho2, hnmlΔ319∷URA3 | This study |
| CTY1377 | MATa, CTY1-1A, opi3, hnmlΔ319∷URA3 | This study |
| CTY1461 | MATα, ura3-52, Δhis3-200, ade2, ade3, leu2, sec14Δp∷hisG, YEp(SEC14) | This study |
| CTY1469 | MATα, CTY1461, hnmlΔp∷URA3 | This study |
| CTY1470 | MATα, CTY1461, hnmlΔp∷URA3, cho2Δ409∷HIS3 | This study |
| CTY1471 | MATα, CTY1461, hnmlΔp∷URA3, opi3Δ93∷HIS3 | This study |
| CTY1473 | MATα, ura3-52, Δhis3-200, sec14-1ts, ino4 | This study |
| CTY1485 | MATα, ura3-52,Δhis3-200, lys2-801am, Δtrpl, sec14-1ts, hnmlΔ319∷URA3, spo14Δ∷URA3, opi3 | This study |
| CTY1498 | MATa, CTY182, hnmlΔp∷URA3 | This study |
| CTY1499 | MATa, CTY1-1A, hnmlΔp∷URA3 | This study |
| CTY1500 | MATa, CTY1-1A, cho2, hnmlΔp∷URA3 | This study |
| CTY1501 | MATa, CTY1-1A, opi3, hnm1Δp∷URA3 | This study |
| CTY1502 | MATa, CTY182, hnmlΔp∷HIS3 | This study |
| CTY1504 | MATa, CTY1-1A, cho2, hnmlΔp∷HIS3 | This study |
| CTY1505 | MATa, CTY1-1A, opi3, hnmlΔp∷HIS3 | This study |
| CTY1506 | MATa, CTY182, hnmlΔp∷HIS3, spo14Δ∷URA3 | This study |
| CTY1507 | MATa, CTY1-1A, hnmlΔp∷URA3, spo14Δ∷HIS3 | This study |
| CTY1512 | MATa, CTY1-1A, cho2, hnmlΔp∷HIS3,, spo14Δ∷URA3 | This study |
| CTY1513 | MATa, CTY1-1A, opi3, hnmlΔp∷HIS3,,spo14Δ∷URA3 | This study |
| CTY1519 | MATα, CTY1485, YCp(SPO14) | This study |
| CTY1520 | MATα, CTY1485, YCp(spo14K→H) | This study |
| CTY1521 | MATα, CTY1485, YCp(spo14ΔN) | This study |
| CTY1522 | MATα, CTY1485, YEp(spo14K→H) | This study |
| CTY1523 | MATα, CTY1485, YEp(spo14ΔN) | This study |
| CTY1528 | MATa, CTY1-1A/YEp(PPGK∷PLB1) | This study |
| CTY1529 | MATa, CTY1-1A YEp(PIK1) | This study |
| CTYD174 |
MATa, ura3-52,
Δhis3-200, lys2-801am, TRP1, sec14-1ts,
css1 MATα, ura3-52 Δhis3-200 lys2-801am Δtrp1 sec14-1ts cho2Δ∷HIS3 |
This study |
| CTYD175 |
MATa, ura3-52, Δhis3-200,
lys2-801am, ADE2, TRP1, sec14-1ts,
css2 MATα, ura3-52 Δhis3-200 LYS2 ade2 Δtrp1 sec14-1ts opi3Δ∷URA3 |
This study |
| CTYD176 |
MATa, ura3-52, Δhis3-200,
lys2-801am, sec14-1ts,
ino4Δ∷URA3 MATα, ura3-52 Δhis3-200 LYS2 sec14-1ts css3 |
Table 2.
Plasmids
| Plasmid | Designation | Origin |
|---|---|---|
| YCp(OPI3, HISΔ3) | pCTY749 | This study |
| YCp(CHO2, URA3) | pCTY750 | This study |
| YCp(INO4, URA3) | pCTY780 | This study |
| YCp(SPO14, URA3) | pCTY747 | This study |
| YCp(SPO14K→H, HISΔ3) | pCTY793 | This study |
| YCp(SPO14ΔN, HISΔ3) | pCTY794 | This study |
| YEp(SPO14K→H, HISΔ3) | pCTY795 | This study |
| YEp(SPO14ΔN, HISΔ3) | pCTY796 | This study |
| YCp(PPGK∷PLB1, URAΔ3) | pCTY775 | This study |
| YEp(PIK1, URAΔ3) | pCTY797 | Walch-Solimena and Novick (1999) |
Isolation of css Mutations
sec14-1ts strains were cultured in liquid I+C− media at 26°C, culture aliquots were spread plated onto I+C− agar, and the plates were incubated at 35°C for 5 d. Revertant colonies were isolated, patched onto I+C− plates, and replica plated onto I+C+ and I+C− agar plates. The replicas were incubated at 37°C for 36 h and scored for growth relative to the sec14-1ts control strain. The revertants whose growth was improved on I+C− agar but not on I+C+ agar were saved. These css mutants were subsequently confirmed by their ability to form single colonies on I+C−, but not I+C+, agar at 35°C.
Invertase Assay and Immunoprecipitation of Carboxypeptidase Y (CPY)
Invertase assays were performed as described by Salama et al. (1990). CPY was precipitated from cell-free lysates prepared from radiolabeled yeast strains exactly as described previously (Bankaitis et al., 1989; Cleves et al., 1991; Fang et al., 1996).
Briefly, appropriate yeast strains were grown in Wickerham's minimal media to mid-logarithmic phase at 26°C, shifted to 37°C for 2 h, and pulse-radiolabeled with 35S-labeled amino acids for 30 min. Proteins were precipitated with trichloroacetic acid and solubilized in SDS buffer. Immunoprecipitation of CPY antigen, and separation and analysis of different CPY species by SDS-PAGE and phosphorimaging have been described (Bankaitis et al., 1989; Cleves et al., 1991; Fang et al., 1996).
Phospholipid Determinations
For measurements of steady-state phospholipid compositions, appropriate yeast strains were grown for five to six generations at 26°C in I+C− or I+C+media in the presence [32P]orthophosphate (10 μCi/ml). In pulse-radiolabeling experiments, appropriate yeast strains were grown in I+C− or I+C+ media to mid-logarithmic phase at 26°C, and shifted to 33.5°C for 2 h. [32P]Orthophosphate was then added into the media to 10 μCi/ml and cells incubated in the presence of the label at 33.5°C for 20 min. Incorporation of label was terminated by addition of trichloroacetic acid to 5% and phospholipid extraction, resolution by two-dimensional paper chromatography, and quantification of individual phospholipid species was performed as described in detail elsewhere (McGee et al., 1994; Rivas et al., 1999; Li et al., 2000).
RESULTS
Isolation of Mutations that Suppress sec14-1ts Growth Defects in a Choline-sensitive Manner
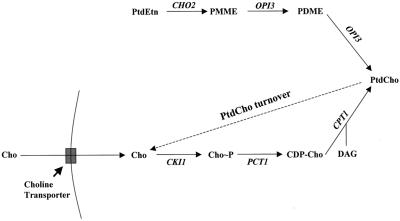
We previously described the rationale for a genetic screen designed to identify genes involved in promoting PtdCho turnover (Xie et al., 1998). Briefly, defects in the CDP-choline pathway for PtdCho biosynthesis relieve the normally essential Sec14p requirement for Golgi secretory function and cell viability (Figure 1A; Cleves et al., 1991a,b). This bypass Sec14p effect is observed even when yeast are grown in choline-free (C−) media (Cleves et al., 1991b). Under these conditions, the CDP-choline pathway simply serves as a salvage pathway that scavenges the choline liberated by PtdCho turnover and reuses it in a round of PtdCho resynthesis. Because yeast cannot synthesize choline de novo, this cycle does not contribute to net cellular PtdCho synthesis. On the basis of those data we reasoned that defects in pathways for PtdCho turnover that liberate free choline should impede metabolic flux through the CDP-choline pathway when yeast are grown in C−, but not in C+, media. Such defects elicit bypass Sec14p phenotypes that are sensitive to choline in the medium (Figure 1A).
Figure 1.
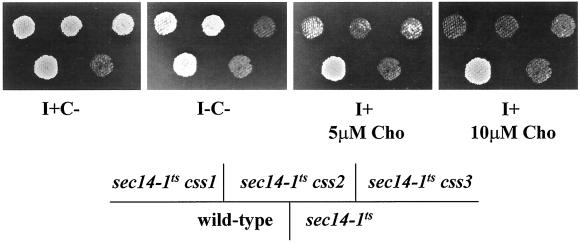
(A) The two pathways for PtdCho biosynthesis in yeast. Mutations in CDP-choline pathway, but not in PtdEtn-methylation pathway, suppress the essential requirement of Sec14p for Golgi secretory function and cell viability. CDP-choline pathway activity is supported by endogenous choline derived from PtdCho turnover, and by exogenous choline, which is captured by the choline transporter. In the absence of exogenous choline, PtdCho turnover represents the sole pathway for choline production. Genetic designations for the structural genes encoding enzymes for PtdCho synthesis and turnover are given at the corresponding execution points. The role of the choline transporter is also illustrated. (B) Choline-sensitive suppressors of sec14 (css). Indicated strains were patched on YPD plate and incubated at 26°C for 24 h. The cell patches were then replica plated onto minimal plates supplemented with 1 mM inositol but no choline (I+C−), or with 1 mM inositol and choline at indicated concentrations (I+ 5 μM Cho and I+ 10 μM Cho), or neither inositol nor choline (I−C−). The replica plates were then incubated at 37°C for 48 h. Isogenic strains were used: CTY182 (wild-type), CTY1-1A (sec14-1ts), CTY1374 (sec14-1ts css1), CTY1375 (sec14-1ts css2), CTY1473 (sec14-1ts css3).
One of the genes we expected to identify in this screen for choline-sensitive suppressors of sec14 defects (css mutations) was the structural gene encoding phospholipase D (PLD). A direct test of this predicted outcome was made possible by finding that the nonessential SPO14 is the PLD structural gene (Rose et al., 1995). Counter to expectations, we found that PLD activity is unconditionally essential for all known mechanisms of bypass Sec14p (Xie et al., 1998). This finding indicated that the role of PLD in bypass Sec14p is more complex than anticipated, and suggested that we did not fully comprehend the relationship between PtdCho metabolism and Sec14p function in yeast.
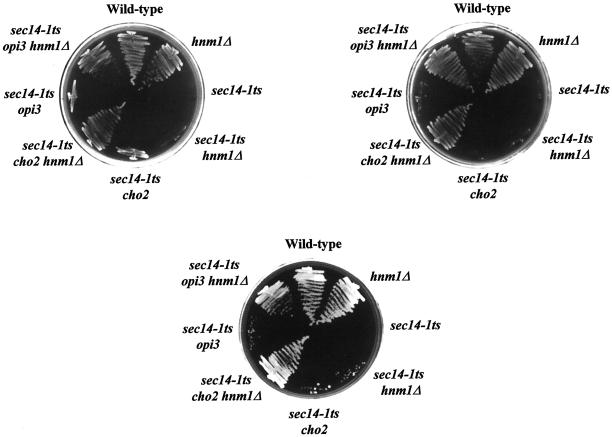
To further investigate how PtdCho metabolism might interface with Sec14p function, we isolated and characterized css mutations (see MATERIALS AND METHODS). A total of 43 independent and spontaneously arising css mutants was isolated. Standard dominance tests, meiotic segregation, and genetic complementation analyses were performed to assign these mutations into complementation groups. A difficulty we encountered in such analyses of css mutants was that growth of all sec14-1ts css mutants is extremely poor on I+C− plates at 37°C. Indeed, these mutants fail to form single colonies under these conditions, although single colonies are formed at 35°C. Reliable visualization of the improved residual growth of sec14-1ts css mutants on I+C− plates under conditions restrictive for growth of sec14-1ts parental strains required replica plating at 37°C (Figure 3A). Streak plating of sec14-1ts css mutants for isolated colonies at 35°C was also used to confirm the results gathered by replica plating.
Figure 3.
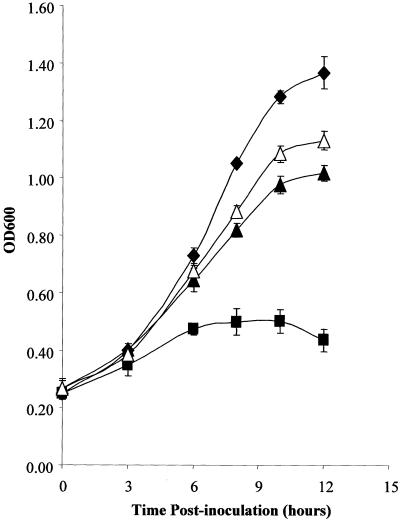
(A) Prevention of choline uptake enables PtdEtn-methylation pathway dysfunction to efficiently suppress sec14-1ts growth defects. Appropriate strains were streaked on indicated plates and incubated at 37°C for 48 h. Strains used were: CTY182 (wild type), CTY1-1A (sec14-1ts), CTY1374 (sec14-1ts cho2), CTY1375 (sec14-1ts opi3), CTY1498 (hnm1Δ::URA3), CTY1499 (sec14-1ts hnm1Δ::URA3), CTY1500 (sec14-1ts cho2 hnm1Δ::URA3), CTY1501 (sec14-1ts opi3 hnm1Δ::URA3). (B) PtdEtn-methylation pathway mutations efficiently restore growth to the sec14-1ts strain at 37°C in liquid I+C− media. Appropriate strains were picked up from freshly streaked YPD plates, inoculated into liquid defined media supplemented with inositol but no choline, and incubated at 37°C with shaking. Aliquots were collected for determination of OD600 at indicated times. Strains used were CTY182 (wild-type; closed diamonds), CTY1-1A (sec14-1ts; closed squares), CTY1375 (sec14-1ts opi3; closed triangles), CTY1377 (sec14-1ts opi3 hnm1Δ::URA3; open triangles). (C) Choline recapture visualized by cross-feeding. Patches of a sec14-1ts opi3 strain and a sec14-1ts opi3 hnm1Δ strain (choline feeders) were deposited on a lawn of sec14-1ts opi3 strain (indicator) on an I+C− plate and incubated at 37°C for 36 h. The growth of indicator cells surrounding the sec14-1ts opi3 hnm1Δ feeder was inhibited, as indicated by a halo surrounding the feeder. This inhibition did not occur when the sec14-1ts opi3 strain, which is competent for choline reuptake, was used as feeder. No halo was observed when the incubation temperature is 26°C or when the sec14-1ts opi3 hnm1Δ strain was used as indicator. Strains used were isogenic and included: CTY1375 (sec14-1ts opi3), CTY1500 (sec14-1ts opi3 hnm1Δ::URA3). (D) Rate of PtdCho biosynthesis via CDP-choline pathway correlates with the suppression of sec14 growth defects in I+C+ or I+C− media. Indicated strains were cultured to early logarithmic phase at 26°C in either I+C+ (black bars) or I+C− minimal media (open bars). Cultures were subsequently shifted to 33.5°C for 1 h, and pulse-radiolabeled with [32P]orthophosphate (10 μCi/ml) at 33.5°C for 20 min. Bulk glycerophospholipids were extracted, resolved, and quantitated by phosphoimaging. Incorporation of 32P into each phospholipid species is indicated as a percentage of total incorporation of radiolabel into extractable phospholipid. The strains used were isogenic and included: CTY182 (wild-type), CTY1-1A (sec14-1ts), CTY102 (sec14-1ts pct1-2), CTY1374 (sec14-1ts cho2), CTY1499 (sec14-1ts hnm1Δ::URA3), CTY1500 (sec14-1ts cho2 hnm1Δ::URA3). These data represent averages from at least three independent experiments.
A combination of dominance tests and complementation analyses demonstrated that all 43 css mutations are recessive to wild type and define three complementation groups. These were designated css1 (37 representatives), css2 (2 representatives), and css3 (4 representatives), respectively. Meiotic segregation analyses further demonstrated that each individual complementation group corresponds to a linkage group, indicating that the 43 css mutations identify three unlinked genes.
As shown in Figure 1B, the growth of all sec14-1ts css mutants is exquisitely sensitive to exogenous choline at restrictive temperatures. Introduction of low concentrations of choline into agar plates (5 μM final concentration) results in a strong inhibition of growth, whereas increasing exogenous choline to a concentration of 10 μM inhibits growth completely (Figure 1B). Choline concentrations as low as 1 μM also detectably inhibit growth. By contrast, exogenous ethanolamine (1 mM) has no effect on the growth of sec14-1ts css strains, irrespective of whether inositol is present in the medium (unpublished data). Finally, css3 mutants, although inositol prototrophs, nonetheless require inositol for manifestation of the css phenotype. All sec14-1ts css3 mutants fail to grow on I−C− medium at 37°C. Depletion of inositol from the medium has no effect on growth of sec14-1ts css1 and sec14-1ts css2 mutants, however (Figure 1B).
css Mutants Are Defective in PtdEtn-Methylation Pathway Activity
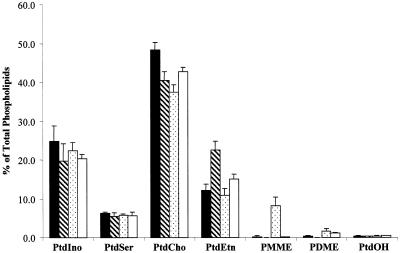
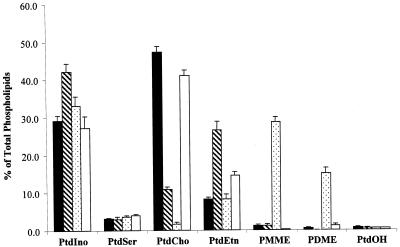
To investigate whether css mutations influence PtdCho turnover, we first analyzed the bulk phospholipid profiles of css mutant strains (see MATERIALS AND METHODS). Unexpectedly, all css mutants displayed profiles diagnostic of compromised activity of the PtdEtn-methylation pathway for PtdCho biosynthesis. Even in I+C+ media, where the CDP-choline pathway is a major contributor to PtdCho biosynthesis, all css mutants exhibit reduced PtdCho levels. Moreover, css1 mutants exhibit a twofold increase in bulk PtdEtn, whereas css2 mutants accumulate phosphatidylmonomethylethanolamine (PMME; Figure 2A). PMME is an intermediate in the conversion of PtdEtn to PtdCho via the PtdEtn-methylation pathway. In I+C− media, where the PtdEtn-methylation pathway is the sole route for net synthesis of bulk PtdCho, defects in the activity of this pathway are most dramatic. Under these conditions, we record a fourfold reduction in bulk PtdCho and a threefold elevation in bulk PtdEtn in css1 mutants, whereas css2 mutants exhibited a 30-fold reduction in bulk PtdCho and 30-fold elevations in levels of both PMME and phosphatidyldimethylethanolamine (PDME; Figure 2B). PDME is produced from PMME, and is the immediate precursor to PtdCho in the PtdEtn-methylation pathway (Figure 1A). Finally, css3 mutants display only modest reductions in bulk PtdCho and modest accumulations of PtdEtn under these conditions (Figure 2B). Inositol depletion has no significant effect on the phospholipid profiles of css1 and css2 strains, regardless of whether choline is present in the medium or not. Inositol depletion evokes a significant reduction in bulk PtdIno levels in css3 mutants, however (our unpublished data).
Figure 2.
Steady-state phospholipid profiles of css mutants in defined I+C+ media. Cells were grown for five to six generations at 26°C in I+C+ (A) or I+C− media (B) supplemented with [32P]orthophosphate to 10 μCi/ml. Bulk glycerophospholipids were extracted, resolved, and quantitated by phosphorimaging. Incorporation of 32P into each phospholipid species is indicated as a percentage of total incorporation of radiolabel into extractable phospholipid. Strains used were isogenic and included: CTY1-1A (sec14-1ts: black bars), CTY1374 (sec14-1ts css1; hatched bars), CTY1375 (sec14-1ts css2, stippled bars), CTY1473 (sec14-1ts css3; open bars). These data represent the averages of three independent experiments. PtdSer, phosphatidylserine.
CSS Genes Represent Structural Genes for Enzymes and Regulators of the PtdEtn-Methylation Pathway
The phospholipid profiles obtained for each css mutant provided strong clues for the molecular identities of the CSS genes. The accumulation of PtdEtn at the expense of PtdCho strongly implicated css1 mutations as alleles of CHO2, the PtdEtn methyltransferase structural gene (Figure 1A). This enzyme catalyzes the methylation of PtdEtn to produce PMME. The observed accumulation of both PMME and PDME in lieu of PtdCho suggested that css2 mutations are OPI3 alleles. OPI3 encodes a distinct phospholipid methyltransferase, which catalyzes the methylation of PMME to PDME and PDME to PtdCho (Figure 1A). Finally, the css3 profiles, when coupled with their inositol-dependent css phenotypes, strongly suggested these are hypomorphic alleles of INO2 or INO4. These genes encode transcription factors required for expression of not only CHO2 and OPI3 but also of INO1 whose product is required for de novo synthesis of inositol in yeast.
That css1, css2, and css3 mutations represent alleles of CHO2, OPI3, and INO4, respectively, is formally demonstrated by the collective weight of three lines of evidence. First, we find that low copy plasmids bearing individual CHO2, OPI3, or INO4 genes specifically complement css1, css2, and css3 mutations, respectively. Second, we find that naive cho2Δ, opi3Δ, and ino4Δ alleles themselves exhibit css phenotypes when introduced into sec14-1ts strains (our unpublished data). Third, meiotic segregation analyses indicate a tight genetic linkage between css1 and cho2Δ::HIS3, css2 and opi3Δ::HIS3, and css3 and ino4Δ::URA3. In these linkage experiments, diploid strains CTYD174 (css1 s14-1ts/cho2Δ sec14-1ts), CTYD175 (css2 sec14-1ts/opi3Δ sec14-1ts), and CTYD176 (css3 sec14-1ts/ino4Δ sec14-1ts) were generated (Table 1). Each of these diploids exhibits css phenotypes, consistent with CSS1/CHO2, CSS2/OPI3, and CSS3/INO4 allelic assignments. Analysis of at least 20 tetrads derived from each diploid confirmed these allelic assignments. In all cases, only parental di-type asci were recovered (4:0 css [Ts+]:0 CSS [ts−] spores). From this point forward, we use the CHO2, OPI3, and INO4 genetic nomenclature for CCS1, CSS2, and CSS3, respectively.
Because ino4 mutations were encountered in the screen for css mutants, and because the INO4 and INO2 gene products cooperate in regulating expression of CHO2 and OPI3, we also tested whether ino2Δ mutations elicit css phenotypes. As expected, sec14-1ts ino2Δ strains closely resemble sec14-1ts ino4Δ mutants from the standpoint that these exhibit css phenotypes that are considerably weaker than those associated with either sec14-1ts cho2 or sec14-1ts opi3 strains (our unpublished data). Although both the INO4 and INO2 gene products are required for optimal expression of CHO2 and OPI3, ino4Δ and ino2Δ mutations result in only modest reductions in PtdEtn-methylation pathway activity in vivo (Figure 2, A and B; unpublished data). Because ino4Δ and ino2Δ mutants exhibit the weakest css phenotypes, these collective data indicate that strength of the css phenotype is inversely proportional to PtdEtn-methylation pathway activity. In the following characterizations, we limit our analyses to cho2 and opi3 mutants.
PtdEtn-Methylation Pathway Defects Efficiently Restore Growth to Sec14p-deficient Yeast When Choline Reuptake Is Blocked by Inactivation of the Choline Transporter
Because PLD is activated in Sec14p-deficient yeast cells, we considered the possibility that PLD activity and the efficient recapture of excreted choline cooperate to drive CDP-choline pathway activity when yeast are grown in choline-free media. We reasoned that such a choline reuptake mechanism might obscure bypass Sec14p phenotypes that would otherwise be associated with defects in PtdEtn-methylation pathway function. A basic prediction of this hypothesis is that prevention of choline reuptake will potently improve the ability of PtdEtn-methylation pathway defects to promote Sec14p-independent cell growth.
To test this hypothesis, we determined whether disruption of the single high-affinity choline transporter of yeast (HNM1 gene product; Nikawa et al., 1990) improves growth of sec14-1ts cho2 and sec14-1ts opi3 strains at 37°C. The phenotypic data clearly demonstrate that hnm1Δ not only significantly improves the growth of sec14-1ts cho2 and sec14-1ts opi3 mutants on I+C− plates but also enables these strains to grow well on choline-rich I+C+ and YPD plates at 37°C (Figure 3A). Hnm1p dysfunction completely blocks the uptake of exogenous choline by yeast cells (Nikawa et al., 1990) and also impedes the uptake of exogenous ethanolamine (Nikawa et al., 1986). Because even high concentrations of exogenous ethanolamine (1 mM) have no effect on the weak rescue of sec14-1ts growth defects by cho2 and opi3 on I+C− media at 35°C or 37°C (see above), we conclude that diminished choline reuptake is the basis for the observed effects.
Combined Defects in Activities of the PtdEtn-Methylation Pathway and the Choline Transporter Bypass the Essential Cellular Requirement for Sec14p
We used a plasmid shuffle strategy to determine whether the combination of PtdEtn-methylation pathway mutations and choline transporter defects is sufficient to effect rescue of sec14Δ (see MATERIALS AND METHODS). We introduced an hnm1Δ::URA3 allele into a sec14Δ::hisG strain (CTY1469) carrying an endogenous YEp(SEC14 LEU2) plasmid that is required for the viability of this strain because this plasmid covers the unconditionally lethal sec14Δ::hisG allele. Subsequently, either cho2Δ::HIS3 or opi3Δ::HIS3 alleles were introduced into CTY1469 under conditions where the YEp(SEC14 LEU2) plasmid was subject to selection for leucine prototrophy. These cho2Δ::HIS3 and opi3Δ::HIS3 derivative strains (CTY1470 and CTY1471; Table 1) were then streaked onto YPD plates to relieve the nutritional selection pressure for YEp(SEC14 LEU2). The ability of these yeast strains to spontaneously cure the plasmid was assessed by monitoring the recovery of white colonies/colony sectors that exhibited unselected Leu− phenotypes. We find that, although the parental control strain CTY1469 is unable to cure the plasmid, both CTY1470 and CTY1471 readily do so. These data demonstrate that cho2Δ::HIS3 and opi3Δ::HIS3 alleles exert efficient bypass Sec14p phenotypes under conditions where choline uptake/reuptake is prevented. Remarkably, hnm1Δ renders deficiency in PtdEtn-methylation pathway function as potent a mechanism for bypass Sec14p as is CDP-choline pathway dysfunction.
PtdEtn-Methylation Pathway Defects Restore Growth to Sec14p-deficient Yeast in Liquid Media
Our demonstration that choline recapture dramatically attenuates the bypass Sec14p phenotype of PtdEtn-methylation pathway deficiencies suggests that the context in which bypass Sec14p mutants are selected has a significant bearing on what types of bypass Sec14p mutants are recovered. Initial selections involved plating of sec14-1ts cells and selecting for revertants that grew as single colonies on solid medium at 37°C (Cleves et al., 1989, 1991b). Efficient mechanisms of choline reuptake could potentially operate under these conditions because diffusion of choline away from cells is slower in choline-free agar than it is in the corresponding liquid medium. However, choline recapture should be much less efficient in liquid medium. Thus, PtdEtn-methylation pathway defects are predicted to effect bypass Sec14p in choline-free liquid medium, even in the face of choline transporter activity.
To test this possibility, appropriate strains were picked from freshly streaked YPD plates, inoculated into I+C− media, and incubated at 37°C. Culture growth was monitored by measuring the OD600 of the culture as a function of time. The sec14-1ts strain exhibits an initial doubling time of ∼6 h after which it ceases growing (Figure 3B). The sec14-1ts opi3 strain, however, grows nearly as robustly as the wild-type control strain under these conditions and the culture reaches saturation ∼12 h after inoculation (Figure 3B). Thus, in contrast to what is observed on solid growth media, the opi3 allele efficiently rescues sec14-1ts-associated growth defects when the test is performed in choline-free liquid media. As expected, the hnm1Δ allele does not further improve the growth of sec14-1ts opi3 strains in I+C− liquid medium at 37°C (Figure 3B).
To confirm that extracellular choline is growth inhibitory to the sec14-1ts opi3 strain, we supplemented a base I+C− liquid medium with choline to final concentrations of 1 and 10 μM. The growth of sec14-1ts opi3 cells at 37°C in these media was then assessed. Choline at a concentration of 1 μM effects a marked inhibition of cell growth, whereas 10 μM choline abolishes cell growth completely (our unpublished data). These collective data indicate that a choline transporter-mediated mechanism for choline recapture from the extracellular milieu, and the subsequent salvage of recaptured choline through the CDP-choline pathway for PtdCho biosynthesis, significantly obscure the bypass Sec14p phenotype associated with PtdEtn-methylation pathway dysfunction.
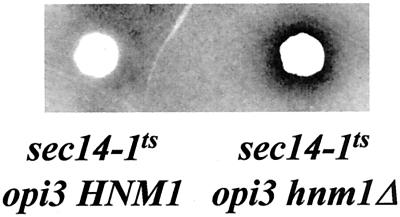
Demonstration of Choline Recapture on Solid Media
To test the idea that choline transporter activity mediates efficient choline recapture when cells are cultured on solid I+C− medium, we designed a sensitive bioassay to score the net leakage of choline from cells in the presence and absence of the choline transporter. The exquisite sensitivity of sec14-1ts opi3 strains to the growth inhibitory effects of exogenous choline at restrictive temperatures (Figure 1B) forms the basis for a cross-feeding assay designed to demonstrate a paracrine uptake of excreted choline. In this assay, we deposited a heavy bolus of sec14-1ts opi3 or sec14-1ts opi3 hnm1Δ yeast cells (as choline feeders) onto a lawn of sec14-1ts opi3 cells (as indicator) on I+C− plates, and incubated the plates at 37°C for 36 h. These incubation conditions result in PLD activation and subsequent stimulation of PtdCho hydrolysis to PtdOH and free choline. Leakage of choline from the feeder colony is manifested by the inhibition of growth of the surrounding indicator cells. As shown in Figure 3C, no halo of inhibition is formed when the feeder possesses a wild-type HNM1 gene. This result indicates that little if any choline escaped from the feeder colony. By contrast, introduction of hnm1Δ into the feeder strain results in formation of a clearly discernable halo surrounding the feeder colony. Experiments where sec14-1ts cho2 cells are used as feeder, or where sec14-1ts cho2 cells are used as indicator yield similar results (our unpublished data).
Measurements of PtdCho Synthesis
The collective results indicate that inhibition of choline reuptake is required for efficient down-regulation of PtdCho synthesis in sec14-1ts cho2 and sec14-1ts opi3 mutants at 37°C. We expected that choline recapture sustains PtdCho synthesis by driving activity of the CDP-choline pathway. This hypothesis predicts that metabolic flux through the CDP-choline pathway is low in sec14-1ts cho2 and sec14-1ts opi3 mutants incubated under conditions permissive for bypass Sec14p, but that CDP-choline pathway activity is high under conditions restrictive for cho2- and opi3-mediated bypass Sec14p.
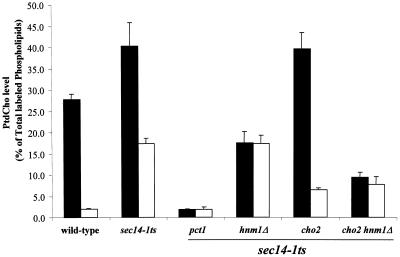
We tested these predictions by monitoring CDP-choline pathway activity in sec14-1ts cho2 mutants cultured in I+C− and I+C+ media, i.e., permissive and restrictive conditions for bypass Sec14p, respectively. We also performed these experiments with hnm1Δ derivatives of these strains for which bypass Sec14p is no longer a function of the choline content of the medium. As positive and negative controls for CDP-choline pathway activity, we used isogenic sec14-1ts and sec14-1ts pct1 mutants, respectively. To measure metabolic flux through the CDP-choline pathway without the complication of adding radiolabeled choline to the medium, we monitored the incorporation of 32P into PtdCho in a 20-min pulse radiolabeling experiment at a semipermissive temperature for sec14-1ts strains (33.5°C). In this regimen, PLD-dependent PtdCho turnover is stimulated and CDP-choline pathway activity is the predominant contributor to PtdCho synthesis (McGee et al., 1994; Rivas et al., 1999; Li et al., 2000).
As shown in Figure 3D, a comparison of the rates of 32P incorporation into PtdCho in SEC14 and sec14-1ts strains cultured in I+C+ versus I+C− medium demonstrates that CDP-choline pathway activity is highly stimulated by inclusion of choline in the medium. Of the total extractable lipid phosphate, the wild-type strain cultured in I+C+ and I+C− medium incorporates 27.7 ± 1.3 and 2.0 ± 0.17% of the 32P radiolabel into PtdCho, respectively. For the isogenic sec14-1ts strain, 40.3 ± 5.5 and 17.3 ± 1.3% of the radiolabel is incorporated into PtdCho under these same conditions, respectively. The increased rate of CDP-choline pathway activity in the sec14-1ts strain is in agreement with previous reports that Sec14p down-regulates this pathway (McGee et al., 1994; Skinner et al., 1995). Genetic inactivation of the CDP-choline pathway in the sec14-1ts pct1 mutant reduces the rate of PtdCho biosynthesis to nearly undetectable levels when cells are cultured in I+C+ or I+C− medium (1.8 ± 0.15 and 1.8 ± 0.6% of extractable lipid 32P incorporated into PtdCho, respectively). Introduction of the hnm1Δ allele into the sec14-1ts mutant reduced CDP-choline pathway activity in cells grown in I+C+ medium approximately fourfold from 40.3 ± 5.5 to 17.5 ± 2.7% of extractable lipid 32P incorporated into PtdCho, respectively. This level of incorporation was similar to that recorded for sec14-1ts hnm1Δ cells incubated in choline-free medium (17.3 ± 2.0%; Figure 3D). These levels of PtdCho synthesis are intermediate between the values recorded for the sec14-1ts and sec14-1ts pct1 mutants. The residual activity of the CDP-choline pathway measured in the hnm1Δ derivatives likely represents salvage of an intracellular choline pool that is generated by PtdCho turnover, but is not excreted from the cells.
The PtdCho profile of the sec14-1ts cho2 mutant is essentially indistinguishable from that of the isogenic sec14-1ts control when the strains are incubated in I+C+ medium (Figure 3D). However, CDP-choline pathway activity in the cho2 mutant is clearly reduced relative to the positive control when cells are incubated in I+C− medium (6.5 ± 0.31 versus 17.3 ± 1.3 of extractable lipid 32P incorporated into PtdCho, respectively). Introduction of the hnm1Δ lesion into the sec14-1ts cho2 derivative reduces CDP-choline pathway activity in cells grown in I+C+ medium (9.4 ± 1.1% of extractable lipid 32P incorporated into PtdCho) to a level resembling that recorded for sec14-1ts cho2 hnm1Δ cells incubated in choline-free medium (7.7 ± 1.9% of extractable lipid 32P incorporated into PtdCho; Figure 3D). Thus, in accord with expectations, metabolic flux through the CDP-choline pathway is low when sec14-1ts cho2 mutants are incubated under conditions permissive for bypass Sec14p, and CDP-choline pathway activity is high under conditions restrictive for cho2-mediated bypass Sec14p.
Golgi Secretory Function in Sec14p-deficient PtdEtn-Methylation Pathway Mutants
To determine whether the bypass Sec14p phenotypes associated with cho2 and opi3 alleles extend to rescue of sec14-1ts-associated secretory defects, we used efficiency of invertase secretion as an indicator of yeast secretory competence. This measurement is quantified by an invertase secretion index that relates the percentage of secreted invertase relative to the total amount of invertase produced by the cells (Bankaitis et al., 1989; Franzusoff and Schekman, 1989; Salama et al., 1990). In these experiments, we quantify the secretory efficiencies of yeast strains cultured at 37°C in I+C− liquid growth media (i.e., conditions where cho2 and opi3 alleles efficiently rescue sec14-1ts-associated growth defects). These values are then compared with the corresponding secretory efficiencies measured in I+C+ liquid media (i.e., restrictive conditions for cho2- and opi3-mediated rescue of sec14-1ts growth defects).
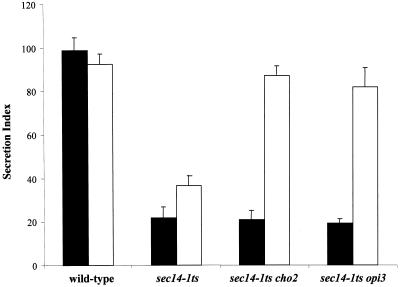
Wild-type yeast cells secreted invertase rapidly and efficiently when cultured in either I+C− or I+C+ liquid media. As shown in Figure 4A, the secretion indices for the wild-type strain are 99 ± 7.0 and 98.7 ± 6.1% in I+C− and I+C+ conditions, respectively. By contrast, the secretion index for the isogenic sec14-1ts strain is 21.7 ± 5.1% in I+C+ medium. This reduced secretory index reflects accumulation of invertase in the lumen of the yeast Golgi complex. Interestingly, the secretion index of the sec14-1ts strain is modestly, but significantly, improved to 36.6 ± 4.7% simply by culturing the strain in I+C− medium (Figure 4A).
Figure 4.
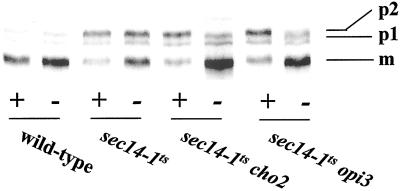
(A) Efficiency of invertase secretion at 37°C for PtdEtn-methylation pathway mutants grown in I+C+ (black bars) and I+C− media (open bars). Secretion index was calculated as (extracellular invertase/total invertase × 100%) (Salama et al., 1990), and the values presented represent the averages of triplicate determinations from at least three independent experiments. The secretion indices of wild-type strain and sec14-1ts strain represent the secretory efficiency under Sec14p-proficient and Sec14p-deficient conditions, respectively. Strains used were CTY182 (wild type), CTY1-1A (sec14-1ts), CTY1374 (sec14-1ts cho2), CTY1375 (sec14-1ts opi3). (B) Trafficking of CPY through the secretory pathway to vacuole in I+C+ and I+C− media. Appropriate strains were grown at 26°C to early logarithmic phase in the indicated media (I+C+ media are indicated by “+” and I+C− media was indicated by “−” below the lane), shifted to 37°C for 2 h, and pulse-radiolabeled with 35S-amino acids at 37°C for 30 min. Radiolabeled CPY species were recovered, resolved, and quantitated as described (Fang et al., 1996). The p1 (ER), p2 (Golgi), and mature vacuolar (m) forms of CPY are indicated at right. Strains used included CTY182 (wild type), CTY1-1A (sec14-1ts), CTY1374 (sec14-1ts cho2), and CTY1375 (sec14-1ts opi3).
Secretion indices recorded for the sec14-1ts cho2 and sec14-1ts opi3 strains cultured in I+C+ medium are 21.0 ± 4.1 and 19.2 ± 2.1%, respectively. These values are indistinguishable from those measured for the isogenic sec14-1ts strain cultured under the same conditions, and these secretory defects are consistent with the choline-sensitive growth of cho2 and opi3 strains at temperatures restrictive for function of the sec14-1ts gene product. By contrast, the invertase secretion indices of the sec14-1ts cho2 and sec14-1ts opi3 strains cultured in I+C− liquid medium are 87.2 ± 4.4 and 81.7 ± 9.1%, respectively (Figure 4A). These values are very similar to those recorded for the wild-type strain, indicating that cho2 and opi3 individually restore near wild-type efficiencies of invertase secretion to Sec14p-deficient cells when these were cultured in choline-free media. Introduction of hnm1Δ into sec14-1ts cho2 and sec14-1ts opi3 strains also restores wild-type invertase secretion profiles to these mutants, regardless of the choline-content of the medium (our unpublished data).
In an independent evaluation of Golgi secretory function, we employed pulse-chase methods to monitor the trafficking of carboxypeptidase Y (CPY) through the secretory pathway to vacuole. As illustrated in Figure 4B, the wild-type strain grown in either I+C+ or I+C− liquid media accumulates predominantly the 61 kDa mature form CPY (mCPY). Only trace amounts of the 67 kDa and the 69 kDa endoplasmic reticulum and Golgi precursor forms (p1 and p2 CPY, respectively) are observed. Because mCPY represents the vacuolar form of the enzyme, these data demonstrate the rapid trafficking of CPY through the yeast secretory pathway to the vacuole.
The isogenic sec14-1ts mutant cultured in I+C+ medium, however, accumulates ∼70% of the total labeled CPY as p2 CPY (Figure 4B). These results diagnose the defective transport of CPY from the Golgi complex in Sec14p-deficient strains. This trafficking block is substantially relieved when the sec14-1ts strain is challenged with the restrictive temperature in I+C− medium. Under these conditions, some 40% of the total labeled CPY is in the p2 form, whereas the remaining fraction is delivered to the vacuole and recovered as mCPY (Figure 4B). Thus, as in the invertase secretion measurements, simple omission of choline from the medium effects a detectable suppression of the secretory defects associated with Sec14p deficiency.
When incubated in I+C+ medium at 37°C, sec14-1ts cho2 and sec14-1ts opi3 strains exhibit CPY profiles that are indistinguishable from those recorded for the isogenic sec14-1ts mutant. That is, ∼70% of the total labeled CPY accumulates in the p2 form. When the experiment is performed in I+C− medium, the CPY profiles of both the sec14-1ts cho2 and sec14-1ts opi3 mutants are indistinguishable from those recorded for the wild-type strain. Nearly all of the labeled CPY is recovered as mCPY (Figure 4B). Again, hnm1Δ restores wild-type efficiencies of CPY trafficking to the vacuole in sec14-1ts cho2 and sec14-1ts opi3 strains irrespective of the choline content of the medium (our unpublished data). These collective data emphasize the point that PtdCho synthesis is toxic to yeast Golgi secretory function in the absence of a functional Sec14p. Evidence that at least one aspect of this toxicity involves an intrinsic toxicity of PtdCho itself is presented below.
Bypass Sec14p Phenotype Associated with cho2 hnm1Δ and opi3 hnm1Δ Alleles Is PLD-dependent
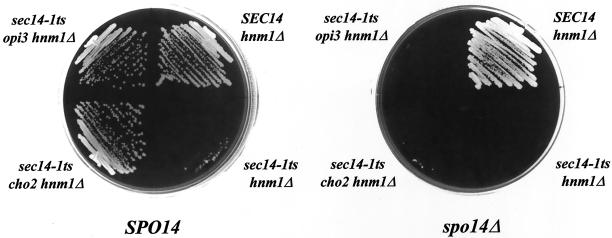
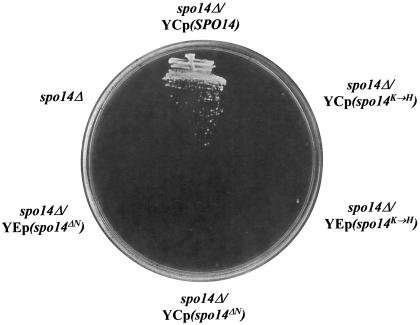
We previously demonstrated that all known pathways for bypass Sec14p exhibit the dual requirement for PLD catalytic activity, and an ability of PLD to properly access its PtdCho substrate in vivo (Xie et al., 1998). It was therefore of interest to determine whether the bypass Sec14p effected by PtdEtn-methylation pathway defects exhibits a similar PLD requirement. To investigate this issue, a spo14Δ allele was introduced into sec14-1ts cho2 hnm1Δ and into sec14-1ts opi3 hnm1Δ strains, and the growth phenotypes of these strains were assessed on YPD plates at 37°C. As shown in Figure 5A, spo14Δ has no effect on the growth of the SEC14 hnm1Δ control strain, but this mutation prevents growth of both the sec14-1ts cho2 hnm1Δ and the sec14-1ts opi3 hnm1Δ strain. Neither expression of a catalytic-dead spo14K→H PLD form (Sung et al., 1997) nor of a catalytically active form of PLD that fails to localize to membranes (spo14ΔN ;Rudge et al., 1998), complements the spo14Δ-associated ts− growth phenotype of these strains (Figure 5B). Finally, this acquired ts− phenotype reflects a reimposition of a Sec14p requirement for growth as evidenced by the fact that SEC14 cho2 hnm1Δ spo14Δ and SEC14 opi3 hnm1Δ spo14Δ mutants grow well under these conditions (unpublished data).
Figure 5.
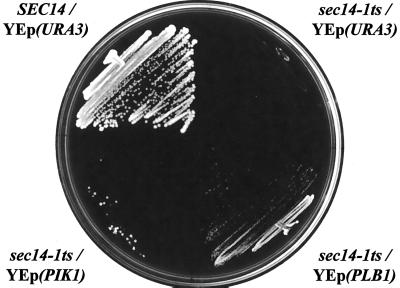
Role of phospholipase D in suppression of sec14 defects by PtdEtn-methylation pathway dysfunction. (A) Role of PLD (SPO14 gene product). The indicated strains were streaked onto YPD plates, incubated at 37°C for 48 h, and the growth of each strain was then recorded. Isogenic strains were used and these included CTY1499 (sec14-1ts hnm1Δ::URA3), CTY1502 (hnm1Δ::HIS3), CTY1504 (sec14-1ts cho2 hnm1Δ::HIS3), CTY1505 (sec14-1ts opi3 hnm1Δ::HIS3), CTY1506 (hnm1Δ::HIS3 spo14Δ::URA3), CTY1507 (sec14-1ts hnm1Δ::URA3 spo14Δ::HIS3), CTY1512 (sec14-1ts cho2 hnm1Δ::HIS3 spo14Δ::URA3), CTY1513 (sec14-1ts opi3 hnm1Δ::HIS3 spo14Δ::URA3). (B) The catalytic activity of PLD is necessary, but not sufficient, for the suppression of sec14 defects by PtdEtn-methylation pathway defects. The sec14-1ts opi3 hnm1Δ::URA3 spo14Δ::URA3 strain and its derivative strains carrying either a wild-type SPO14 gene on low copy plasmid (YCp), or a mutant spo14 gene on low copy plasmid (YCp) or high copy plasmid (YEp), were streaked on YPD plates and incubated at 37°C for 48 h. The growth of each strain was then recorded. Strains used included CTY1485 (sec14-1ts opi3 hnm1Δ::URA3 spo14Δ::URA3); CTY1519 (CTY1485/YCpSPO14); CTY1520 (CTY1485/YCp spo14K→H); CTY1521 (CTY1485/YCp spo14ΔN); CTY1522 (CTY1485/YEp spo14K→H); CTY1523 (CTY1485/YEp spo14ΔN). (C) Plb1p overexpression improves growth of sec14-1ts mutants at a nonpermissive temperature. Isogenic yeast strains CTY182 (SEC14) and CTY1–1A (sec14-1ts) were transformed with the indicated YEp plasmids. Transformants were isolated and streaked for isolated colonies on YPD medium at 35°C. Growth was scored after 48 h of incubation. Relevant genotypes are shown. The SEC14/YEp(URA3) and sec14-1ts/YEp(URA3) strains represented positive and negative controls for growth, respectively.
These data indicate that bypass Sec14p phenotypes associated with PtdEtn-methylation pathway defects require the participation of a catalytically active PLD that retains the ability to efficiently interface with its PtdCho substrate in vivo.
Relative Efficiencies of Plb1p and Pik1p Overexpression in Rescue of sec14-1ts-associated Growth Defects
PLD catalyzes hydrolysis of PtdCho to produce PtdOH and choline. PLD activity may support the suppression by the breakdown of PtdCho, or by the generation of a downstream metabolite PtdOH/DAG, or both. Present evidence indicates that generation of a downstream metabolite is a contributing factor to bypass Sec14p (Xie et al., 1998; Rivas et al., 1999). Yeast express a PLB1 gene that encodes a protein with both phospholipase B and lysophospholipase activity. Plb1p catalyzes a concerted deacylation of PtdCho and PtdEtn to glycerophosphocholine and glycerophosphoethanolamine, respectively. These compounds are generated without formation of a discrete lysophospholipid intermediate, and are excreted into the medium (Lee et al., 1994; Whit et al., 1994).
To determine whether Plb1p-mediated PtdCho hydrolysis modulates sec14-1ts-associated growth defects, we constructed a multicopy plasmid that directs expression of PLB1 via the powerful promoter of the yeast phosphoglycerate kinase structural gene (PGK). This plasmid, YEp(PPGK::PLB1), was introduced into a sec14-1ts strain and its phenotypic effects were determined. Our expectation was that Plb1p would serve as a negative control in experiments studying the relationship between PtdCho turnover and Sec14p function. Because this phospholipase is predicted to localize to the plasma membrane in an orientation where its active site is exposed to the noncytoplasmic leaflet of this membrane (Whit et al., 1984; Lee et al., 1994), we expected that Plb1p activity would be irrelevant to any Sec14p-dependent pathway. Surprisingly, we found that YEp(PPGK::PLB1) clearly improved the growth of sec14-1ts strains at restrictive temperatures. As shown in Figure 5C, the parental sec14-1ts strain does not grow at all when incubated at 35°C. The YEp(PPGK::PLB1) derivative, however, grows at this temperature and forms isolated colonies. The growth rate is significantly slower than that exhibited by the isogenic wild-type strain. Moreover, YEp(PPGK::PLB1) only partially rescues sec14-1ts growth defects because the effect is not observed at 37°C.
We cannot provide a precise rationale for this unanticipated result, given the expected topology of Plb1p. Perhaps yeast cells accelerate a flipping of PtdCho from the cytoplasmic leaflet of the plasma membrane (or other intracellular membranes, such as the Golgi complex) to the opposing leaflet when a PtdCho deficit is imposed upon the noncytoplasmic leaflet. Alternatively, Plb1p overproduction might result in sustained levels of active Plb1p in the cytoplasm, thereby promoting the abnormal deacylation of PtdCho in the cytoplasmic leaflet of intracellular membranes.
The idiosyncratic nature of the effect notwithstanding, YEp(PPGK::PLB1) effects a stronger suppression of sec14-1ts growth defects than does a YEp(PIK1) plasmid, which drives overexpression of the Pik1p PtdIns 4-kinase (Walch-Solimena and Novick, 1999). Hama et al. (1999) reported that YEp(PIK1) suppresses sec14-1ts growth defects at 34°C and interpreted that result to indicate that the critical function of Sec14p is to stimulate Pik1p-dependent synthesis of PtdIns-4-phosphate. We do not find that YEp(PIK1) exerts a significant effect on sec14-1ts-associated growth defects at 34 or 35°C, yet YEp(PPGK::PLB1) clearly does (Figure 5C). Because Plb1p deacylates PtdCho to products that cannot be directly metabolized to PtdOH or DAG, we conclude that the benefit realized by Sec14p-deficient mutants overproducing Plb1p is related to PtdCho hydrolysis itself. These data suggest an intrinsic toxicity of PtdCho to Sec14p-dependent Golgi function in yeast. This result agrees with our previous demonstration that a mutant Sec14p that retains only its PtdCho-transfer activity, and its ability to down-regulate PtdCho synthesis via the CDP-choline pathway in vivo, fulfills all essential Sec14p functions in vivo (Phillips et al., 1999).
DISCUSSION
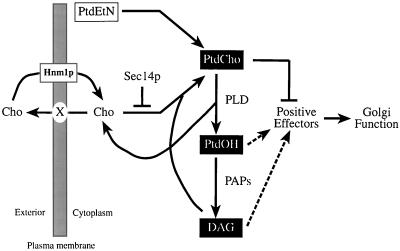
Herein, we describe a body of evidence that indicates an intrinsic toxicity of PtdCho to Golgi function in yeast. The data support the concept that a primary function of Sec14p is to modulate PtdCho biosynthesis so that an appropriate balance is maintained between PtdCho production and a Golgi PtdCho content that is permissive for protein transport from this organelle. Our data also provide a solution to the long-standing puzzle for why defects in the CDP-choline pathway for PtdCho biosynthesis efficiently effect bypass Sec14p, whereas defects in PtdCho synthesis via the PtdEtn methylation pathway do not (Cleves et al., 1991b). Namely, that a cycle of PtdCho turnover, coupled to choline excretion and reuptake, activates the CDP-choline pathway and obscures the bypass Sec14p phenotype that would normally be associated with inactivation of the PtdEtn-methylation pathway (Figure 6).
Figure 6.
PtdCho toxicity for Sec14p-dependent Golgi secretory function. We propose PtdCho is toxic to Sec14p-dependent Golgi secretory function because of its negative effects on the activities of positive downstream effectors. Defects in PtdCho biosynthesis rescue viability of Sec14p-deficient cells when the CDP-choline pathway for PtdCho biosynthesis is inactivated, or is operating at low levels, and PLD is activated. This condition is a function of the efficiency with which the pool of liberated choline that is excreted from cells is recaptured by the choline transporter (Hnm1p). The recaptured choline is metabolically channeled into the CDP-choline pathway. PtdOH and DAG are also produced by PLD; in the former case directly, and in the latter case by PtdOH-phosphohydrolase (PAP)-mediated dephosphorylation of PtdOH. We propose that the combinatorial regulation of PtdCho, DAG, and PtdOH metabolism by Sec14p (or PLD under conditions of Sec14p dysfunction) is required for at least one essential pathway for protein transport from the yeast Golgi complex.
Initial clues regarding pathways for bypass Sec14p that involve PtdEtn-methylation dysfunction came from a genetic screen we had originally devised for the purpose of detecting mutations that compromise PtdCho turnover (Xie et al., 1998). Of the three genes identified by this screen, we found that two correspond to CHO2 and OPI3, i.e., structural genes that encode for distinct phospholipid methyltransferases that are involved in the de novo production of PtdCho from PtdEtn. The third gene encodes a transcription factor, Ino4p, that has long been known to cooperate with its Ino2p binding partner in driving optimal expression of phospholipid biosynthetic genes such as CHO2 and OPI3 (Carman and Zeimetz, 1996). Subsequent tests indicated that ino2 mutations also satisfy the genetic screen. This outcome demonstrates that the assumptions upon which that genetic screen was founded were imprecise. In retrospect, we now appreciate that this screen points the way toward identifying novel pathways for bypass Sec14p that involve the combinatorial interface of pathways for PtdCho biosynthesis, turnover, and mechanisms of choline salvage.
Detailed analyses of these mutant phenotypes reveal unexpected aspects of yeast physiology as these pertain to choline metabolism. Our data indicate that a significant fraction of the choline generated via PLD-mediated hydrolysis of PtdCho is excreted from cells. The fate of this excreted choline determines the activity of the CDP-choline pathway, which functions to scavenge whatever excreted choline is recaptured and reincorporates it into PtdCho. The salvage activity of the CDP-choline pathway fueled by recaptured choline is of primary significance to the phenotypic effects of the PtdEtn methylation pathway as these relate to bypass Sec14p. Under conditions where choline recapture is favored, such as incubation of cells on solid choline-free medium (I+C− plates) or when the yeast high-affinity choline transporter is functional, choline salvage is efficient and the CDP-choline pathway is active. That metabolic flux through the CDP-choline pathway obscures the bypass Sec14p phenotype that would otherwise be associated with PtdEtn-methylation pathway inactivity.
By contrast, cells grown in choline-free liquid media face a rapid outward diffusion of excreted choline into the environment. In cultures with a relatively low cell density, excreted choline rapidly dilutes to concentrations below the Km of the transporter (1 μM; Nikawa et al., 1990). These conditions do not favor choline recapture, the CDP-choline pathway remains largely inactive, and PtdEtn-methylation pathway dysfunction supports Sec14p-independent cell growth and Golgi secretory function. Inactivation of the choline transporter generates the same condition and, in that circumstance, the bypass Sec14p phenotype associated with PtdEtn-methylation pathway defects becomes choline-resistant.
These various data satisfactorily account for why mutations that inactivate the PtdEtn-methylation pathway were not recovered in the classical bypass Sec14p mutant selections of Cleves et al. (1989, 1991b). Those selections were not only performed in choline-replete medium at 37°C, but used agar plates for both mutant selection and phenotypic characterization of mutants. In addition, directed tests of whether PtdEtn-methylation pathway defects could effect bypass Sec14p were performed on I+C− plates at 37°C (Cleves et al., 1991b). It is now clear that selection of bypass Sec14p mutants in I+C− liquid medium does yield mutants individually deficient in activity of either the CDP-choline pathway or the PtdEtn pathway for PtdCho biosynthesis.
Finally, our demonstration that genetic inactivation of either pathway for PtdCho biosynthesis can result in bypass Sec14p, and that accelerated rates of Plb1p-driven PtdCho hydrolysis effect partial suppression of sec14-1ts-associated growth defects, make a case that PtdCho is intrinsically toxic to Golgi function in yeast. This finding supports our early model that Sec14p acts to reduce Golgi PtdCho content (Cleves et al., 1991a,b). This finding also forces us to reconsider our later models that the CDP-choline pathway is uniquely toxic to Golgi secretory function solely on the basis of its consumption of a critical metabolic precursor (i.e., DAG; McGee et al., 1994; Kearns et al., 1997; Rivas et al., 1999). Elucidation of the details for why PtdCho is toxic to yeast Golgi function now defines an important area for future study.
ACKNOWLEDGMENTS
We are very grateful to JoAnne Engebrecht (State University of New York, Stony Brook, NY) for the generous gift of SPO14, spo14K→H, and spo14ΔN plasmids from which the plasmids we used were derived. We are also grateful to Fritz Paltauf (Graz, Austria) for providing a PLB1 clone, and Christiane Walch-Solimena and Peter Novick (Yale University) for making YEp(PIK1) available to us. This work was supported by National Institutes of Health Grant GM44530 awarded to V.A.B.
REFERENCES
- Antonny B, Huber I, Paris S, Chabre M, Cassel D. Activation of ADP-ribosylation factor 1 GTPase-activating protein by phosphatidylcholine-derived diacylglycerols. J Biol Chem, 1997;272:30848–30851. doi: 10.1074/jbc.272.49.30848. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14. gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman GM, Zeimetz GM. Regulation of phospholipid biosynthesis in the yeast Saccharomyces cerevisiae. J Biol Chem. 1996;271:13293–13296. doi: 10.1074/jbc.271.23.13293. [DOI] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Bankaitis VA. Phospholipid transfer proteins: a biological debut. Trends Cell Biol. 1991a;1:30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- Cleves AE, Novick PJ, Bankaitis VA. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol. 1989;109:2939–2950. doi: 10.1083/jcb.109.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991b;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MKY, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A, Schekman R. Functional compartments of the Golgi complex are defined by the sec7 mutation. EMBO J. 1989;8:2695–2702. doi: 10.1002/j.1460-2075.1989.tb08410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkaline cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns BG, Alb JG, Jr, Bankaitis VA. Phosphatidylinositol transfer proteins: the long and winding road to function. Trends Cell Biol. 1998;8:276–282. doi: 10.1016/s0962-8924(98)01281-1. [DOI] [PubMed] [Google Scholar]
- Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. An essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Patton JL, Fido M, Hines LK, Kohlwein SD, Paltauf F, Henry SA, Levin DE. The Saccharomyces cerevisiae PLB1 gene encodes a protein required for lysophospholipase and phospholipase B activity. J Biol Chem. 1994;269:19725–19730. [PubMed] [Google Scholar]
- Li X, Routt SM, Xie Z, Cui X, Fang M, Kearns MA, Bard M, Kirsch DR, Bankaitis VA. Identification of a novel family of nonclassical yeast PITPs whose function modulates phospholipase D activity and Sec14p-independent cell growth. Mol Biol Cell. 2000;11:1989–2005. doi: 10.1091/mbc.11.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MC, Nicaud J-M, Skinner HB, Vergnolle C, Kader JC, Bankaitis VA, Gaillardin C. A phosphatidylinositol/phosphatidylcholine transfer protein is required for differentiation of the dimorphic yeast Yarrowia lipolytica from the yeast to the mycelial form. J Cell Biol. 1994;124:113–127. doi: 10.1083/jcb.125.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee TP, Skinner HB, Whitters EA, Henry SA, Bankaitis VA. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J Cell Biol. 1994;124:273–287. doi: 10.1083/jcb.124.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa J, Hosaka K, Tsukagoshi Y, Yamashita S. Primary structure of the yeast choline transport gene and regulation of its expression. J Biol Chem. 1990;265:15996–16003. [PubMed] [Google Scholar]
- Nikawa J, Tsukagoshi Y, Yamashita S. Cloning of a gene encoding choline transport in Saccharomyces cerevisiae. J Bacteriol. 1986;166:328–330. doi: 10.1128/jb.166.1.328-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SE, Sha B, Topalof L, Xie Z, Alb JG, Klenchin VA, Swigart P, Cockcroft S, Martin TF, Luo M, Bankaitis VA. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol Cell. 1999;4:187–197. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- Rivas MP, Kearns BG, Xie Z, Guo S, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Pleiotropic alterations in lipid metabolism in yeast sac1 mutants: relationship to “bypass Sec14p”and inositol auxotrophy. Mol Biol Cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K, Rudge SA, Frohman MA, Morris AJ, Engebrecht J. Phospholipase D signaling is essential for meiosis. Proc Natl Acad Sci USA. 1995;92:12151–12155. doi: 10.1073/pnas.92.26.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein RJ. One step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rudge SA, Morris AJ, Engebrecht J. Relocalization of phospholipase D activity mediates membrane formation during meiosis. J Cell Biol. 1998;140:81–90. doi: 10.1083/jcb.140.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1983. pp. 1–113. [Google Scholar]
- Salama SR, Cleves AE, Malehorn DE, Whitters EA, Bankaitis VA. Cloning and characterization of Kluyveromyces lactis SEC14, a gene whose product stimulates Golgi secretory function in Saccharomyces cerevisiae. J Bacteriol. 1990;172:4510–4521. doi: 10.1128/jb.172.8.4510-4521.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HB, McGee TP, McMaster CR, Fry MR, Bell RM, Bankaitis VA. Phosphatidylinositol transfer protein stimulates yeast Golgi function by inhibiting choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci USA. 1995;92:112–116. doi: 10.1073/pnas.92.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivas A, Paton-Vogt JL, Bruno V, Griac P, Henry SA. A role for phospholipase D in growth, secretion, and regulation of membrane lipid synthesis in yeast. J Biol Chem. 1998;273:16635–16638. doi: 10.1074/jbc.273.27.16635. [DOI] [PubMed] [Google Scholar]
- Sung TC, Roper RL, Zhang Y, Rudge SA, Temel R, Hammond SM, Morris AJ, Moss B, Engebrecht J, Frohman MA. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J. 1997;16:4519–4530. doi: 10.1093/emboj/16.15.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nature Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- Witt W, Schweingruber ME, Mertsching A. Phospholipase B from the plasma membrane of Saccharomyces cerevisiae. Separation of two forms with different carbohydrate content. Biochim Biophys Acta. 1984;795:108–116. doi: 10.1016/0005-2760(84)90110-3. [DOI] [PubMed] [Google Scholar]
- Xie Z, Fang M, Rivas MP, Faulkner A, Sternweis PC, Engebrecht J, Bankaitis VA. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci USA. 1998;95:12346–12351. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]